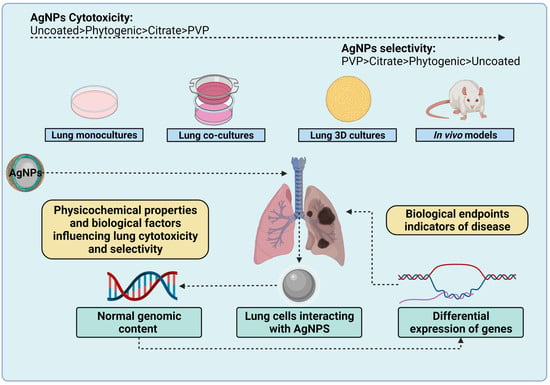
Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health
Abstract
:1. Introduction
2. Relation between Physicochemical Properties and Their Target Biological Models (PP × BM)
2.1. Size
2.2. Surface Reactivity
2.3. Agglomeration
2.4. Ion Release
2.5. Surface Functionalization
2.5.1. Uncoated AgNPs
2.5.2. Phytogenic AgNPs
2.5.3. Citrate-AgNPs
2.5.4. Polyvinylpyrrolidone-AgNPs
3. Biological Factors Involved in AgNPs Cytotoxicity
3.1. Cell Type
3.2. Pulmonary Cell Type
3.2.1. Bronchial Epithelial Cells (BECs)
3.2.2. Alveolar Epithelial Cells
3.2.3. Macrophages
3.3. Biocorona Formation
3.4. Gender
3.5. Cytotoxic Response-Dependent on Extrinsic Factors
3.5.1. Time of Exposure
3.5.2. Concentration/Dose
3.5.3. Model
4. Nanotoxicity Models to Evaluate Lung Cytotoxicity
4.1. Lung Mono-Cultures
4.2. Lung Co-Cultures
4.3. Lung 3D Cultures
4.4. Ex Vivo Lung Models
4.5. In Vivo Lung Models
| AgNPs Coating | Size (nm) | Dose (mg/kg BW) | Time of Exposure | Model | Observed Outcome | Ref |
|---|---|---|---|---|---|---|
| PVP | 10–30 | Daily i.p. of 0.25, 0.5, 1 | 9 d | Male Balb/C mice | Toxic damage in major organs at all doses (lung, liver, spleen, kidney, heart, brain, and testicles) Dose-dependent toxicity on the lung. Thickening of interstitial tissues and focal interstitial pneumonia (0.5 mg/kg BW). Significant interstitial pneumonia with massive cell infiltration and interstitial hemorrhage (1 mg/kg BW). | [127] |
| PVP | 25 | Final dose 0.02 using inhalation chamber | Exposure to 0.7 mg/m3 AgNPs for a half-hour every day until 45 days | Male C57BL/6 mice | Cell cycle arrest in the G2/M phase Upregulation of COX2/PGE2 intracrine pathway Accelerate lung cellular senescence Cause mild fibrosis. | [130] |
| PVP | 50 and 200 | 3.75, 75, 150, 300 μg | 3 and 21 d | Female Wistar rat | Dose-dependent toxicity. DNA double-strand breaks. Damage to alveolar macrophages and endothelial cell destruction Lung inflammation. | [40] |
| PVP and citrate | 20, 60 and 100 nm | 10 μg Ag/mouse | 4 and 24 h | Male ICR mouse | IL-1β and neutrophils in BALF, lung inflammation but do not indicate if PVP- or citrate-AgNps produce it. Size-dependent toxicity for citrate-AgNPs. | [131] |
| Citrate | 20 and 110 nm | 0.5 mg AgNPs/kg BW | 24 h | Sprague Dawley Rats | Size-dependent uptake and toxicity. Ion flux dysfunction, ROS production. Uptake-dependence produces cytoskeleton rearrangement, stiffening of mechanics, and cytoskeleton damage that softens the mechanical profile. | [44] |
| Citrate | 20 and 110 | Single n.a. 7.2 and 5.4 mg/m3 | 6 h | Male Sprague Dawley rats | Presence of silver in tissue macrophages obtained from BALF, 56 days post-exposure. AgNPs are predominantly localized within the lung’s terminal bronchial/alveolar duct junction region associated with extracellular matrix and within epithelial cells. | [128] |
| Citrate | 20 | o.a. 0.25 | 24 h | Male mice CBA/J, C57L/J, MRL/MpJ, NOD/ShiLtJ, NZB/BlNJ, NZO/HlLtJ, NZW/LacJ, PL/J, PWD/PhJ, PWK/PhJ, TALLYHO/JngJ, WSB/EiJ, BALB/cJ, BTBRT + tf/J, C3H/HeJ, C57BL/10J, DBA/2J, FVB/NJ, SJL/J, SM/J, SWR/J, 129S1/SvImJ, A/J, AKR/J, and C57BL/6J. | Strain and treatment-dependent in neutrophils in BALF with the exception of SWR/J, DBA/2J, and SM/J. Lung inflammation | [132] |
| Citrate | 20 nm | Single IV 5 | 1, 3, and 5 d | Male Sprague Dawley rats | Time-dependent Ag accumulation in the lung. Ag+ accelerates the dissolution of citrate-AgNPs by MT overexpression. | [133] |
| Citrate, octreotide (OCT), and Citrate/ OCT/ alginate (ALG) | 22.77 ± 1.1, 78.77 ± 2.3, and 155.99 ± 5.2 nm | Nebulization of 1.27 at a rate of 5 mL/h for 3d (10h/d) | 3 d | Male and female Sprague Dawley rats | AgNPs surface modification with OCT and ALG favors AgNPs accumulation in the lung and enhances interaction with somatostatin receptors (SSRT)in tumor cell lines. | [134] |
| ND | 14–15 nm | 0.05, 0.12, and 0.38 mg/m3 (Low, medium, and high dose respectively) in an inhalation chamber | 6 h/day, 5 days/week for 12 weeks | Male and female Sprague Dawley rats | Accumulation in greater quantity in the lung, and in a dose-dependent manner in the liver, kidney, blood, vessel, eye, and testicle. No effect on the brain High amounts of silver were maintained after 12 weeks in liver, vessel, and eyes | [101] |
| ND | 18–19 nm | 0.049, 0.133 o 0.515 mg/m3 (Low, medium, and high dose respectively) in a whole-body inhalation chamber | 6 hours/day, 5 days/week for 13 weeks | Male and female Sprague Dawley rats | Accumulation in greater quantity in the lung, liver, vessel, kidney, brain, and olfactory bulb Accumulation in a dose-dependent manner 0.7, 1.8, and 4.3 μg silver/kg dry weight of tissue in blood | [99] |
| ND | 15 nm | 0.133 mg/m3 | Laminar horizontal flow, ventilation exchange rate of 20 times/hr) for 6 hr | Female Fischer 344 rats | Accumulation in greater quantity in the lung, nasal cavities, lymph nodes associated with the lungs, and blood. Low in heart, liver, blood vessel, kidney, and brain. Recovery 7 days after exposure. | [125] |
| ND | 18.1–19.6 nm | 0.031, 0.082, 0.116 g/m3 | 6 h/day, 5 days/week for 4 weeks in a nose-only inhalation chamber | Male Sprague Dawley rats | Accumulation in lung with a recovery of half the day after 14.7, 6.4 and 1.6 μg silver/kg dry weight of tissue in blood, followed by a low elimination phase of 60 to 100 days | [126] |
| ND | 20 and 200 | IV single dose to the tail vein of 5 | 24 h, 7 and 28 d | Male Wistar rats | Time-dependent change in concentration of silver in the liver, spleen, kidneys, lungs, and the brain. The highest amount of silver in the lungs was observed after 7 days. Individual AgNPs and AgNPs cluster were found within lung macrophages attached to the alveolar wall and inside the interstitium. AgNPs accumulated in the cytoplasm, mitochondria, and nucleus. | [129] |
| ND | 8–22 | Daily i.p. of 0.01 | 36 d | female severe combined immunodeficient (SCID) mice | Apoptosis. Significant tumor growth decrease after 36 days of treatment. No toxicological effects studied. | [135] |
| ND | 27.9–33.4 and 57.3–33.4 | * More information in the paper | 40 min | Rat (no defined strain or sex) | Neutrophil increase in BALF with a size and dose-dependent response. Lung inflammation. | [136] |
| ND | 20 nm | i.i. 50 μg AgNPs/rat | 7 and 28 d | Male Sprague Dawley rats | Lung parenchyma injury, alveolar collapse, parenchymal fibrosis. Partial recovery after 28d but persistence of inflammatory/fibrosis response. | [137] |
| ND | 10–20 | i.i. 200 μg per rat | (1) Once a day for 7 days (2) Single intratracheal instillation | Male Sprague Dawley rats | Enhancement of oxidative stress, mitochondrial dynamic imbalance. Thickening of the alveolar septa, accumulation of macrophages in the alveoli, formation of pulmonary bullae and pulmonary consolidation, the disintegration of the mitochondrial cristae, and swelling of the mitochondria. | [138] |
4.6. Human Exposure
5. Lung Models as a Tool for Evaluating ADME to Predict Environmental Implications
6. Mechanisms of AgNPs Cytotoxicity on the Lungs
6.1. Differentially Expressed Genes (DEGs) Associated with Lung Cytotoxicity
6.2. Trojan Horse Mechanism Exerting Lung Cell Death Mainly by p53 Apoptotic Pathway
7. Impact of Model Complexity in Lung Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr. 2017, 38, 179–192. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. Bionanoscience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Nicolae-Maranciuc, A.; Chicea, D.; Chicea, L.M. Ag Nanoparticles for Biomedical Applications—Synthesis and Characterization—A Review. Int. J. Mol. Sci. 2022, 23, 5778. [Google Scholar] [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of silver nanoparticles utilizing various biological systems: Mechanisms and applications—A review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [Green Version]
- Drake, P.L.; Hazelwood, K.J. Exposure-related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Rim, K.T.; Song, S.W.; Kim, H.Y. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Saf. Health Work 2013, 4, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Godugu, C.; Patel, A.R.; Desai, U.; Andey, T.; Sams, A.; Singh, M. AlgiMatrixTM Based 3D Cell Culture System as an In-Vitro Tumor Model for Anticancer Studies. PLoS One 2013, 8, e53708. [Google Scholar] [CrossRef] [Green Version]
- Gliga, A.R.; Di Bucchianico, S.; Lindvall, J.; Fadeel, B.; Karlsson, H.L. RNA-sequencing reveals long-term effects of silver nanoparticles on human lung cells. Sci. Rep. 2018, 8, 6668. [Google Scholar] [CrossRef]
- Yeşilot, Ş.; Aydın Acar, Ç. Silver nanoparticles; a new hope in cancer therapy? East. J. Med. 2019, 24, 111–116. [Google Scholar] [CrossRef]
- Bao, M.; Jiang, G. Differential expression and functional analysis of lung cancer gene expression datasets: A systems biology perspective. Oncol. Lett. 2019, 18, 776–782. [Google Scholar] [CrossRef]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomedicine 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [Green Version]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Raja, G.; Jang, Y.-K.; Suh, J.-S.; Kim, H.-S.; Ahn, S.H.; Kim, T.-J. Microcellular Environmental Regulation of Silver Nanoparticles in Cancer Therapy: A Critical Review. Cancers 2020, 12, 664. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Duong, C.N.; Vestweber, D. Mechanisms Ensuring Endothelial Junction Integrity Beyond VE-Cadherin. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.A.; Anderson, D.S.; Van Winkle, L.S.; Pinkerton, K.E.; Guo, T. Evolution of Silver Nanoparticles in the Rat Lung Investigated by X-ray Absorption Spectroscopy. J. Phys. Chem. A 2015, 119, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundarakrishnan, A.; Chen, Y.; Black, L.D.; Aldridge, B.B.; Kaplan, D.L. Engineered cell and tissue models of pulmonary fibrosis. Adv. Drug Deliv. Rev. 2018, 129, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, C. Biofabrication of silver nanoparticles and their combined effect with low intensity ultrasound for treatment of lung cancer. J. Photochem. Photobiol. B Biol. 2018, 181, 122–126. [Google Scholar] [CrossRef]
- Lee, J.A.; Yeo, M.-K.; Kim, S.S. Hydra protein reduces the toxicity of Ag–PVP nanoparticles in a 3D A549 cell line. Mol. Cell. Toxicol. 2020, 16, 73–81. [Google Scholar] [CrossRef]
- öfdahl, A.; Jern, A.; Flyman, S.; Kåredal, M.; Karlsson, H.L.; Larsson-Callerfelt, A.-K. Silver Nanoparticles Alter Cell Viability Ex Vivo and in Vitro and Induce Proinflammatory Effects in Human Lung Fibroblasts. Nanomaterials 2020, 10, 1868. [Google Scholar] [CrossRef]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L.; Swain, K.A. Health effects related to nanoparticle exposures: Environmental, health and safety considerations for assessing hazards and risks. Pharmacol. Ther. 2008, 120, 35–42. [Google Scholar] [CrossRef]
- Ha, M.K.; Shim, Y.J.; Yoon, T.H. Effects of agglomeration on in vitro dosimetry and cellular association of silver nanoparticles. Environ. Sci. Nano 2018, 5, 446–455. [Google Scholar] [CrossRef]
- Ha, M.K.; Chung, K.H.; Yoon, T.H. Heterogeneity in Biodistribution and Cytotoxicity of Silver Nanoparticles in Pulmonary Adenocarcinoma Human Cells. Nanomaterials 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodorou, I.G.; Ryan, M.P.; Tetley, T.D.; Porter, A.E. Inhalation of silver nanomaterials—Seeing the risks. Int. J. Mol. Sci. 2014, 15, 23936–23974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, I.-L.; Hsieh, Y.-K.; Wang, C.-F.; Chen, I.-C.; Huang, Y.-J. Trojan-Horse Mechanism in the Cellular Uptake of Silver Nanoparticles Verified by Direct Intra- and Extracellular Silver Speciation Analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Souza, L.R.R.; da Silva, V.S.; Franchi, L.P.; de Souza, T.A.J. Toxic and Beneficial Potential of Silver Nanoparticles: The Two Sides of the Same Coin. In Cellular and Molecular Toxicology of Nanoparticles; Springer: Cham, Switzerland, 2018; pp. 251–262. [Google Scholar]
- Zhang, W.; Xiao, B.; Fang, T. Chemical transformation of silver nanoparticles in aquatic environments: Mechanism, morphology and toxicity. Chemosphere 2018, 191, 324–334. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag+ ion release in the cytosol. Nanomedicine Nanotechnology, Biol. Med. 2015, 11, 731–739. [Google Scholar] [CrossRef]
- Konduru, N.V.; Molina, R.M.; Swami, A.; Damiani, F.; Pyrgiotakis, G.; Lin, P.; Andreozzi, P.; Donaghey, T.C.; Demokritou, P.; Krol, S.; et al. Protein corona: Implications for nanoparticle interactions with pulmonary cells. Part. Fibre Toxicol. 2017, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Tariba Lovaković, B.; Barbir, R.; Pem, B.; Goessler, W.; Ćurlin, M.; Micek, V.; Debeljak, Ž.; Božičević, L.; Ilić, K.; Pavičić, I.; et al. Sex-related response in mice after sub-acute intraperitoneal exposure to silver nanoparticles. NanoImpact 2021, 23, 100340. [Google Scholar] [CrossRef]
- Alessandrini, F.; Vennemann, A.; Gschwendtner, S.; Neumann, A.; Rothballer, M.; Seher, T.; Wimmer, M.; Kublik, S.; Traidl-Hoffmann, C.; Schloter, M.; et al. Pro-Inflammatory versus Immunomodulatory Effects of Silver Nanoparticles in the Lung: The Critical Role of Dose, Size and Surface Modification. Nanomaterials 2017, 7, 300. [Google Scholar] [CrossRef] [Green Version]
- Blanco, J.; Lafuente, D.; Gómez, M.; García, T.; Domingo, J.L.; Sánchez, D.J. Polyvinyl pyrrolidone-coated silver nanoparticles in a human lung cancer cells: Time- and dose-dependent influence over p53 and caspase-3 protein expression and epigenetic effects. Arch. Toxicol. 2017, 91, 651–666. [Google Scholar] [CrossRef]
- Rosário, F.; Hoet, P.; Nogueira, A.J.A.; Santos, C.; Oliveira, H. Differential pulmonary in vitro toxicity of two small-sized polyvinylpyrrolidone-coated silver nanoparticles. J. Toxicol. Environ. Heal. -Part A Curr. Issues 2018, 81, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver Nanoparticles Induce Mitochondrial Protein Oxidation in Lung Cells Impacting Cell Cycle and Proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Fayez, H.; El-Motaleb, M.A.; Selim, A.A. Synergistic Cytotoxicity Of Shikonin-Silver Nanoparticles As An Opportunity For Lung Cancer. J. Label. Compd. Radiopharm. 2020, 63, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, S.; Datta, H.K.; Chaudhuri, S.; Malik, D.; Bandyopadhyay, A. In-vitro anti-cancer activity of shape controlled silver nanoparticles (AgNPs) in various organ specific cell lines. J. Mol. Liq. 2017, 242, 757–766. [Google Scholar] [CrossRef]
- Sunil Gowda, S.N.; Rajasowmiya, S.; Vadivel, V.; Banu Devi, S.; Celestin Jerald, A.; Marimuthu, S.; Devipriya, N. Gallic acid-coated sliver nanoparticle alters the expression of radiation-induced epithelial-mesenchymal transition in non-small lung cancer cells. Toxicol. Vitr. 2018, 52, 170–177. [Google Scholar] [CrossRef]
- Zhang, D.; Ramachandran, G.; Mothana, R.A.; Siddiqui, N.A.; Ullah, R.; Almarfadi, O.M.; Rajivgandhi, G.; Manoharan, N. Biosynthesized silver nanoparticles using Caulerpa taxifolia against A549 lung cancer cell line through cytotoxicity effect/morphological damage. Saudi J. Biol. Sci. 2020, 27, 3421–3427. [Google Scholar] [CrossRef]
- Tian, S.; Saravanan, K.; Mothana, R.A.; Ramachandran, G.; Rajivgandhi, G.; Manoharan, N. Anti-cancer activity of biosynthesized silver nanoparticles using Avicennia marina against A549 lung cancer cells through ROS/mitochondrial damages. Saudi J. Biol. Sci. 2020, 27, 3018–3024. [Google Scholar] [CrossRef]
- Mittal, J.; Pal, U.; Sharma, L.; Verma, A.K.; Ghosh, M.; Sharma, M.M. Unveiling the cytotoxicity of phytosynthesised silver nanoparticles using Tinospora cordifolia leaves against human lung adenocarcinoma A549 cell line. IET Nanobiotechnology 2020, 14, 230–238. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Kim, J.-H.H. Combination Effect of Silver Nanoparticles and Histone Deacetylases Inhibitor in Human Alveolar Basal Epithelial Cells. Molecules 2018, 23, 2046. [Google Scholar] [CrossRef] [Green Version]
- Fard, N.N.; Noorbazargan, H.; Mirzaie, A.; Hedayati Ch, M.; Moghimiyan, Z.; Rahimi, A. Biogenic synthesis of AgNPs using Artemisia oliveriana extract and their biological activities for an effective treatment of lung cancer. Artif. Cells Nanomedicine Biotechnol. 2018, 46, S1047–S1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanakumar, K.; Chelliah, R.; Hu, X.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Antioxidant, anti-lung cancer, and anti-bacterial activities of Toxicodendron vernicifluum. Biomolecules 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyayama, T.; Fujiki, K.; Matsuoka, M. Silver nanoparticles induce lysosomal-autophagic defects and decreased expression of transcription factor EB in A549 human lung adenocarcinoma cells. Toxicol. Vitr. 2018, 46, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Schlinkert, P.; Casals, E.; Boyles, M.; Tischler, U.; Hornig, E.; Tran, N.; Zhao, J.; Himly, M.; Riediker, M.; Oostingh, G.J.; et al. The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J. Nanobiotechnology 2015, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávalos, A.; Haza, A.I.; Mateo, D.; Morales, P. Effects of silver and gold nanoparticles of different sizes in human pulmonary fibroblasts. Toxicol. Mech. Methods 2015, 25, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kletting, S. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX 2018, 211–222. [Google Scholar] [CrossRef]
- Zhang, F.; Aquino, G.V.; Dabi, A.; Bruce, E.D. Assessing the translocation of silver nanoparticles using an in vitro co-culture model of human airway barrier. Toxicol. Vitr. 2019, 56, 1–9. [Google Scholar] [CrossRef]
- Guo, C.; Buckley, A.; Marczylo, T.; Seiffert, J.; Römer, I.; Warren, J.; Hodgson, A.; Chung, K.F.; Gant, T.W.; Smith, R.; et al. The small airway epithelium as a target for the adverse pulmonary effects of silver nanoparticle inhalation. Nanotoxicology 2018, 12, 539–553. [Google Scholar] [CrossRef] [Green Version]
- Fizeșan, I.; Cambier, S.; Moschini, E.; Chary, A.; Nelissen, I.; Ziebel, J.; Audinot, J.-N.; Wirtz, T.; Kruszewski, M.; Pop, A.; et al. In vitro exposure of a 3D-tetraculture representative for the alveolar barrier at the air-liquid interface to silver particles and nanowires. Part. Fibre Toxicol. 2019, 16, 14. [Google Scholar] [CrossRef]
- Poh, T.Y.; Ali, N.A.T.B.M.; Mac Aogáin, M.; Kathawala, M.H.; Setyawati, M.I.; Ng, K.W.; Chotirmall, S.H. Inhaled nanomaterials and the respiratory microbiome: Clinical, immunological and toxicological perspectives. Part. Fibre Toxicol. 2018, 15, 1–16. [Google Scholar] [CrossRef]
- Stater, E.P.; Sonay, A.Y.; Hart, C.; Grimm, J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 2021, 16, 1180–1194. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Durham, P.G.; Anselmo, A.C. Inorganic nanoparticles and the microbiome. Nano Res. 2018, 11, 4936–4954. [Google Scholar] [CrossRef]
- Hamilton, R.F.; Buckingham, S.; Holian, A. The Effect of Size on Ag Nanosphere Toxicity in Macrophage Cell Models and Lung Epithelial Cell Lines Is Dependent on Particle Dissolution. Int. J. Mol. Sci. 2014, 15, 6815–6830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic silver nanoparticles: Synthesis and application as antibacterial and antifungal agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.; Pereira, T.M.; Bonatto, C.C. Frontiers and perspectives in the green synthesis of silver nanoparticles. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 137–164. ISBN 9780081025796. [Google Scholar]
- Jeevanandam, J.; Kulabhusan, P.K.; Sabbih, G.; Akram, M.; Danquah, M.K. Phytosynthesized nanoparticles as a potential cancer therapeutic agent. 3 Biotech 2020, 10, 535. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Kim, I.; Lee, B.-T.; Kim, H.-A.; Kim, K.-W.; Kim, S.D.; Hwang, Y.-S. Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere 2016, 143, 99–105. [Google Scholar] [CrossRef]
- Roh, J.; Umh, H.N.; Sim, J.; Park, S.; Yi, J.; Kim, Y. Dispersion stability of citrate- and PVP-AgNPs in biological media for cytotoxicity test. Korean J. Chem. Eng. 2013, 30, 671–674. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef]
- Robinson, B.V.; Sullivan, F.M.; Borzelleca, J.F.; Schwartz, S.L. PVP; Routledge: Oxfordshire, UK, 2018; ISBN 9780203741672. [Google Scholar]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhang, L.; Yang, Q.; Wang, Z. Polyvinylpyrrolidone as an Efficient Stabilizer for Silver Nanoparticles. Chinese J. Chem. 2014, 32, 909–913. [Google Scholar] [CrossRef]
- Ha, M.K.; Kwon, S.J.; Choi, J.S.; Nguyen, N.T.; Song, J.; Lee, Y.; Kim, Y.E.; Shin, I.; Nam, J.W.; Yoon, T.H. Mass Cytometry and Single-Cell RNA-seq Profiling of the Heterogeneity in Human Peripheral Blood Mononuclear Cells Interacting with Silver Nanoparticles. Small 2020, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Holmila, R.; Wu, H.; Lee, J.; Tsang, A.W.; Singh, R.; Furdui, C.M. Integrated redox proteomic analysis highlights new mechanisms of sensitivity to silver nanoparticles. Mol. Cell. Proteomics 2021, 20, 100073. [Google Scholar] [CrossRef]
- Haase, A.; Dommershausen, N.; Schulz, M.; Landsiedel, R.; Reichardt, P.; Krause, B.-C.; Tentschert, J.; Luch, A. Genotoxicity testing of different surface-functionalized SiO2, ZrO2 and silver nanomaterials in 3D human bronchial models. Arch. Toxicol. 2017, 91, 3991–4007. [Google Scholar] [CrossRef]
- Gao, W.; Li, L.; Wang, Y.; Zhang, S.; Adcock, I.M.; Barnes, P.J.; Huang, M.; Yao, X. Bronchial epithelial cells: The key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology 2015, 20, 722–729. [Google Scholar] [CrossRef]
- Betts, J.G.; Desaix, P.; Johnson, E.; Johnson, J.E.; Korol, O.; Kruse, D.; Poe, B.; Wise, J.A.; Womble, M.; Young, K.A. Anatomy and Physiology; Rice University, Ed.; OpenStax: Houston, TX, USA, 2014; ISBN 9781938168130. [Google Scholar]
- Schyns, J.; Bureau, F.; Marichal, T. Lung Interstitial Macrophages: Past, Present, and Future. J. Immunol. Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Ward, H.E.; Nicholas, T.E. Alveolar type I and type II cells. Aust. N. Z. J. Med. 1984, 14, 731–734. [Google Scholar] [CrossRef]
- Mason, R.J. Epithelial Cells | Type II Cells. In Encyclopedia of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2006; pp. 138–142. [Google Scholar]
- Bobyk, L.; Tarantini, A.; Beal, D.; Veronesi, G.; Kieffer, I.; Motellier, S.; Valsami-Jones, E.; Lynch, I.; Jouneau, P.-H.; Pernet-Gallay, K.; et al. Toxicity and chemical transformation of silver nanoparticles in A549 lung cells: Dose-rate-dependent genotoxic impact. Environ. Sci. Nano 2021, 8, 806–821. [Google Scholar] [CrossRef]
- Hu, G.; Christman, J.W. Editorial: Alveolar Macrophages in Lung Inflammation and Resolution. Front. Immunol. 2019, 10, 2275. [Google Scholar] [CrossRef] [Green Version]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Dong, L.; Zhou, X.; Hu, L.; Yin, Y.; Liu, J. Simultaneous size characterization and mass quantification of the in vivo core-biocorona structure and dissolved species of silver nanoparticles. J. Environ. Sci. 2018, 63, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, J. The biocorona: A challenge for the biomedical application of nanoparticles. Nanotechnol. Rev. 2017, 6, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, M.H.; Pawlina, W. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology, 8th ed.; LWW: Philadelphia, PA, USA, 2018; ISBN 1496383427. [Google Scholar]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Hu, G.; Jiao, B.; Shi, X.; Valle, R.P.; Fan, Q.; Zuo, Y.Y. Physicochemical properties of nanoparticles regulate translocation across pulmonary surfactant monolayer and formation of lipoprotein corona. ACS Nano 2013, 7, 10525–10533. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Sharma, A.K.; Loeschner, K.; Jacobsen, N.R. Pulmonary toxicity of silver vapours, nanoparticles and fine dusts: A review. Regul. Toxicol. Pharmacol. 2020, 115, 104690. [Google Scholar] [CrossRef]
- Kato, T.; Yashiro, T.; Murata, Y.; Herbert, D.; Oshikawa, K.; Bando, M.; Ohno, S.; Sugiyama, Y. Evidence that exogenous substances can be phagocytized by alveolar epithelial cells and transported into blood capillaries. Cell Tissue Res. 2003, 311, 47–51. [Google Scholar] [CrossRef]
- Ray, J.L.; Fletcher, P.; Burmeister, R.; Holian, A. The role of sex in particle-induced inflammation and injury. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2020, 12, 1–21. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Endocrine regulation of lung disease and inflammation. Exp. Biol. Med. 2018, 243, 1313–1322. [Google Scholar] [CrossRef] [Green Version]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Zhang, S.; Huang, Y.; Zhang, T.; Liu, X.; Hu, Y.; Zhang, Z.; Tang, M. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J. Appl. Toxicol. 2012, 32, 890–899. [Google Scholar] [CrossRef]
- Barbir, R.; Goessler, W.; Ćurlin, M.; Micek, V.; Milić, M.; Vuković, B.; Milić, M.; Ljubojević, M.; Domazet Jurašin, D.; Vinković Vrček, I. Protein Corona Modulates Distribution and Toxicological Effects of Silver Nanoparticles In Vivo. Part. Part. Syst. Charact. 2019, 36, 1–12. [Google Scholar] [CrossRef]
- Gan, J.; Sun, J.; Chang, X.; Li, W.; Li, J.; Niu, S.; Kong, L.; Zhang, T.; Wu, T.; Tang, M.; et al. Biodistribution and organ oxidative damage following 28 days oral administration of nanosilver with/without coating in mice. J. Appl. Toxicol. 2020, 40, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Yoon, J.U.; Kim, D.S.; Jeon, K.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; et al. Subchronic inhalation toxicity of silver nanoparticles. Toxicol. Sci. 2009, 108, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ji, J.H.; Yoon, J.U.; Kim, D.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; Chung, Y.H.; Kim, J.; et al. Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal. Toxicol. 2008, 20, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Sung, J.H.; Ji, J.H.; Lee, J.H.J.S.J.K.; Lee, J.H.J.S.J.K.; Ryu, H.R.; Lee, J.H.J.S.J.K.; Chung, Y.H.; Park, H.M.; Shin, B.S.; et al. Recovery from silver-nanoparticle-exposure-induced lung inflammation and lung function changes in Sprague Dawley rats. Nanotoxicology 2013, 7, 169–180. [Google Scholar] [CrossRef]
- Molleman, B.; Hiemstra, T. Time, pH, and size dependency of silver nanoparticle dissolution: The road to equilibrium. Environ. Sci. Nano 2017, 4, 1314–1327. [Google Scholar] [CrossRef]
- Veronesi, G.; Aude-Garcia, C.; Kieffer, I.; Gallon, T.; Delangle, P.; Herlin-Boime, N.; Rabilloud, T.; Carrière, M. Exposure-dependent Ag+ release from silver nanoparticles and its complexation in AgS2 sites in primary murine macrophages. Nanoscale 2015, 7, 7323–7330. [Google Scholar] [CrossRef]
- Ferdous, Z.; Al-Salam, S.; Greish, Y.E.; Ali, B.H.; Nemmar, A. Pulmonary exposure to silver nanoparticles impairs cardiovascular homeostasis: Effects of coating, dose and time. Toxicol. Appl. Pharmacol. 2019, 367, 36–50. [Google Scholar] [CrossRef]
- Sur, I.; Altunbek, M.; Kahraman, M.; Culha, M. The influence of the surface chemistry of silver nanoparticles on cell death. Nanotechnology 2012, 23, 375102. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Shang, M.; Niu, S.; Zhang, W.; Zhang, B.; Huang, W.; Wu, T.; Zhang, T.; Tang, M.; et al. Mitophagy–lysosomal pathway is involved in silver nanoparticle-induced apoptosis in A549 cells. Ecotoxicol. Environ. Saf. 2021, 208, 111463. [Google Scholar] [CrossRef]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol. In Vitro 2013, 27, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, J.; Buckley, A.; Leo, B.; Martin, N.G.; Zhu, J.; Dai, R.; Hussain, F.; Guo, C.; Warren, J.; Hodgson, A.; et al. Pulmonary effects of inhalation of spark-generated silver nanoparticles in Brown-Norway and Sprague-Dawley rats. Respir. Res. 2016, 17, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costard, L.S.; Hosn, R.R.; Ramanayake, H.; O’Brien, F.J.; Curtin, C.M. Influences of the 3D microenvironment on cancer cell behaviour and treatment responsiveness: A recent update on lung, breast and prostate cancer models. Acta Biomater. 2021, 132, 360–378. [Google Scholar] [CrossRef]
- Voloboueva, L.; Sun, X.; Ouyang, Y.-B.; Giffard, R.G. Cell Culture: Primary Neural Cells. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Garcia-Mouton, C.; Hidalgo, A.; Cruz, A.; Pérez-Gil, J. The Lord of the Lungs: The essential role of pulmonary surfactant upon inhalation of nanoparticles. Eur. J. Pharm. Biopharm. 2019, 144, 230–243. [Google Scholar] [CrossRef]
- Wiemann, M.; Vennemann, A.; Blaske, F.; Sperling, M.; Karst, U. Silver Nanoparticles in the Lung: Toxic Effects and Focal Accumulation of Silver in Remote Organs. Nanomaterials 2017, 7, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Kim, J.-H.; Hong, K. Cytotoxic Potential and Molecular Pathway Analysis of Silver Nanoparticles in Human Colon Cancer Cells HCT116. Int. J. Mol. Sci. 2018, 19, 2269. [Google Scholar] [CrossRef] [Green Version]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [Green Version]
- Hiemstra, P.S.; Grootaers, G.; van der Does, A.M.; Krul, C.A.M.; Kooter, I.M. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol. Vitr. 2018, 47, 137–146. [Google Scholar] [CrossRef]
- Pearson, J.P.; Chater, P.I.; Wilcox, M.D. The properties of the mucus barrier, a unique gel—How can nanoparticles cross it? Ther. Deliv. 2016, 7, 229–244. [Google Scholar] [CrossRef]
- Abramenko, N.; Demidova, T.B.; Krutyakov, Y.A.; Zherebin, P.M.; Krysanov, E.Y.; Kustov, L.M.; Peijnenburg, W. The effect of capping agents on the toxicity of silver nanoparticles to Danio rerio embryos. Nanotoxicology 2019, 13, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radiom, M.; Sarkis, M.; Brookes, O.; Oikonomou, E.K.; Baeza-Squiban, A.; Berret, J.-F. Pulmonary surfactant inhibition of nanoparticle uptake by alveolar epithelial cells. Sci. Rep. 2020, 10, 19436. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, G.; De Luca, A.; Jose, S.S.; Antonini, M.; Teloni, I.; Fric, J.; Zelante, T. Using Lung Organoids to Investigate Epithelial Barrier Complexity and IL-17 Signaling During Respiratory Infection. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza Carvalho, C.; Daum, N.; Lehr, C.-M. Carrier interactions with the biological barriers of the lung: Advanced in vitro models and challenges for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2014, 75, 129–140. [Google Scholar] [CrossRef]
- Bhowmick, R.; Derakhshan, T.; Liang, Y.; Ritchey, J.; Liu, L.; Gappa-Fahlenkamp, H. A Three-Dimensional Human Tissue-Engineered Lung Model to Study Influenza A Infection. Tissue Eng. Part A 2018, 24, 1468–1480. [Google Scholar] [CrossRef]
- Sauer, U.G.; Vogel, S.; Aumann, A.; Hess, A.; Kolle, S.N.; Ma-Hock, L.; Wohlleben, W.; Dammann, M.; Strauss, V.; Treumann, S.; et al. Applicability of rat precision-cut lung slices in evaluating nanomaterial cytotoxicity, apoptosis, oxidative stress, and inflammation. Toxicol. Appl. Pharmacol. 2014, 276, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hirn, S.; Haberl, N.; Loza, K.; Epple, M.; Kreyling, W.G.; Rothen-Rutishauser, B.; Rehberg, M.; Krombach, F. Proinflammatory and cytotoxic response to nanoparticles in precision-cut lung slices. Beilstein J. Nanotechnol. 2014, 5, 2440–2449. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.S.; Kim, J.K.; Kim, Y.; Kim, H.P.; Kim, H.S.; Ahn, K.; Lee, J.H.; Faustman, E.M.; Gulumian, M.; Kelman, B.; et al. Mode of silver clearance following 28-day inhalation exposure to silver nanoparticles determined from lung burden assessment including post-exposure observation periods. Arch. Toxicol. 2020, 94, 773–784. [Google Scholar] [CrossRef]
- Moradi-Sardareh, H.; Basir, H.R.G.; Hassan, Z.M.; Davoudi, M.; Amidi, F.; Paknejad, M. Toxicity of silver nanoparticles on different tissues of Balb/C mice. Life Sci. 2018, 211, 81–90. [Google Scholar] [CrossRef]
- Liu, Y.X.; Karsai, A.; Anderson, D.S.; Silva, R.M.; Uyeminami, D.L.; Van Winkle, L.S.; Pinkerton, K.E.; Liu, G.Y. Single-Cell Mechanics Provides an Effective Means To Probe in Vivo Interactions between Alveolar Macrophages and Silver Nanoparticles. J. Phys. Chem. B 2015, 119, 15118–15129. [Google Scholar] [CrossRef] [PubMed]
- Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Oczkowski, M.; Krawczyńska, A.; Chwastowska, J.; Sadowska-Bratek, M.; Chajduk, E.; Wojewódzka, M.; Dušinská, M.; et al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J. Appl. Toxicol. 2012, 32, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chai, Q.; Xu, S.; Li, Q.; Wu, T.; Chen, S.; Wu, L. Silver nanoparticle-activated COX2/PGE2 axis involves alteration of lung cellular senescence in vitro and in vivo. Ecotoxicol. Environ. Saf. 2020, 204, 111070. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Miyayama, T.; Hirano, S. Difference in the toxicity mechanism between ion and nanoparticle forms of silver in the mouse lung and in macrophages. Toxicology 2015, 328, 84–92. [Google Scholar] [CrossRef]
- Scoville, D.K.; Botta, D.; Galdanes, K.; Schmuck, S.C.; White, C.C.; Stapleton, P.L.; Bammler, T.K.; MacDonald, J.W.; Altemeier, W.A.; Hernandez, M.; et al. Genetic determinants of susceptibility to silver nanoparticle-induced acute lung inflammation in mice. FASEB J. 2017, 31, 4600–4611. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Lai, Y.; Zhou, H.; Yan, B.; Liu, J. The biodistribution and transformation of nanoparticulate and ionic silver in rat organs in vivo. NanoImpact 2020, 20, 100265. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Khan, R.A.; Alhowail, A.H.; Alqasoumi, A.; Sajid, S.M.; Mohammed, A.M.; Alsharidah, M.; Al Rugaie, O.; Mousa, A.M. Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model. Nanotechnol. Rev. 2022, 11, 266–283. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Ma, S.; Liu, Y.; Li, D.; Huang, H.; Jiang, S.; Cheng, S.; Wu, W.; Zhang, K.; et al. Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int. J. Nanomedicine 2016, 11, 1879–1887. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.F.; Wang, W.M.; Chen, C.Y.; Lu, T.H.; Liao, C.M. Assessing human exposure risk and lung disease burden posed by airborne silver nanoparticles emitted by consumer spray products. Int. J. Nanomedicine 2019, 14, 1687–1703. [Google Scholar] [CrossRef]
- Roda, E.; Bottone, M.G.; Biggiogera, M.; Milanesi, G.; Coccini, T. Pulmonary and hepatic effects after low dose exposure to nanosilver: Early and long-lasting histological and ultrastructural alterations in rat. Toxicol. Reports 2019, 6, 1047–1060. [Google Scholar] [CrossRef]
- Ma, W.; He, S.; Ma, H.; Jiang, H.; Yan, N.; Zhu, L.; Bang, J.J.; Li, P.A.; Jia, S. Silver Nanoparticle Exposure Causes Pulmonary Structural Damage and Mitochondrial Dynamic Imbalance in the Rat: Protective Effects of Sodium Selenite. Int. J. Nanomedicine 2020, Volume 15, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.S.; Patchin, E.S.; Silva, R.M.; Uyeminami, D.L.; Sharmah, A.; Guo, T.; Das, G.K.; Brown, J.M.; Shannahan, J.; Gordon, T.; et al. Influence of particle size on persistence and clearance of aerosolized silver nanoparticles in the rat lung. Toxicol. Sci. 2015, 144, 366–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toome, K.; Willmore, A.M.A.; Paiste, P.; Tobi, A.; Sugahara, K.N.; Kirsimäe, K.; Ruoslahti, E.; Braun, G.B.; Teesalu, T. Ratiometric: In vivo auditioning of targeted silver nanoparticles. Nanoscale 2017, 9, 10094–10100. [Google Scholar] [CrossRef] [PubMed]
- Sampath, G.; Govarthanan, M.; Rameshkumar, N.; Krishnan, M.; Alotaibi, S.H.; Nagarajan, K. A comparative analysis of in vivo toxicity, larvicidal and catalytic activity of synthesized silver nanoparticles. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Delay, M.; Dolt, T.; Woellhaf, A.; Sembritzki, R.; Frimmel, F.H. Interactions and stability of silver nanoparticles in the aqueous phase: Influence of natural organic matter (NOM) and ionic strength. J. Chromatogr. A 2011, 1218, 4206–4212. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cortés, S.; Francioso, O.; Ciavatta, C.; García-Ramos, J.V.; Gessa, C. pH-Dependent Adsorption of Fractionated Peat Humic Substances on Different Silver Colloids Studied by Surface-Enhanced Raman Spectroscopy. J. Colloid Interface Sci. 1998, 198, 308–318. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Z.; Deng, B. Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms. Water Res. 2016, 88, 403–427. [Google Scholar] [CrossRef] [Green Version]
- Cumberland, S.A.; Lead, J.R. Particle size distributions of silver nanoparticles at environmentally relevant conditions. J. Chromatogr. A 2009, 1216, 9099–9105. [Google Scholar] [CrossRef]
- Diegoli, S.; Manciulea, A.L.; Begum, S.; Jones, I.P.; Lead, J.R.; Preece, J.A. Interaction between manufactured gold nanoparticles and naturally occurring organic macromolecules. Sci. Total Environ. 2008, 402, 51–61. [Google Scholar] [CrossRef]
- Baalousha, M.; Manciulea, A.; Cumberland, S.; Kendall, K.; Lead, J.R. Aggregation and surface properties of Iron Oxides Nanoparticles: Influence of pH and natural organic matter. Environ. Toxicol. Chem. 2008, 27, 1875. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnology 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Jaggi, S.; Varghese, E.; Lall, S.; Bhowmik, A.; Rai, A. Identification of Differentially Expressed Genes in RNA-seq Data of Arabidopsis thaliana: A Compound Distribution Approach. J. Comput. Biol. 2016, 23, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.P.; Miaskowski, C.; Dhruva, A.A.; Flowers, E.; Kober, K.M. Mechanisms and Measurement of Changes in Gene Expression. Biol. Res. Nurs. 2018, 20, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Spira, B. Current Methods of Gene Expression Analysis and Quantification. Curr. Pharm. Anal. 2005, 1, 243–249. [Google Scholar] [CrossRef]
- Wong, M.L.; Medrano, J.F. Real-time PCR for mRNA quantitation. Biotechniques 2005, 39, 75–85. [Google Scholar] [CrossRef]
- Nossa, R.; Costa, J.; Cacopardo, L.; Ahluwalia, A. Breathing in vitro: Designs and applications of engineered lung models. J. Tissue Eng. 2021, 12, 204173142110086. [Google Scholar] [CrossRef]
- Jalink, K.; Cheng, S.S.Y.; Ben Ireland, S.; Louise Meunier, M.A.F. Silver nanoparticle uptake in the human lung assessed through in-vitro and in-silico methods. Environ. Pollut. 2020, 259, 113880. [Google Scholar] [CrossRef]
- Morais, M.; Teixeira, A.L.; Dias, F.; Machado, V.; Medeiros, R.; Prior, J.A.V. Cytotoxic Effect of Silver Nanoparticles Synthesized by Green Methods in Cancer. J. Med. Chem. 2020, 63, 14308–14335. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Liu, J.; Kang, Y.; Lin, Y.; Li, D.; Shao, L. Interactions of nanomaterials with ion channels and related mechanisms. Br. J. Pharmacol. 2019, 176, 3754–3774. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, B.; Arellano-García, M.E.; Radilla-Chávez, P.; Salas-Vargas, D.S.; Toledano-Magaña, Y.; Casillas-Figueroa, F.; Luna Vazquez-Gomez, R.; Pestryakov, A.; García-Ramos, J.C.; Bogdanchikova, N. Cytokinesis-Block Micronucleus Assay Using Human Lymphocytes as a Sensitive Tool for Cytotoxicity/Genotoxicity Evaluation of AgNPs. ACS Omega 2020. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M.M.; Snyder, C.M.; Sloop, J.; Solst, S.R.; Donati, G.L.; Spitz, D.R.; Furdui, C.M.; Singh, R. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part. Fibre Toxicol. 2021, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Wiley, H.S. A systems perspective of heterocellular signaling. Essays Biochem. 2018, 62, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, G.; Hayashi, M. In vivo rodent micronucleus assay: Protocol, conduct and data interpretation. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2000, 455, 155–166. [Google Scholar] [CrossRef]




| Coating | Size (nm) | Concentration (μg/mL) | IC50 (μg/mL) a | Exposure Time (h) | Cell Line | Outcomes | Cytotoxic Response | Ref |
|---|---|---|---|---|---|---|---|---|
| Monocultures | ||||||||
| PVP | 20 | 0, 10, 25, 50, 100 and 200 | 100 | 24, 48, 72 | A549 | Gene and protein expression decreases of p53, p21, MDM,2, and caspase 3. Mitochondrial ROS production. Global acetylation levels decrease on tails of histone H3 protein. Global DNA methylation increases. Late apoptosis/necrosis increase after 48 h. HMOX1 has a high expression on A5 and 49, might it render them less susceptible to ROS-induced cell death early-stage apoptosis. | Concentration-, and Time-dependency. | [41] |
| PVP | 10, 20 | 5–10 | 10 nm: 56.4 20 nm: >100 | 24, 48 | A549 | Severe ADN damage. Cell cycle arrest, increase in several of cells at S and sub-G1 phases (DNA repair mechanism more effective on 10 nm AgNPs). A decrease in cell viability. Increase of late-apoptotic and necrotic cells at 100 μg/mL. | Size-, Concentration-, and Time-dependency. | [42] |
| PVP | 23 | 1–10 | NS | 24, 48, 72 | A549 Calu-1 BEAS-2B NCI-H358 | Cell cycle arrest. Cell viability decreased in all cell lines except NCI-H358. Mitochondrial ROS production and protein oxidation, particularly on AgNPs sensitive cell lines. Decrease in cellular ATP levels. Cell arrest on G2 and S-phase for A549 and Calu-1 and S-phase for BEAS-2B. NCI-H358 cells did not show cell cycle changes related to AgNPs exposure. | Concentration-, Time-, and Cell type dependency. | [43] |
| PVP | 25 | 0.4, 1, 4, 10 | >100 | 240 | NHLF MRC-5 | Moderate acute toxicity for MRC-5 and cellular senescence using sub-toxic concentrations associated with β-galactosidase (SA-β-gal) activity and heterochromatin foci (SAHF) Expression of SASP and inflammatory genes G2/M phase arrest completed after 10 days 685 transcripts upregulated and 718 transcripts downregulated in RNA-seq global mRNA levels Potential role of the COX2-PGE2 pathway in AgNPs-induced lung cellular senescence. COX2-PGE2 pathway regulated by p65 and highly differentiated. BCL-2 downregulated by AgNPs subsequently undergoes apoptosis. | Concentration-, and Cell type-dependent. | [44] |
| PVP | 50 and 200 | 5.6, 11.5, 22.5, 45 | NR | 16 | NR8383 | Increase of lactate deshydrogenase (LDH) and glucuronidase (GLU) activity. TNH-α increase at lower concentration of 50 nm citrate-AgNP and at the higher concentration of PVP-AgNP | Concentration-dependent | [40] |
| Shikonin | 20 | 0.078–10 | 2.4 ± 0.11 | 24 | A549 | Cell viability and proliferation decrease. | Concentration-, and | [45] |
| Acacia nilotica, NG, or TKP | 10–78 | 10–100 | Wi38: 86.15 A549: 65.85 | 12, 24, 48 | A549 Wi38 | Cytotoxic selective to cancer cells. Inhibition of cell cycle. ROS mediated apoptosis. | Cell-type-dependent. | [46] |
| Gallic acid | 10–30 | 5, 25, 50, 100, 200 | 46.5 | 24 | A549 | Effective in treating the radiation toxicity and resistance developed by the cancer cells during cancer treatment. Cell viability decrease. Epithelial-Mesenchymal Transition suppression. | Concentration-dependent. | [47] |
| Caulerpa taxifolia | 10–100 | 10–100 | 40,000 | 24 | A549 | Morphological damage and condensation morphology. Cell death. Apoptosis/necrosis induction. | Concentration-dependent. | [48] |
| Avicennia marina | 10–20 | 10–80 | 50,000 | 24 | A549 | Cancer cell growth inhibition. Damage to the mitochondrial membrane. ROS. | Concentration-dependent. | [49] |
| Tinospora cordifolia | 25–50 | 25, 50, 75, 100, 150 | 100 | 12, 24, and 48 | A549 | Cell viability decrease. Cytomorphological changes. Apoptosis. Nuclear damage. ROS. Loss of mitochondrial membrane potential (ψm). | Concentration-, and Time-dependent. | [50] |
| Wogonin | 5, 40 | 2–10 μM 1–5 μM (Ag content) | 5 nm: 2 μM 40 nm: 6 μM | 24 and 48 | A549 | Cell viability decrease. ROS. Activation of the mitochondrial apoptotic pathway. DNA damage. Activation of Caspase-9 and Caspase-3. Secretion of pro-inflammatory markers such as TNFα. | Concentration-, and size-dependent. | [51] |
| Artemisia oliveriana | 10.63 | 5, 25, 50, 100 and 200 | A549: 3.6 MRC-5: 10 | 24 | A549 MRC-5 | Cell viability decrease. Apoptotic genes Bax, Casp3, Casp9, and miR-192 expression increase. Anti-apoptotic gene Bcl-2 expression decrease. Cell cycle shift to sub-G1 phase. Antioxidant activity. Fewer effects on normal cells (MRC-5). Fragmentation of the genomic DNA. | Concentration-, and Cell-Type dependent. | [52] |
| Toxicodendron vernicifluum | 2–40 | 5, 10, 20, 40, 80, 160, 320 | A549: >320 NiH3T3: 160 | 24 | A549 NIH3T3 | Cell viability decreased on A549 but not on mouse embryo cells. ROS mediated apoptosis on A549. 95% Cell death at the maximum concentration for A549. | Concentration-, and Cell type-dependent. | [53] |
| Citrate | 10, 75 | 1 | Not specified | 144 | BEAS-2B | 719 down-regulated and 998 up-regulated genes after exposure. DNA damage, Cell cycle arrest on G1. Fibrosis induction. EMT (epithelial-mesenchymal transition). Cell transformation is indicative of an oncogenic phenotype. | Concentration-, Size-dependent-, and Time-dependent. | [14] |
| Citrate | 60 | 50, 100, 200 | 200 μg Ag/mL | 24 | A549 HPSAEpiC | Lysosomal pH alkalization (dysfunction) and autophagosome formation. Inhibition of autophagic flux. Inhibition of Transcriptional Factor EB (TFEB) expression. Concentration-dependence increase of p62 and LC3B-II proteins. | Concentration-, And Cell type-dependent. | [54] |
| Citrate, chitosan | 7–10 | 6.25 × 1012, 1.25 × 1012, 2.5 × 1012, 5 × 1012 NPs/mL | NHBE: 0.7 μg/cm2 A549 and BEAS-2B: not in range | 0.5, 4, and 24 hours | A549. BEAS-2B. NHBE. | No cytotoxicity was observed on A549 and NHBE; not responsive to Transepithelial/transendothelial electrical resistance (TEER) change. Higher cytotoxicity resistance for NHBE compared with the other cells. ROS production is most prominent in A549. | Concentration-, Cell type-, and Coating dependent | [55] |
| Citrate | 10, 75 | 2 and 10 | 10 | 24 and 48 | HLF-1 | Decrease in cell viability. Reduction of metabolic activity. Procollagen and proinflammatory cytokine secretion. | Time-dependent-, Concentration-, and size-dependent | [28] |
| Uncoated | 4.7, 42 | 0.84–2000 | 4.7 nm: 7700 42 nm: 1150,000 | 24 | HbPF | Decrease in HPF viability. Reduction in cell mitochondrial activity and LDH leakage. ROS production and oxidative stress. No statistically significant changes in SOD activity. GSH depletion. | Size-dependent. | [56] |
| Co-cultures | ||||||||
| Starch | 20 ± 4 | 7.25 μg, 41.25 μg (Nebulization) | Out of range | 24 | hAELVi and THP-1 | High viability. Problems with determination. | Concentration-, and Cell type-dependent. | [57] |
| Garcinia mangostana | 12 | 2.5 μg/mL | Out of range | 24 | A549 with BEAS-2B | Cell viability decreased for A549. BEAS-2B is highly resistant. | Cell type-dependent. | |
| Tannic acid | 50 ± 4 | 3 mg/L, 30 mg/L | Out of range | 24 | Calu-3, EA.hy926, and THP-1 | High toxicity at high concentration treatment. Pro-inflammatory markers IL-6, IL-8, and TNF-α significant secretion reduction. | Cell type-, and Concentration-dependent. | [58] |
| 3D-cultures | ||||||||
| Uncoated | 14 | 1.5, 4.4 and 13.2 ng/cm2. | LDH (not specified) | 6 and 24 | Organotypic-reconstituted 3D human primary small airway epithelial cell | Neutrophil accumulation. Macrophage levels modestly increased. SLC26A4 mucin gene production overexpressed. Duox1 expression increased (Small airway epithelial repair and bronchiolar re-epithelialization). Ect2, sftpa1, sftpd, muc1, and cftr epithelial-specific genes increase. MT1A and MT2A were upregulated (Cellular defense systems are in place to mitigate the effects of metal ion exposure), and metal overload. mir146, mir155, mir21 and mir224 (inflammatory process). NOXO1 and SOD2 ROS, mitochondrial disruption, DNA damage, cell cycle regulation, G2/M phase cell cycle arrest. The inflammatory process, Immunomodulatory response, and tissue remodeling. | Concentration-dependent. | [59] |
| Uncoated | 20, 200 | 0.05, 0.5, 5 μg/cm2 | Out of range | 6 and 24 | 3D model representative of the alveolar barrier | ROS, cell death Increased level of mRNA Antioxidant and anti-inflammatory HMOX-1. Nuclear translocation of the transcription factor NF-kB in endothelial cells. Inflammation, increase in the mRNA levels of IL-6 and IL-8. | Concentration-, and Size-dependent. | [60] |
| PVP | 10–20 | 40 | Out of range | 24 | 3D and 2D A549 model | Apoptosis/Necrosis No effects on p53, Bax, and Caspase-3. Slightly reduced expression of Bcl-xL and NF-kB genes. Cells within 3D cultures were less affected by nanomaterials than in 2D cell cultures. Less affected when combined with hydra protein (ROS entrapment). | Concentration-, size-, and Model-dependent. | [27] |
| Ways of Exposure | Average Blood Levels | Study Population (Individuals) |
|---|---|---|
| Population in general that does not work with silver | 1 μg Ag/L | 26 |
| Silver material manufacturers | 0.00035 and 0.00135 mg Ag/m3, blood levels of 0.34 and 0.30 μg Ag/L | 2 |
| Recovery of silver from x-rays and photographic films. | 0.085 and 1 mg Ag/m3, 0.03 y 0.17 mg Ag/m3 resulting in blood levels of 49 y 79 μg Ag/L, respectively. | 2 |
| Exposed to silver oxides and silver nitrates | Media: 19.5 μg/L; range: 11–84 μg/L | 30 |
| Silver powder manufacturing | Media: 10 μg/L; range: 0.5–62 μg/L | 25 |
| Recovery of silver in waste | Media: 10 μg/L | 21 |
| Scrap silver recovery, coin silver refinery, jewelry production | Media: 10 μg/L; range: 0.1–23 μg/L | 98 |
| Smelting, refining, and manufacturing of silver salts | Media: 11 μg/L | 37 |
| Exposed to silver aerosol | 154.4 μg/L | 1 |
| Differentially Expressed Genes (DEGs) | Cellular Response Pathways | AgNP Size (nm) | AgNP Coating | Cell Line | Ref |
|---|---|---|---|---|---|
| p53 ↓, p21↓, Mdm2↓, caspase-3↓ | Cell damage DNA damage Apoptosis | 20 | PVP | A549 | [41] |
| ATM protein ↑, Heme oxygenase-1↑ | Cell cycle DNA damage Apoptosis | 10, 20 | PVP | A549 | [42] |
| Bax↑, Casp3↑, Casp9↑, miR-192↑, Bcl-2↓ | Cell cycle Apoptosis | 10.63 | Artemisia oliveriana | A549 MRC-5 | [52] |
| 685 transcripts upregulated and 718 transcripts downregulated in RNA-seq global mRNA levels Bcl-2↓ | Cell growth (Senescence) Cell Cycle | 25 | PVP | Normal human lung fibroblast (NHLF) MRC-5 | [130] |
| p53↑, p21↑, Bid↑, Bax↑, Bak ↑, Cyt C↑, Bcl-2↓, Bcl-xL↓ | Enriched signaling pathways; MAPK2, TNF, IL17, P13k-AKT, NF- Kappa B, Apoptosis | 5, 40 | Wogonin | A549 | [113] |
| 719 genes were down-regulated, and 998 genes were up-regulated. Collagen related (COL1A1, COL1A2, COL6A2, COL11A1, COL16A1, COL18A1, COL21A1) ↑ COL17A1 ↓, MMP2 matrix-metallopeptidase involved in the degradation of collagen (IV, V, VII, X) ↑ MMP11, and MMP19 inhibition of metalloproteases ↑ TGFβ1, an important pro-fibrotic growth factor and a key regulator of lung fibrosis and its receptor TGFBR1 ↑, BAMBI ↓, AGTR1, PGF, and PDGF ↑, CDH1 ↑, CDH 12 ↓, NOTCH3 ↑, MMP2 ↑, MRas ↑, HIF1α ↓, Antioxidant enzymes such as glutathione-S-transferases (GSTM1, GSTM2, GSTM3, GSTT2/GSTT2B) ↑, M NQO1 ↑, EPHX1 ↑, CAT ↓ | Enriched pathways related to: Carcinogenesis, Hepatic fibrosis, ROS, regulation of epithelial mesenchymal transition. | 10, 75 | Citrate | BEAS-2B | [14] |
| TFEB↓, LC3B-II↑, LAMP1→, P62↑, C, Bax↑, Bcl-xL↓, C, Casp3→, NF-kB↓, p53→ | Apoptosis Necrosis | 10, 20 | PVP | 3D and 2D A549 model with and without hydra protein | [27] |
| 493 differentially regulated transcripts SLC26A4↑, Duox1↑, Ect2↑, sftpa1↑, sftpd↑, muc1↑, cftr↑, MTA1 and MTA2 ↑, NOXO1 and SOD2↑, mir146, mir155, mir21 and mir 224↑ | Nrf2 Regulation of inflammatory processes Regulation of metals DNA damage cell cycle regulation Inflammatory process Immunomodulatory response ROS Tissue remodeling Metal overload. | 14 | Uncoated | Organotypic-reconstituted 3D human primary small airway epithelial cell | [59] |
| HMOX-1↑, NQO1↓, SOD1↑ MT-1A, MT-1B and MT-2A↑, Casp7 ↑, FAS↑, HSP70↑, GST↓, VCAM1↑, ICAM-1↓, NF-kB↓, IL-6↑, COX-2↓, N | Nrf2 regulates inflammatory processes Metal binding antioxidant metallothionein Apoptosis | 20, 200 | Uncoated | 3D model representative of the alveolar barrier | [60] |
| Drp1↑, p-Drp1↑, Opa1↓, Mfn2↓, Casp3↑ | Fission Fusion Apoptotic | 10–20 | Uncoated | Sprague Dawley Rats | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Vega, J.G.; García-Ramos, J.C.; Chavez-Santoscoy, R.A.; Castillo-Quiñones, J.E.; Arellano-Garcia, M.E.; Toledano-Magaña, Y. Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health. Nanomaterials 2022, 12, 2316. https://doi.org/10.3390/nano12132316
González-Vega JG, García-Ramos JC, Chavez-Santoscoy RA, Castillo-Quiñones JE, Arellano-Garcia ME, Toledano-Magaña Y. Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health. Nanomaterials. 2022; 12(13):2316. https://doi.org/10.3390/nano12132316
Chicago/Turabian StyleGonzález-Vega, Jesús Gabriel, Juan Carlos García-Ramos, Rocio Alejandra Chavez-Santoscoy, Javier Emmanuel Castillo-Quiñones, María Evarista Arellano-Garcia, and Yanis Toledano-Magaña. 2022. "Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health" Nanomaterials 12, no. 13: 2316. https://doi.org/10.3390/nano12132316
APA StyleGonzález-Vega, J. G., García-Ramos, J. C., Chavez-Santoscoy, R. A., Castillo-Quiñones, J. E., Arellano-Garcia, M. E., & Toledano-Magaña, Y. (2022). Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health. Nanomaterials, 12(13), 2316. https://doi.org/10.3390/nano12132316