Architecting Nanostructured Co-BTC@GO Composites for Supercapacitor Electrode Application
Abstract
:1. Introduction
2. Experimental
2.1. Materias
2.2. Synthesis of Graphene Oxide
2.3. Synthesis of Co-BTC
2.4. Synthesis of Co-BTC@GO
2.5. Morphological Characterization
3. Result and Discussion
3.1. Morphological Characterization
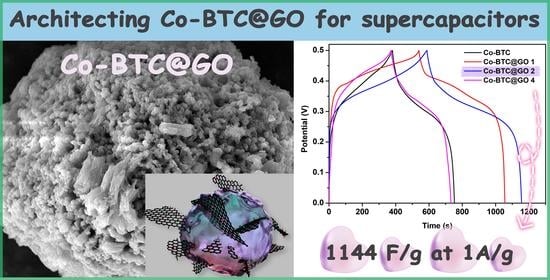
3.2. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Zhou, S.; Yu, C.; Huang, H.; Zhao, J.; Dai, L.; Qiu, J. Rapid and Energy-Efficient Microwave Pyrolysis for High-Yield Production of Highly-Active Bifunctional Electrocatalysts for Water Splitting. Energy Environ. Sci. 2020, 13, 545–553. [Google Scholar] [CrossRef]
- Huang, H.; Yu, C.; Zhao, C.; Han, X.; Yang, J.; Liu, Z.; Li, S.; Zhang, M.; Qiu, J. Iron-Tuned Super Nickel Phosphide Microstructures with High Activity for Electrochemical Overall Water Splitting. Nano Energy 2017, 34, 472–480. [Google Scholar] [CrossRef]
- Wang, K.B.; Bi, R.; Wang, Z.K.; Chu, Y.; Wu, H.; Wang, K.B. Metal-Organic Frameworks with Different Spatial Dimensions for Supercapacitors. New J. Chem. 2020, 44, 3147–3167. [Google Scholar] [CrossRef]
- Huang, H.; Jung, H.; Jun, H.; Woo, D.Y.; Han, J.W.; Lee, J. Design of Grain Boundary Enriched Bimetallic Borides for Enhanced Hydrogen Evolution Reaction. Chem. Eng. J. 2021, 405, 126977. [Google Scholar] [CrossRef]
- Gautam, K.P.; Acharya, D.; Bhatta, I.; Subedi, V.; Das, M.; Neupane, S.; Kunwar, J.; Chhetri, K.; Yadav, A.P. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. [Google Scholar] [CrossRef]
- Wan, L.; Shamsaei, E.; Easton, C.D.; Yu, D.; Liang, Y.; Chen, X.; Abbasi, Z.; Akbari, A.; Zhang, X.; Wang, H. ZIF-8 Derived Nitrogen-Doped Porous Carbon/Carbon Nanotube Composite for High-Performance Supercapacitor. Carbon 2017, 121, 330–336. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, X.; Qiao, Z.; Jung, J.; Zhu, Y.; Lu, Y.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of Carbon-Based Electrical Double-Layer Capacitors. Nat. Commun. 2014, 5, 3317. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-Doped Activated Carbon for a High Energy Hybrid Supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable Sieving of Ions Using Graphene Oxide Membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef]
- Xiong, S.; Shi, Y.; Chu, J.; Gong, M.; Wu, B.; Wang, X. Preparation of High-Performance Covalently Bonded Polyaniline Nanorods/Graphene Supercapacitor Electrode Materials Using Interfacial Copolymerization Approach. Electrochim. Acta 2014, 127, 139–145. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, X.; Chen, P. Biological and Chemical Sensors Based on Graphene Materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colomba, L.; Ruoff, R.S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; Chang, M.; Peng, Z.; Li, C.; He, X.; Yang, Y.; Liu, Y.; Lu, Y. Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review. Nanomaterials 2021, 11, 2771. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. Porous Graphene Materials: The Chemistry and Promising Applications of Graphene and Porous Graphene Materials (Adv. Funct. Mater. 41/2020). Adv. Funct. Mater. 2020, 30, 2070275. [Google Scholar] [CrossRef]
- Wang, J.; Ding, B.; Hao, X.; Xu, Y.; Wang, Y.; Shen, L.; Dou, H.; Zhang, X. A Modified Molten-Salt Method to Prepare Graphene Electrode with High Capacitance and Low Self-Discharge Rate. Carbon 2016, 102, 255–261. [Google Scholar] [CrossRef]
- Eigler, S.; Grimm, S.; Enzelberger-Heim, M.; Müller, P.; Hirscha, A. Graphene Oxide: Efficiency of Reducing Agents. Chem. Commun. 2013, 49, 7391–7393. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Mao, S.; Zhou, G.; Zhang, Z.; Wen, Z.; Huang, X.; Ci, S.; Chen, J. A High-Performance Catalyst Support for Methanol Oxidation with Graphene and Vanadium Carbonitride. Nanoscale 2015, 7, 1031–1307. [Google Scholar] [CrossRef]
- Meng, F.L.; Guo, Z.; Huang, X.J. Graphene-Based Hybrids for Chemiresistive Gas Sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion Sieving in Graphene Oxide Membranes via Cationic Control of Interlayer Spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Qian, Y.; Shang, J.; Liu, D.; Yang, G.; Wang, X.; Chen, C.; Kou, L.; Lei, W. Enhanced Ion Sieving of Graphene Oxide Membranes via Surface Amine Functionalization. J. Am. Chem. Soc. 2021, 143, 5080–5090. [Google Scholar] [CrossRef] [PubMed]
- Mi, B. Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef]
- Liu, Z. Ion Sieving in Graphene Oxide Membranes via Precise Cationic “Binding”. Acta Phys. Chim. Sin. 2018, 34, 731–732. [Google Scholar] [CrossRef]
- Leong, Z.Y.; Han, Z.; Wang, G.; Li, D.S.; Yang, S.A.; Yang, H.Y. Electric Field Modulated Ion-Sieving Effects of Graphene Oxide Membranes. J. Mater. Chem. A 2021, 9, 244–253. [Google Scholar] [CrossRef]
- Zou, L.; Hou, C.C.; Liu, Z.; Pang, H.; Xu, Q. Superlong Single-Crystal Metal-Organic Framework Nanotubes. J. Am. Chem. Soc. 2018, 140, 15393–15401. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Jiang, H.; Zhang, X.; Xu, Q. Metal-Organic Framework (MOF) as a Template for Syntheses of Nanoporous Carbons as Electrode Materials for Supercapacitor. Carbon 2010, 48, 456–463. [Google Scholar] [CrossRef]
- Cao, X.; Tan, C.; Sindoro, M.; Zhang, H. Hybrid Micro-/Nano-Structures Derived from Metal-Organic Frameworks: Preparation and Applications in Energy Storage and Conversion. Chem. Soc. Rev. 2017, 46, 2660–2677. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Yang, F.; Ji, L.; Xu, L.; Zhang, C. Polytriphenylamine Derivative with High Free Radical Density as the Novel Organic Cathode for Lithium Ion Batteries. J. Mater. Chem. A 2014, 2, 20083–20088. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Gong, K.; Xu, B.; Mei, H.; Zhang, H.; Zhang, J.; Kang, Z.; Yan, Y.; Sun, D. Temperature Controlled Diffusion of Hydroxide Ions in 1D Channels of Ni-MOF-74 for Its Complete Conformal Hydrolysis to Hierarchical Ni(OH)2 Supercapacitor Electrodes. Nanoscale 2019, 11, 9598–9607. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, S.; Zhou, S.; Xu, H.; Zhao, J.; Wang, J.; Li, Y. An Electrochromic Supercapacitor Based on an MOF Derived Hierarchical-Porous NiO Film. Nanoscale 2020, 12, 8934–8941. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 1–14. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, F.; Xiong, W.; Li, X.; Yuan, A.; Pang, H. Strategies to Improve Electrochemical Performances of Pristine Metal-organic Frameworks-based Electrodes for Lithium/Sodium-ion Batteries. SmartMat 2021, 2, 488–518. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal Organic Frameworks for Energy Storage and Conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kim, M.K.; Cho, K.; Woo, J.Y.; Lee, Y.; Han, S.H.; Byun, D.; Choi, W.; Lee, J.K. One-Step Catalytic Synthesis of CuO/Cu2O in a Graphitized Porous C Matrix Derived from the Cu-Based Metal-Organic Framework for Li- and Na-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 19514–19523. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zhou, H.; Tong, Y.; Wang, J.; Zhu, M.; Chen, T.; Yuan, A. Facile Fabrication of MOF-Derived Octahedral CuO Wrapped 3D Graphene Network as Binder-Free Anode for High Performance Lithium-Ion Batteries. Chem. Eng. J. 2017, 313, 1623–1632. [Google Scholar] [CrossRef]
- Yan, X.; Li, X.; Yan, Z.; Komarneni, S. Porous Carbons Prepared by Direct Carbonization of MOFs for Supercapacitors. Appl. Surf. Sci. 2014, 308, 306–310. [Google Scholar] [CrossRef]
- Ke, F.S.; Wu, Y.S.; Deng, H. Metal-Organic Frameworks for Lithium Ion Batteries and Supercapacitors. J. Solid State Chem. 2015, 223, 109–121. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. A New Straightforward Uncalcined Approach for Morphology Modulating to Enhance the Electrical Capacity Performance of Co-MOF. Electrochim. Acta 2021, 389, 138684. [Google Scholar] [CrossRef]
- Chhetri, K.; Kim, T.; Acharya, D.; Muthurasu, A.; Dahal, B.; Bhattarai, R.M.; Lohani, P.C.; Pathak, I.; Ji, S.; Ko, T.H.; et al. Hollow Carbon Nanofibers with Inside-Outside Decoration of Bi-Metallic MOF Derived Ni-Fe Phosphides as Electrode Materials for Asymmetric Supercapacitors. Chem. Eng. J. 2022, 450, 138363. [Google Scholar] [CrossRef]
- Hong, J.; Park, S.J.; Kim, S. Synthesis and Electrochemical Characterization of Nanostructured Ni-Co-MOF/Graphene Oxide Composites as Capacitor Electrodes. Electrochim. Acta 2019, 311, 62–71. [Google Scholar] [CrossRef]
- Ehrnst, Y.; Ahmed, H.; Komljenovic, R.; Massahud, E.; Shepelin, N.A.; Sherrell, P.C.; Ellis, A.V.; Rezk, A.R.; Yeo, L.Y. Acoustotemplating: Rapid Synthesis of Freestanding Quasi-2D MOF/Graphene Oxide Heterostructures for Supercapacitor Applications. J. Mater. Chem. A 2022, 10, 7058–7072. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Wu, Q.; Zhang, Q.; Wang, X. MOF-Derived Ni(OH)2 Nanocubes/GO For High-Performance Supercapacitor. ChemistrySelect 2019, 4. [Google Scholar] [CrossRef]
- Li, S.; Shi, C.; Pan, Y.; Wang, Y. 2D/2D NiCo-MOFs/GO Hybrid Nanosheets for High-Performance Asymmetrical Supercapacitor. Diam. Relat. Mater. 2021, 115, 108358. [Google Scholar] [CrossRef]
- Van Ngo, T.; Moussa, M.; Tung, T.T.; Coghlan, C.; Losic, D. Hybridization of MOFs and Graphene: A New Strategy for the Synthesis of Porous 3D Carbon Composites for High Performing Supercapacitors. Electrochim. Acta 2020, 329, 135104. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal-Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.K.; Mishra, S.; Kim, K.H.; Deep, A. Metal-Organic Frameworks and Their Composites as Efficient Electrodes for Supercapacitor Applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Yutkin, M.P.; Peresypkina, E.V.; Virovets, A.V.; Hasegawa, Y.; Nishihara, H.; Fedin, V.P. Synthesis, Structure, and Magnetic Properties of the Cobalt(II) 1,3,5-Benzenetricarboxylate Layered Coordination Polymer. Russ. Chem. Bull. 2007, 56, 1782–1786. [Google Scholar] [CrossRef]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Flexible Graphene-Polyaniline Composite Paper for High-Performance Supercapacitor. Energy Environ. Sci. 2013, 6, 1185–1191. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Yin, G.; Lu, Y.; Yin, J. Cu2O@reduced Graphene Oxide Composite for Removal of Contaminants from Water and Supercapacitors. J. Mater. Chem. 2011, 21, 10645–10648. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Fan, X.; Ling, Z.; Qiu, J.; Gogotsi, Y. Facile Fabrication of MWCNT-Doped NiCoAl-Layered Double Hydroxide Nanosheets with Enhanced Electrochemical Performances. J. Mater. Chem. A 2013, 1, 1963–1968. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced Asymmetric Supercapacitors Based on Ni(OH) 2/Graphene and Porous Graphene Electrodes with High Energy Density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Yang, J.; Ma, Z.; Gao, W.; Wei, M. Layered Structural Co-Based MOF with Conductive Network Frames as a New Supercapacitor Electrode. Chem. A Eur. J. 2017, 23, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Z.H.; Wang, Z.G.; Zhang, F.X.; Jin, J. Layered α-Co(OH)2 Nanocones as Electrode Materials for Pseudocapacitors: Understanding the Effect of Interlayer Space on Electrochemical Activity. Adv. Funct. Mater. 2013, 23, 2758–2764. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Bin Wu, H.; Yu, L.; Madhavi, S.; Lou, X.W. A Flexible Quasi-Solid-State Asymmetric Electrochemical Capacitor Based on Hierarchical Porous V2O5 Nanosheets on Carbon Nanofibers. Adv. Energy Mater. 2015, 5, 1500753. [Google Scholar] [CrossRef]
- Zhu, G.; He, Z.; Chen, J.; Zhao, J.; Feng, X.; Ma, Y.; Fan, Q.; Wang, L.; Huang, W. Highly Conductive Three-Dimensional MnO2-Carbon Nanotube-Graphene-Ni Hybrid Foam as a Binder-Free Supercapacitor Electrode. Nanoscale 2014, 6, 1079–1085. [Google Scholar] [CrossRef]
- Ramachandran, R.; Saranya, M.; Velmurugan, V.; Raghupathy, B.P.C.; Jeong, S.K.; Grace, A.N. Effect of Reducing Agent on Graphene Synthesis and Its Influence on Charge Storage towards Supercapacitor Applications. Appl. Energy 2015, 153, 22–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Sun, Y.; Han, P.; Wang, J.; Ding, X.; Zhang, X.; Yang, H. MoO2@Cu@C Composites Prepared by Using Polyoxometalates@Metal-Organic Frameworks as Template for All-Solid-State Flexible Supercapacitor. Electrochim. Acta 2016, 188, 490–498. [Google Scholar] [CrossRef]







| Material | Capacitance | Scan Rate/Current Density | Electrolyte | Reference |
|---|---|---|---|---|
| CNTs@Co-BTC | 553.3 F/g | 1 A/g | 1M LiOH | [39] |
| Ni-Co-MOF/GO | 447.2 F/g | 1 A/g | 6M KOH | [41] |
| 2DMOF/rGO film | 292.5 F/g | 0.7 A/g | 1M H2SO4 | [42] |
| 2D/2D NiCo-MOF/GO | 413.61 C/g | 0.5 A/g | 2M KOH | [44] |
| L-rGO-C-MOF | 390 F/g | 5 mV/s | 1M NaNO3 | [45] |
| Ni-BTC@GO 2 | 1144 F/g | 1 A/g | 3M KOH | This work |
| Rs (Ω) | Rct (Ω) | |
|---|---|---|
| Co-BTC | 1.07 | 0.90 |
| Error (%) | 1.3228 | 14.6740 |
| Co-BTC@GO 2 | 0.65 | 0.26 |
| Error | 1.0077 | 14.5030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Yang, A.; Zhang, W.; Nie, J.; Wang, T.; Gong, J.; Wang, Y.; Ji, Y. Architecting Nanostructured Co-BTC@GO Composites for Supercapacitor Electrode Application. Nanomaterials 2022, 12, 3234. https://doi.org/10.3390/nano12183234
Chen T, Yang A, Zhang W, Nie J, Wang T, Gong J, Wang Y, Ji Y. Architecting Nanostructured Co-BTC@GO Composites for Supercapacitor Electrode Application. Nanomaterials. 2022; 12(18):3234. https://doi.org/10.3390/nano12183234
Chicago/Turabian StyleChen, Tianen, Allen Yang, Wei Zhang, Jinhui Nie, Tingting Wang, Jianchao Gong, Yuanhao Wang, and Yaxiong Ji. 2022. "Architecting Nanostructured Co-BTC@GO Composites for Supercapacitor Electrode Application" Nanomaterials 12, no. 18: 3234. https://doi.org/10.3390/nano12183234
APA StyleChen, T., Yang, A., Zhang, W., Nie, J., Wang, T., Gong, J., Wang, Y., & Ji, Y. (2022). Architecting Nanostructured Co-BTC@GO Composites for Supercapacitor Electrode Application. Nanomaterials, 12(18), 3234. https://doi.org/10.3390/nano12183234