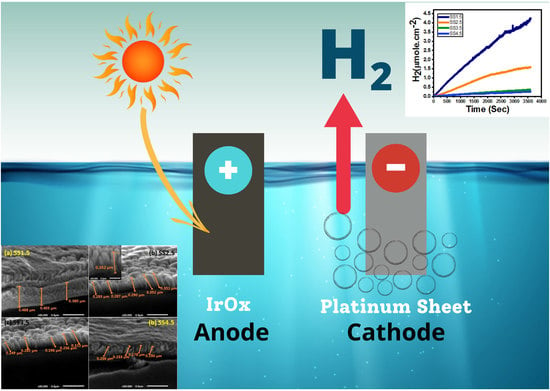
The Influence of Electrode Thickness on the Structure and Water Splitting Performance of Iridium Oxide Nanostructured Films
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Preparation
2.2. Characterization and Photocatalytic Performance
3. Results and Discussion
3.1. Film Morphology and Thickness Evaluation
3.2. Optical Properties and Raman Spectra
4. Photoelectrochemical (PEC) Water Splitting Measurements
4.1. Photoelectrochemical Behaviour of IrOx, Stability, Number of Hydrogen Moles, and the Benchmark Efficiency
4.2. PEC Behaviour of the SS1.5 Photocathode under the Effect of Monochromatic Light Illumination and Photoelectrochemical Efficiencies
4.3. Effect of Temperature and Thermodynamic Parameters
4.4. PEC Impedance Spectroscopy (PEC-IS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, D.; Graziano, M.; Hanrahan, B. A Nanostructured Anti-Reflective Iridium Oxide Coating for Water Oxidation. J. Phys. Chem. C 2018, 122, 12207–12214. [Google Scholar] [CrossRef]
- Gao, J.; Xu, C.-Q.; Hung, S.-F.; Liu, W.; Cai, W.; Zeng, Z.; Jia, C.; Chen, H.M.; Xiao, H.; Li, J.; et al. Breaking Long-Range Order in Iridium Oxide by Alkali Ion for Efficient Water Oxidation. J. Am. Chem. Soc. 2019, 141, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Lin, H.; Fan, Z.; Ma, L.; Shao, Q.; Ji, Y.; Zheng, F.; Geng, S.; Yang, S.-Z.; Kong, N.; et al. Iridium metallene oxide for acidic oxygen evolution catalysis. Nat. Commun. 2021, 12, 6007. [Google Scholar] [CrossRef] [PubMed]
- Dettelbach, K.E.; Kolbeck, M.; Huang, A.; He, J.; Berlinguette, C.P. Rapid Quantification of Film Thickness and Metal Loading for Electrocatalytic Metal Oxide Films. Chem. Mater. 2017, 29, 7272–7277. [Google Scholar] [CrossRef]
- Chandra, D.; Takama, D.; Masaki, T.; Sato, T.; Abe, N.; Togashi, T.; Kurihara, M.; Saito, K.; Yui, T.; Yagi, M. Highly Efficient Electrocatalysis and Mechanistic Investigation of Intermediate IrOx(OH)y Nanoparticle Films for Water Oxidation. ACS Catal. 2016, 6, 3946–3954. [Google Scholar] [CrossRef]
- Minguzzi, A.; Lugaresi, O.; Achilli, E.; Locatelli, C.; Vertova, A.; Ghigna, P.; Rondinini, S. Observing the oxidation state turnover in heterogeneous iridium-based water oxidation catalysts. Chem. Sci. 2014, 5, 3591. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, J.; Park, Y.S.; Yang, J.; Jang, M.J.; Jeong, J.; Choe, S.; Lee, J.W.; Kwon, J.-D.; Choi, S.M. Electrodeposition of High-Surface-Area IrO2 Films on Ti Felt as an Efficient Catalyst for the Oxygen Evolution Reaction. Front. Chem. 2020, 8, 593272. [Google Scholar] [CrossRef]
- Geiger, S.; Kasian, O.; Shrestha, B.R.; Mingers, A.M.; Mayrhofer, K.J.J.; Cherevko, S. Activity and Stability of Electrochemically and Thermally Treated Iridium for the Oxygen Evolution Reaction. J. Electrochem. Soc. 2016, 163, F3132–F3138. [Google Scholar] [CrossRef]
- Hou, X.; Takahashi, R.; Yamamoto, T.; Lippmaa, M. Microstructure analysis of IrO2 thin films. J. Cryst. Growth 2017, 462, 24–28. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, Z.; He, H.; Li, Y.; You, L.; Zheng, H. An improved method of preparing iridium oxide electrode based on carbonate-melt oxidation mechanism. Sens. Actuators B 2018, 261, 316–324. [Google Scholar] [CrossRef]
- Chung, T.-W.; Hsieh, M.-T.; Tso, K.-C.; Kuo, S.-H.; Cheng, C.-T.; Yu, J.; Chan, T.-S.; Wu, P.-W.; Chen, P.-C. Synthesis and characterization of iridium oxide thin film via a pre-coordination step for bio-stimulating electrode application. Ceram. Int. 2020, 46, 18648–18655. [Google Scholar] [CrossRef]
- Chen, C.C.; Herhold, A.B.; Johnson, C.S.; Alivisatos, A.P. Size Dependence of Structural Metastability in Semiconductor Nanocrystals. Science 1997, 276, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Abe, Y.; Kawamura, M.; Kim, K.H. Electrochromic properties of iridium oxide thin films prepared by reactive sputtering in O2 or H2O atmosphere. J. Vac. Sci. Technol. B 2015, 33, 041204. [Google Scholar] [CrossRef]
- Matienzo, D.J.D.; Settipani, D.; Instuli, E.; Kallio, T. Active IrO2 and NiO Thin Films Prepared by Atomic Layer Deposition for Oxygen Evolution Reaction. Catalysts 2020, 10, 92. [Google Scholar] [CrossRef]
- Korkmaz, S.; Tezel, F.M.; Kariper, I.A. Synthesis and characterization of GO/IrO2 thin film supercapacitor. J. Alloys Compd. 2018, 754, 14–25. [Google Scholar] [CrossRef]
- Sachse, R.; Pfluger, M.; Velasco-Velez, J.-J.; Sahre, M.; Radnik, J.; Bernicke, M.; Bernsmeier, D.; Hodoroaba, V.-D.; Krumrey, M.; Strasser, P.; et al. Assessing Optical and Electrical Properties of Highly Active IrOx Catalysts for the Electrochemical Oxygen Evolution Reaction via Spectroscopic Ellipsometry. ACS Catal. 2020, 10, 14210–14223. [Google Scholar] [CrossRef]
- Guan, H.; Ke, Q.; Lv, C.; Zeng, N.; Hu, C.; Wang, S.; Ge, X.; Cai, J. Amorphous Iridium Oxide Nanoparticle Films Prepared by Low-temperature Annealing and Plasma Treatment as Highly Efficient Oxygen Evolution Electrocatalysts. Chem. Lett. 2020, 49, 705–708. [Google Scholar] [CrossRef]
- Tummala, R.R. Fundamentals of Microsystems Packaging; McGraw Hill: New York, NY, USA, 2001; ISBN 0071371699. [Google Scholar]
- Shimizu, M.; Fujisawa, H.; Hyodo, S.; Nankashima, S.; Niu, H. Effect of Sputtered Ir and IrO2 electrodes on the Properties of PZT tThin Films Deposited by MOCVD. Mater. Res. Soc. Symp. Proc. Vol. 1997, 493, 159–164. [Google Scholar] [CrossRef]
- Sardarinejad, A.; Maurya, D.K.; Alameh, K. The effects of sensing electrode thickness on ruthenium oxidethin-film pH sensor. Sens. Actuators A 2014, 214, 15–19. [Google Scholar] [CrossRef]
- Liu, X.-H.; Yang, Y.-W.; Liu, X.-M.; Hao, Q.; Wang, L.-M.; Sun, B.; Wu, J.; Wang, D. Confined Synthesis of Oriented Two-Dimensional Ni3(hexaiminotriphenylene)2 Films for Electrocatalytic Oxygen Evolution Reaction. Langmuir 2020, 36, 7528–7532. [Google Scholar] [CrossRef]
- El Sayed, A.M.; El-Gamal, S.; Morsi, W.M.; Mohammed, G. Effect of PVA and copper oxide nanoparticles on the structural, optical, and electrical properties of carboxymethyl cellulose films. J. Mater. Sci. 2015, 50, 4717–4728. [Google Scholar] [CrossRef]
- Shaban, M.; Mustafa, M.; el Sayed, A.M. Structural, optical, and photocatalytic properties of the spray deposited nanoporous CdS thin films; influence of copper doping, annealing, and deposition parameters. Mater. Sci. Semicond. Process. 2016, 56, 329–343. [Google Scholar] [CrossRef]
- Mo, Y.; Stefan, I.C.; Cai, W.-B.; Dong, J.; Carey, P.; Scherson, D.A. In Situ Iridium LIII-Edge X-ray Absorption and Surface Enhanced Raman Spectroscopy of Electrodeposited Iridium Oxide Films in Aqueous Electrolytes. J. Phys. Chem. B 2002, 106, 3681–3686. [Google Scholar] [CrossRef]
- Liao, P.C.; Chen, C.S.; Ho, W.S.; Huang, Y.S.; Tiong, K.K. Characterization of IrO thin films by Raman spectroscopy. Thin Solid Films 1997, 301, 7–11. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Shaban, M. Morphological, surface and optical properties of spin-coated IrOx films; influence of spin speed, annealing and (Cr, La) codoping. Ceram. Int. 2019, 45, 8460–8470. [Google Scholar] [CrossRef]
- Saeed, K.H.; Forster, M.; Li, J.-F.; Hardwick, L.J.; Cowan, A.J. Water oxidation intermediates on iridium oxide electrodes probed by in situ electrochemical SHINERS. Chem. Commun. 2020, 56, 1129. [Google Scholar] [CrossRef]
- Pavlovic, Z.; Ranjan, C.; van Gastel, M.; Schlogl, R. The active site for the water oxidising anodic iridium oxide probed through in situ Raman spectroscopy. Chem. Commun. 2017, 53, 12414–12417. [Google Scholar] [CrossRef]
- Zubkins, M.; Kalendarev, R.; Gabrusenoks, J.; Plaude, A.; Zitolo, A.; Anspoks, A.; Pudzs, K.; Vilnis, K.; Azens, A.; Purans, J. Changes in structure and conduction type upon addition of Ir to ZnO thin films. Thin Solid Films 2017, 636, 694–701. [Google Scholar] [CrossRef]
- Gao, F.; He, J.; Wang, H.; Lin, J.; Chen, R.; Yi, K.; Huang, F.; Lin, Z.; Wang, M. Te-mediated electro-driven oxygen evolution reaction. Nano Res. Energy, 2022; in press. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Mohamed, F.; Ashraf, A.M.; Shaban, M.; Aslam, A.; Khan, P.; Asiri, A.M. Enhanced photoelectrochemical water splitting activity of carbon nanotubes@ TiO2 nanoribbons in different electrolytes. Chemosphere 2019, 238, 124554. [Google Scholar] [CrossRef]
- Choudhary, S.; Upadhyay, S.; Kumar, P.; Singh, N.; Satsangi, V.R.; Shrivastav, R.; Dass, S. Nanostructured bilayered thin films in photoelectrochemical water splitting—A review. Int. J. Hydrogen Energy 2012, 37, 18713–18730. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting–materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed]
- Aboud, A.A.; Shaban, M.; Revaprasadu, N. Effect of Cu, Ni and Pb doping on the photo-electrochemical activity of ZnO thin films. RSC Adv. 2019, 9, 7729–7736. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Nasser, N.; Shaban, M.; Alshaikh, H.; Hamdy, H.; Ahmed, A.M. Effect of Morphology and Plasmonic on Au/ZnO Films for Efficient Photoelectrochemical Water Splitting. Nanomaterials 2021, 11, 2338. [Google Scholar] [CrossRef] [PubMed]
- El-Gharbawy, S.A.; Al-Dossari, M.; Zayed, M.; Saudi, H.A.; Hassaan, M.Y.; Alfryyan, N.; Shaban, M. Fabrication and Characterization of Nanostructured Rock Wool as a Novel Material for Efficient Water-Splitting Application. Nanomaterials 2022, 12, 2169. [Google Scholar] [CrossRef]
- Ma, L.; Cui, H.; Chen, S.; Li, X.; Dong, B.; Zhi, C. Accommodating diverse ions in Prussian blue analogs frameworks for rechargeable batteries: The electrochemical redox reactions. Nano Energy 2021, 81, 105632. [Google Scholar] [CrossRef]
- Mattheis, J.; Werner, J.H.; Rau, U. Finite mobility effects on the radiative efficiency limit of pn-junction solar cells. Phys. Rev. B 2008, 77, 085203. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Saif, A.A.; Poopalan, P. Effect of the film thickness on the impedance behavior of sol–gel Ba0.6Sr0.4TiO3 thin films. Physica B 2011, 406, 1283–1288. [Google Scholar] [CrossRef]
- Shaban, M.; Almohammedi, A.; Saad, R.; el Sayed, A.M. Design of SnO2:Ni,Ir Nanoparticulate Photoelectrodes for Efficient Photoelectrochemical Water Splitting. Nanomaterials 2022, 12, 453. [Google Scholar] [CrossRef]







| Sample | dav. (µm) | (µm ) | Eg (eV) | |
|---|---|---|---|---|
| SS1.5 | 0.417 | 0.026 | 16.04 | 2.925 |
| SS2.5 | 0.281 | 0.020 | 14.05 | 3.00 |
| SS3.5 | 0.244 | 0.017 | 14.35 | 3.03 |
| SS4.5 | 0.193 | 0.014 | 13.78 | 3.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, A.S.; Shaban, M.; Abdelkarem, K.; El Sayed, A.M. The Influence of Electrode Thickness on the Structure and Water Splitting Performance of Iridium Oxide Nanostructured Films. Nanomaterials 2022, 12, 3272. https://doi.org/10.3390/nano12193272
Altowyan AS, Shaban M, Abdelkarem K, El Sayed AM. The Influence of Electrode Thickness on the Structure and Water Splitting Performance of Iridium Oxide Nanostructured Films. Nanomaterials. 2022; 12(19):3272. https://doi.org/10.3390/nano12193272
Chicago/Turabian StyleAltowyan, Abeer S., Mohamed Shaban, Khaled Abdelkarem, and Adel M. El Sayed. 2022. "The Influence of Electrode Thickness on the Structure and Water Splitting Performance of Iridium Oxide Nanostructured Films" Nanomaterials 12, no. 19: 3272. https://doi.org/10.3390/nano12193272
APA StyleAltowyan, A. S., Shaban, M., Abdelkarem, K., & El Sayed, A. M. (2022). The Influence of Electrode Thickness on the Structure and Water Splitting Performance of Iridium Oxide Nanostructured Films. Nanomaterials, 12(19), 3272. https://doi.org/10.3390/nano12193272