N, P Self-Doped Porous Carbon Material Derived from Lotus Pollen for Highly Efficient Ethanol–Water Mixtures Photocatalytic Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pollen Carbon Material
2.2. Characterization
2.3. Photocatalytic (PC) Performances
2.4. Electrochemical (EC) and Photoelectrochemical (PEC) Performance
3. Results and Discussion
3.1. Characterizations of Catalysts
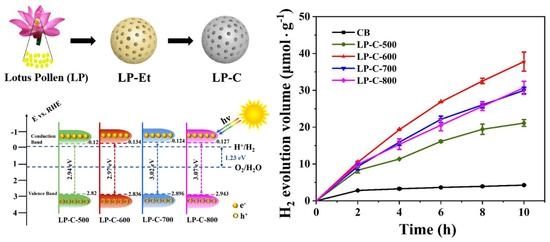
3.2. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ager, J.W.; Shaner, M.; Walczak, K.; Sharp, I.D.; Ardo, S. Experimental Demonstrations of Spontaneous, Solar-Driven Photoelectrochemical Water Splitting. Energy Environ. Sci. 2015, 8, 2799–3050. [Google Scholar] [CrossRef] [Green Version]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Lund, H. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Sofii, Y.K.; Siswanto, E.; Ueda, T.; Wardana, I.N.G. The role of activated carbon in boosting the activity of clitoria ternatea powder photocatalyst for hydrogen production. Int. J. Hydrog. Energy 2020, 45, 22613–22628. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2008, 139, 244–260. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [Green Version]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production of CdS-Cluster-Decorated Graphene Nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1694. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly Efficient Photocatalytic H2Evolution from Water using Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Chen, Y.-X.; Lu, C.-Z. Bio-inspired Materials for Photocatalytic Hydrogen Production. Chin. J. Struct. Chem. 2020, 39, 2123–2130. [Google Scholar]
- Wang, Z.; Smith, A.T.; Wang, W.; Sun, L. Versatile Nanostructures from Rice Husk Biomass for Energy Applications. Angew. Chem. Int. Ed. 2018, 57, 13722–13734. [Google Scholar] [CrossRef]
- Zan, G.; Wu, Q. Biomimetic and Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Mater. 2016, 28, 2099–2147. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S.G. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Ge, S.; Liu, L.; Shao, Q.; Mai, X.; Zhao, C.X.; Hao, L.; Wu, T.; Yu, Z.; Guo, Z. Microwave Solvothermal Fabrication of Zirconia Hollow Microspheres with Different Morphologies Using Pollen Templates and Their Dye Adsorption Removal. Ind. Eng. Chem. Res. 2018, 57, 231–241. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated Graphitic Carbon Nitride Nanosheets as Efficient Catalysts for Hydrogen Evolution Under Visible Light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2008, 19, 292–305. [Google Scholar] [CrossRef]
- Xia, F.; Wang, H.; Jia, Y. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014, 5, 4458. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wei, D.; Zhang, C.; Shao, Q.; Murugadoss, V.; Guo, Z.; Jiang, Q.; Yang, X. An Overview of Oxygen Reduction Electrocatalysts for Rechargeable Zinc-Air Batteries Enabled by Carbon and Carbon Composites. Eng. Sci. 2021, 15, 1–19. [Google Scholar] [CrossRef]
- Li, X.; Guan, B.Y.; Gao, S.; Lou, X.W. A general dual-templating approach to biomass-derived hierarchically porous heteroatom-doped carbon materials for enhanced electrocatalytic oxygen reduction. Energy Environ. Sci. 2019, 12, 648–655. [Google Scholar] [CrossRef]
- Yi, J.; Qing, Y.; Wu, C.; Zeng, Y.; Wu, Y.; Lu, X.; Tong, Y. Lignocellulose-derived porous phosphorus-doped carbon as advanced electrode for supercapacitors. J. Power Sources 2017, 351, 130–137. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2856. [Google Scholar] [CrossRef] [Green Version]
- Kipp, M.A.; Stüeken, E.E. Biomass recycling and Earth’s early phosphorus cycle. Sci. Adv. 2017, 3, 4795. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Lagerkvist, A. Phosphorus recovery from the biomass ash: A review. Renew. Sustain. Energy Rev. 2011, 15, 3588–3602. [Google Scholar] [CrossRef]
- Jinli, Z.; Jiao, W.; Yuanyuan, L.; Ning, N.; Junjie, G.; Feng, Y.; Wei, L. High-performance lithium iron phosphate with phosphorus-doped carbon layers for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2043–2049. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, T.O.; Zhang, D. Biotemplated Materials for Sustainable Energy and Environment: Current Status and Challenges. ChemSusChem 2011, 4, 1344–1387. [Google Scholar] [CrossRef]
- Cheng, Z.; Fang, W.; Zhao, T.; Fang, S.; Bi, J.; Liang, S.; Li, L.; Yu, Y.; Wu, L. Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution on Phosphorus-Doped Covalent Triazine-Based Frameworks. ACS Appl. Mater. Interfaces 2018, 10, 41415–41421. [Google Scholar] [CrossRef]
- Zhao, J.K.; Wei, D.N.; Zhang, X.B.; Zhang, S.G.; Zhang, C.; Yang, X.J. Biomass-derived hierarchical N, P codoped porous 3D-carbon framework@TiO2 hybrids as advanced anode for lithium-ion batteries. J. Colloid Interface Sci. 2021, 606, 577–587. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, W.; Su, H.; Fan, T.; Zhu, S.; Liu, Q.; Zhang, D. Morphology Genetic Materials Templated from Natural Species. Adv. Mater. 2015, 27, 464–478. [Google Scholar] [CrossRef]
- Wei, L.; Tian, K.; Jin, Y.; Zhang, X.; Guo, X. Three-dimensional porous hollow microspheres of activated carbon for high-performance electrical double-layer capacitors. Microporous Mesoporous Mater. 2016, 227, 210–228. [Google Scholar] [CrossRef]
- Sun, L.; Fu, Y.; Tian, C.; Yang, Y.; Wang, L.; Yin, J.; Ma, J.; Wang, R.; Fu, H. Isolated Boron and Nitrogen Sites on Porous Graphitic Carbon Synthesized from Nitrogen-Containing Chitosan for Supercapacitors. ChemSusChem 2014, 7, 1637–1646. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Gawdzik, B.; Tascón, J. Phosphorus-containing carbons: Preparation, properties and utilization. Carbon 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, S.; Pan, D.; Shao, Q.; Lin, J.; Wang, Z.; Hu, Z.; Wu, T.; Guo, Z. Solvothermal synthesis, characterization and photocatalytic property of zirconium dioxide doped titanium dioxide spinous hollow microspheres with sunflower pollen as bio-templates. J. Colloid Interface Sci. 2018, 529, 111–121. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Amati, M.; Llorente, B.; Mudimela, P.; Colomer, J.-F.; Ghijsen, J.; Ewels, C.; Snyders, R.; Gregoratti, L.; Bittencourt, C. Nitrogen ion casting on vertically aligned carbon nanotubes: Tip and sidewall chemical modification. Carbon 2014, 77, 319–328. [Google Scholar] [CrossRef]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of Phosphorus-Doped Graphene and its Multifunctional Applications for Oxygen Reduction Reaction and Lithium Ion Batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Barakat, T.; Wang, T.; Krief, A.; Zeng, Y.; Laboureur, M.; Fusaro, L.; Liao, H.; Su, B. Synergistic effects of carbon doping and coating of TiO2 with exceptionalphotocurrent enhancement for high performance H2 production from water splitting. J. Energy Chem. 2021, 56, 141–151. [Google Scholar] [CrossRef]
- Wood, D.L.; Tauc, J. Weak Absorption Tails in Amorphous Semiconductors. Phys. Rev. B 1972, 5, 3144–3151. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Z.; Sun, H.; Chen, F.; Xu, X.; Wu, Z.; Li, P.; Ge, W. Enhanced Photocatalytic H2 Production on Three-Dimensional Porous CeO2/Carbon Nanostructure. ACS Sustain. Chem. Eng. 2018, 6, 9691–9698. [Google Scholar] [CrossRef]
- Zha, D.-W.; Li, L.-F.; Pan, Y.-X.; He, J.-B. Coconut shell carbon nanosheets facilitating electron transfer for highly efficient visible-light-driven photocatalytic hydrogen production from water. Int. J. Hydrog. Energy 2016, 41, 17370–17379. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Huang, S.; Huang, X.; Xu, B.; Hu, L.; Cui, H.; Ruan, S.; Zeng, Y.-J. Bio-inspired carbon doped graphitic carbon nitride with booming photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 246, 61–71. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, F. Enhanced visible light photocatalytic H2 production activity of g-C3N4 via carbon fiber. Appl. Surf. Sci. 2015, 358, 287–295. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Lu, H.; Lin, T.; Ruan, S.; Zhao, X.S.; Zeng, Y.-J. Improving the Visible-Light Photocatalytic Activity of Graphitic Carbon Nitride by Carbon Black Doping. ACS Omega 2018, 3, 15009–15017. [Google Scholar] [CrossRef] [PubMed]










| Samples | SBET (m2/g) | Smic (m2/g) | Vt (cm3/g) | Vmic (cm3/g) | Vmic/Vt |
|---|---|---|---|---|---|
| LP-C-500 | 1.039 | 0.612 | 0.000238 | 0.000236 | 0.991 |
| LP-C-600 | 81.685 | 76.066 | 0.033854 | 0.029557 | 0.873 |
| LP-C-700 | 2.353 | 0.885 | 0.004092 | 0.003322 | 0.871 |
| LP-C-800 | 350.454 | 298.074 | 0.150731 | 0.114759 | 0.761 |
| Samples | Co-Catalyst | Photocatalyst Dosage (mg) | Solvent | Reaction Parameters | H2 Production Activity (μmol·g−1·h−1) | Photoresponse Cut-off Wavelength/Band Gap | Ref. |
|---|---|---|---|---|---|---|---|
| Nano-CeO2/carbon | Nano-CeO2 | 100 | MeOH/H2O | 300 W Xe lamp | 120 | Null/3.08 eV | [47] |
| Pt/CSC | Pt | 100 | 20 vol% Lactic acid | 300 W Xe lamp/visible light | 0 | Null | [48] |
| 1.5CCN650 | Pt | 10 | TEOA/H2PtCl6/H2O | Xe lamp/λ > 420 nm | 1889 | Null | [49] |
| CF-C3N4 | Pt | 50 | TEOA/H2O | 350 W Xe lamp/ 180 mW/cm2 | 1080 | Null | [50] |
| Carbon black/g-C3N4 | Pt | 10 | TEOA/H2O | Null | 234 | Null | [51] |
| Carbon Black | Null | 10 | EtOH/H2O | LED light/100 mW/cm2 | 0.4 | 300 nm/Null | This work |
| LP-C-600 | Null | 10 | EtOH/H2O | LED light/100 mW/cm2 | 3.5 | 600 nm/2.97 eV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.-W.; Jiang, X.; Chen, Y.-X.; Lin, S.-W.; Lu, C.-Z. N, P Self-Doped Porous Carbon Material Derived from Lotus Pollen for Highly Efficient Ethanol–Water Mixtures Photocatalytic Hydrogen Production. Nanomaterials 2022, 12, 1744. https://doi.org/10.3390/nano12101744
Zhou J-W, Jiang X, Chen Y-X, Lin S-W, Lu C-Z. N, P Self-Doped Porous Carbon Material Derived from Lotus Pollen for Highly Efficient Ethanol–Water Mixtures Photocatalytic Hydrogen Production. Nanomaterials. 2022; 12(10):1744. https://doi.org/10.3390/nano12101744
Chicago/Turabian StyleZhou, Jing-Wen, Xia Jiang, Yan-Xin Chen, Shi-Wei Lin, and Can-Zhong Lu. 2022. "N, P Self-Doped Porous Carbon Material Derived from Lotus Pollen for Highly Efficient Ethanol–Water Mixtures Photocatalytic Hydrogen Production" Nanomaterials 12, no. 10: 1744. https://doi.org/10.3390/nano12101744
APA StyleZhou, J. -W., Jiang, X., Chen, Y. -X., Lin, S. -W., & Lu, C. -Z. (2022). N, P Self-Doped Porous Carbon Material Derived from Lotus Pollen for Highly Efficient Ethanol–Water Mixtures Photocatalytic Hydrogen Production. Nanomaterials, 12(10), 1744. https://doi.org/10.3390/nano12101744