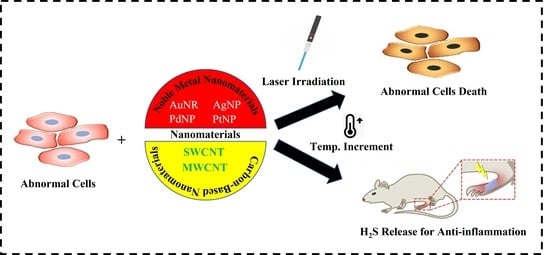
Recent Insights into NIR-Light-Responsive Materials for Photothermal Cell Treatments
Abstract
:1. Introduction
2. Application of Nanomaterials to Control Cell Behavior under Light Exposure
2.1. Metal Nanomaterials
2.2. Carbon-Based Nanomaterials
3. Functions of Nanomaterials to Control Inflammation
4. Mechanisms of Cell Behavioral Control Using Nanomaterials under Light Irradiation
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Deng, Y.; Luo, H.; Zhu, A.; Ke, H.; Yang, H.; Chen, H. Light-Responsive Nanoparticles for Highly Efficient Cytoplasmic Delivery of Anticancer Agents. ACS Nano 2017, 11, 12134–12144. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Xu, X.D.; Jia, H.Z.; Lei, Q.; Luo, G.F.; Cheng, S.X.; Zhuo, R.X.; Zhang, X.Z. Therapeutic nanomedicine based on dual-intelligent functionalized gold nanoparticles for cancer imaging and therapy in vivo. Biomaterials 2013, 34, 8798–8807. [Google Scholar] [CrossRef]
- Sun, I.C.; Eun, D.K.; Koo, H.; Ko, C.Y.; Kim, H.S.; Yi, D.K.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.H. Tumor-Targeting Gold Particles for Dual Computed Tomography/Optical Cancer Imaging. Angew. Chem. Int. Ed. 2011, 50, 9348–9351. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.A.; Encina, E.R.; Stefani, F.D. Optical properties of metallic nanoparticles: Manipulating light, heat and forces at the nanoscale. Nanoscale 2011, 3, 4042–4059. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Sung, D.; Kim, J.; Kim, B.T.; Wang, T.; An, S.S.A.; Seo, S.W.; Yi, D.K. Silica nanoparticle-based dual imaging colloidal hybrids: Cancer cell imaging and biodistribution. Int. J. Nanomed. 2015, 10, 215–225. [Google Scholar]
- Ramasamy, M.; Zhu, Y.; Paik, U.; Yi, D.K. Synthesis and anti-bacterial activity of AuNRs–PS–MNPs. Mater. Lett. 2014, 137, 479–482. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jungsuwadee, P.; Vore, M.; Butterfield, D.A.; St Clair, D.K. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol. Interv. 2007, 7, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Edison, M.N.; Johns, C.M. Acute and Chronic Cutaneous Reactions to Radiotherapy. In Radiation Therapy for Skin Cancer; Cognetta, A., Jr., Mendenhall, W., Eds.; Springer: New York, NY, USA, 2013; pp. 55–69. [Google Scholar]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-Infrared Resonant Nanoshells for Combined Optical Imaging and Photothermal Cancer Therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Hornos Carneiro, M.F.; Barbosa, F. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Health Part B: Crit. Rev. 2016, 19, 129–148. [Google Scholar] [CrossRef]
- Capasso, L.; Camatini, M.; Gualtieri, M. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicol. Lett. 2014, 226, 28–34. [Google Scholar] [CrossRef]
- Gojova, A.; Lee, J.T.; Jung, H.S.; Guo, B.; Barakat, A.I.; Kennedy, I.M. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhal. Toxicol. 2009, 21, 123–130. [Google Scholar] [CrossRef]
- Lee, S.; Yun, H.S.; Kim, S.H. The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials 2011, 32, 9434–9443. [Google Scholar] [CrossRef]
- Sumbayev, V.V.; Yasinska, I.M.; Garcia, C.P.; Gilliland, D.; Lall, G.S.; Gibbs, B.F.; Bonsall, D.R.; Varani, L.; Rossi, F.; Calzolai, L. Gold nanoparticles downregulate interleukin-1β-induced pro-inflammatory responses. Small 2013, 9, 472–477. [Google Scholar] [CrossRef]
- Higby, G.J. Gold in medicine. Gold Bull. 1982, 15, 130–140. [Google Scholar] [CrossRef]
- Brown, D.M.; Johnston, H.; Gubbins, E.; Stone, V.J. Cytotoxicity and cytokine release in rat hepatocytes, C3A cells and macrophages exposed to gold nanoparticles–effect of biological dispersion media or corona. J. Biomed. Nanotechnol. 2014, 10, 3416–3429. [Google Scholar] [CrossRef]
- Kelly, S.; Bombardieri, M.; Humby, F. Angiogenic gene expression and vascular density are reflected in ultrasonographic features of synovitis in early rheumatoid arthritis: An observational study. Arthritis Res Ther. 2015, 17, 58. [Google Scholar] [CrossRef]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic Properties of Gold Nanoparticles. Clin. Cancer Res. 2005, 11, 3530–3534. [Google Scholar] [CrossRef]
- Sasidharan, S.L.; Khee, C.S.; Young, Z. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar]
- Yi, D.K.; Sun, I.C.; Ryu, J.H.; Koo, H.; Park, C.W.; Youn, I.C.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.H. Matrix Metalloproteinase Sensitive Gold Nanorod for Simultaneous Bioimaging and Photothermal Therapy of Cancer. Bioconjugate Chem. 2010, 21, 2173–2177. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Zeng, Y.; Hu, R.; Wang, L.; Gu, D.; He, J.; Wu, S.Y.; Ho, H.P.; Li, X.; Qu, J.; Gao, B.Z.; et al. Recent advances in surface plasmon resonance imaging: Detection speed, sensitivity, and portability. Nanophotonics 2017, 6, 1017–1030. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Kaushal, S.; Nanda, S.S.; Yi, D.K.; Ju, H. Effects of Aspect Ratio Heterogeneity of an Assembly of Gold Nanorod on Localized Surface Plasmon Resonance. J. Phys. Chem. Lett. 2020, 11, 5972–5979. [Google Scholar] [CrossRef]
- Mallick, S.; Sun, I.C.; Kim, K.; Yi, D.K. Silica Coated Gold Nanorods for Imaging and Photo-Thermal Therapy of Cancer Cells. J. Nanosci. Nanotechnol. 2013, 13, 3223–3229. [Google Scholar] [CrossRef]
- Kim, C.B.; Yi, D.K.; Kim, P.S.S.; Lee, W.; Kim, M.J. Rapid Photothermal Lysis of the Pathogenic Bacteria, Escherichia Coli Using Synthesis of Gold Nanorods. J. Nanosci. Nanotechnol. 2009, 9, 2841–2845. [Google Scholar] [CrossRef]
- Wang, T.; Yeom, K.S.; Nanda, S.S.; An, S.S.A.; Yi, D.K. Cancer Cell Growth in the Near Infrared Region by Using Silica Coated Gold Nanorods. Nano 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Nanda, S.S.; Wang, T.; Yoon, H.Y. Enhanced proliferation of rabbit chondrocytes by using a well circulated nanoshock system. Sci. Rep. 2021, 11, 19388. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, S.S.; Yi, D.K.; Kim, K. Magnetic, optical gold nanorods for recyclable photothermal ablation of bacteria. J. Mater. Chem. B 2014, 2, 981–988. [Google Scholar] [CrossRef]
- Nanda, S.S.; Wang, T.; Hossain, M.I.; Yoon, H.Y.; Selvan, S.T.; Kim, K.; Yi, D.K. Gold-Nanorod-Based Scaffolds for Wound-Healing Applications. ACS Appl. Nano Mater. 2022, 5, 8640–8648. [Google Scholar] [CrossRef]
- Boca, S.C.; Potara, M.; Gabudean, A.M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef] [PubMed]
- Curcio, A.; de Walle, A.V.; Benassai, E.; Serrano, A.; Luciani, N.; Menguy, N.; Manshian, B.B.; Sargsian, A.; Soenen, S.; Espinosa, A.; et al. Massive Intracellular Remodeling of CuS Nanomaterials Produces Nontoxic Bioengineered Structures with Preserved Photothermal Potential. ACS Nano 2021, 15, 9782–9795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yan, L.; Xue, J.; Zhang, K.; Xu, F.-J. Degradable one-dimensional dextran-iron oxide nanohybrids for MRI-guided synergistic gene/photothermal/magnetolytic therapy. Nano Today 2021, 38, 101118. [Google Scholar] [CrossRef]
- Robinson, J.T.; Hong, G.S.; Liang, Y.Y.; Zhang, B.; Yaghi, O.K.; Dai, H.J. In Vivo Fluorescence Imaging in the Second Near-Infrared Window with Long Circulating Carbon Nanotubes Capable of Ultrahigh Tumor Uptake. J. Am. Chem. Soc. 2012, 134, 10664–10669. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity Assessments of Multisized Gold and Silver Nanoparticles in Zebrafish Embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Yi, D.K. A study of optothermal and cytotoxic properties of silica coated Au nanorods. Mater. Lett. 2011, 65, 2319–2321. [Google Scholar] [CrossRef]
- Nanda, S.S.; Hembram, K.P.S.S.; Lee, J.-K.; Kim, K.; Selvan, S.T.; Yi, D.K. Experimental and Theoretical Structural Characterization of Cu–Au Tripods for Photothermal Anticancer Therapy. ACS Appl. Nano Mater. 2019, 2, 3735–3742. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Halas, N.J.; Lal, S.; Chang, W.S.; Link, S.; Nordlander, P. Plasmons in strongly coupled metallic nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef]
- Cheng, L.C.; Huang, J.H.; Chen, H.M.; Lai, T.C.; Yang, K.Y.; Liu, R.S.; Hsiao, M.; Chen, C.H.; Her, L.J.; Tsai, D.P. Seedless, silver-induced synthesis of star-shaped gold/silver bimetallic nanoparticles as high efficiency photothermal therapy reagent. J. Mater. Chem. 2012, 22, 2244–2253. [Google Scholar] [CrossRef]
- Goodman, A.M.; Cao, Y.; Urban, C.; Neumann, O.; Ayala-Orozco, C.; Knight, M.W.; Joshi, A.; Nordlander, P.; & Halas, N.J. The surprising in vivo instability of near-IR-absorbing hollow Au-Ag nanoshells. ACS Nano 2014, 8, 3222–3231. [Google Scholar] [CrossRef]
- Johnston, H.J. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010, 40, 328–346. [Google Scholar] [CrossRef]
- Franco-Molina, M.A. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 148–154. [Google Scholar] [CrossRef]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef]
- Rubio-Ruiz, B.; Pérez-López, A.M.; Bray, T.L.; Lee, M.; Serrels, A.; Prieto, M.; Arruebo, M.; Carragher, N.O.; Sebastián, V.; Unciti-Broceta, A. High-Precision Photothermal Ablation Using Biocompatible Palladium Nanoparticles and Laser Scanning Microscopy. ACS Appl. Mater. Interfaces 2018, 10, 3341–3348. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef]
- Tang, S.; Chen, M.; Zheng, N. Sub-10-nm Pd Nanosheets with Renal Clearance for Efficient Near-Infrared Photothermal Cancer Therapy. Small 2014, 10, 3139–3144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.W.; Fan, S.X.; Wang, F.; Sun, L.D.; Zheng, X.Y.; Yan, C.H. Porous Pd nanoparticles with high photothermal conversion efficiency for efficient ablation of cancer cells. Nanoscale 2014, 6, 4345–4351. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D.; et al. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.V.; Huynh, T.C.; Oh, J. Photothermal responsive porous membrane for treatment of infected wound. Polymers 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Gil, Y.G.; Kang, S.; Chae, A.; Kim, Y.K.; Min, D.H.; Jang, H. Synthesis of porous Pd nanoparticles by therapeutic chaga extract for highly efficient tri-modal cancer treatment. Nanoscale 2018, 10, 19810–19817. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Bui, N.Q.; Moorthy, M.S.; Lee, K.D.; Oh, J. Synthesis and In Vitro Performance of Polypyrrole-Coated Iron–Platinum Nanoparticles for Photothermal Therapy and Photoacoustic Imaging. Nanoscale Res. Lett. 2017, 12, 570. [Google Scholar] [CrossRef]
- Chen, C.-L.; Kuo, L.-R.; Lee, S.-Y.; Hwu, Y.-K.; Chou, S.-W.; Chen, C.-C.; Chang, F.-H.; Lin, K.-H.; Tsai, D.-H.; Chen, Y.-Y. Photothermal cancer therapy via femtosecond-laser-excited FePt nanoparticles. Biomaterials 2013, 34, 1128–1134. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Zhang, J.; Wang, X.; Wang, Y.; Ge, H.; Yan, W.; Huang, Q.; Xiao, J.; Zhang, Q.; et al. Trifolium-like Platinum Nanoparticle-Mediated Photothermal Therapy Inhibits Tumor Growth and Osteolysis in a Bone Metastasis Model. Small 2015, 11, 2080–2086. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Zhang, J.; Zhang, W.; Foda, M.F.; Dai, X.; Han, H. Ultrasmall Peptide-Coated Platinum Nanoparticles for Precise NIR-II Photothermal Therapy by Mitochondrial Targeting. ACS Appl. Mater. Interfaces 2020, 12, 39434–39443. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Huang, Q.; Xiao, J.; Zhang, Q.; Cheng, Y. A degradable hydrogel formed by dendrimer-encapsulated platinum nanoparticles and oxidized dextran for repeated photothermal cancer therapy. J. Mater. Chem. B 2018, 6, 2474–2480. [Google Scholar] [CrossRef]
- Yang, W.; Thordarson, P.; Gooding, J.J.; Ringer, S.P.; Braet, F. Carbon nanotubes for biological and biomedical applications. Nanotechnology 2007, 18, 412001. [Google Scholar] [CrossRef]
- Chen, X.; Tam, U.C.; Czlapinski, J.L.; Lee, G.S.; Rabuka, D.; Zettl, A.; Bertozzi, C.R. Interfacing carbon nanotubes with living cells J. Am. Chem. Soc. 2006, 128, 6292–6293. [Google Scholar] [CrossRef]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef]
- Song, Y.; Xu, C.; Wei, W.; Ren, J.; Qu, X. Light regulation of peroxidase activity by spiropyran functionalized carbon nanotubes used for label-free colorimetric detection of lysozyme. Chem. Commun. 2011, 47, 9083–9085. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.Y.; Wang, H.L.; Casalongue, H.S.; Vinh, D.; Dai, H.J. Ultrasmall Reduced GrapheneOxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, J.J.; Nie, Z. Harnessing the collective properties of nanoparticle ensembles for cancer theranostics. Nano Res. 2014, 7, 1719–1730. [Google Scholar] [CrossRef]
- Antaris, A.L.; Robinson, J.T.; Yaghi, O.K.; Hong, G.S.; Diao, S.; Luong, R.; Dai, H.J. Ultra-Low Doses of Chirality Sorted (6,5) Carbon Nanotubes for Simultaneous Tumor Imaging and Photothermal Therapy. ACS Nano 2013, 7, 3644–3652. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Cheng, L.; Lee, S.-T.; Liu, Z. Noble Metal Coated Single-Walled Carbon Nanotubes for Applications in Surface Enhanced Raman Scattering Imaging and Photothermal Therapy. J. Am. Chem. Soc. 2012, 134, 7414–7422. [Google Scholar] [CrossRef]
- Moon, H.K.; Lee, S.H.; Choi, H.C. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 2009, 3, 3707–3713. [Google Scholar] [CrossRef]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor Metastasis Inhibition by Imaging-Guided Photothermal Therapy with Single-Walled Carbon Nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, X.; Chen, T.; Sun, S.; Shi, Z.; Liu, J.; Ji, X.; Zeng, X.; Guan, J.; Mei, L.; et al. Renal-Clearable Ultrasmall Polypyrrole Nanoparticles with Size-Regulated Property for Second Near-Infrared Light-Mediated Photothermal Therapy. Adv. Funct. Mater. 2021, 31, 2008362. [Google Scholar] [CrossRef]
- Cui, H.; Miao, S.; Esworthy, T. A novel near-infrared light responsive 4D printed nanoarchitecture with dynamically and remotely controllable transformation. Nano Res. 2019, 12, 1381–1388. [Google Scholar] [CrossRef]
- Park, E.J.; Bae, E.; Yi, J.; Kim, Y.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C.; Park, K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [Google Scholar] [CrossRef]
- Chen, H.W.; Su, S.F.; Chien, C.T.; Lin, W.H.; Yu, S.L.; Chou, C.C.; Chen, J.J.W.; Yang, P.C. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. 2006, 20, 2393–2395. [Google Scholar] [CrossRef]
- Meng, H.; Chen, Z.; Xing, G.; Yuan, H.; Chen, C.; Zhao, F.; Zhang, C.; Zhao, Y. Ultrahigh reactivity provokes nanotoxicity: Explanation of oral toxicity of nano-copper particles. Toxicol. Lett. 2007, 175, 102–110. [Google Scholar] [CrossRef]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Cho, H.Y.; Han, B.S.; Kim, S.H.; Kim, H.O.; Lim, Y.T.; Chung, B.H. Acute Toxicity and Pharmacokinetics of 13??Nm-Sized PEG-Coated Gold Nanoparticles. Toxicol. Appl. Pharmacol. 2009, 236, 16. [Google Scholar] [CrossRef]
- Semete, B.; Booysen, L.; Lemmer, Y.; Kalombo, L.; Katata, L.; Verschoor, J.; Swai, H.S. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 662–671. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; He, X.; Yang, X.; Li, L.; Liu, S.; Zou, Z.; Wang, K.; Liu, J. Near-infrared photothermal release of hydrogen sulfide from nanocomposite hydrogels for anti-inflammation applications. Chin. Chem. Lett. 2020, 31, 787–791. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Liang, M.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011, 6, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Elder, A.; Yang, H.; Gwiazda, R.; Teng, X.; Thurston, S.; He, H.; Oberdörster, G. Testing Nanomaterials of Unknown Toxicity: An Example Based on Platinum Nanoparticles of Different Shapes. Adv. Mater. 2007, 19, 3124–3129. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Kamaly, N.; Fredman, G.; Subramanian, M.; Gadde, S.; Pesic, A.; Cheung, L.; Fayad, Z.A.; Langer, R.; Tabas, I.; Cameron Farokhzad, O. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 6506–6511. [Google Scholar] [CrossRef]
- Ainslie, K.M.; Thakar, R.G.; Bernards, D.A.; Desai, T.A. Inflammatory Response to Implanted Nanostructured Materials. In Biological Interactions on Materials Surfaces; Springer: New York, NY, USA, 2009; pp. 355–371. [Google Scholar]
- Kruss, S.; Erpenbeck, L.; Amschler, K.; Mundinger, T.A.; Boehm, H.; Helms, H.-J.; Friede, T.; Andrews, R.K.; Schön, M.P.; Spatz, J.P. Adhesion Maturation of Neutrophils on Nanoscopically Presented Platelet Glycoprotein Ibα. ACS Nano 2013, 7, 9984–9996. [Google Scholar] [CrossRef]
- Park, E.J.; Shim, H.W.; Lee, G.H.; Kim, J.H.; Kim, D.W. Comparison of toxicity between the different-type TiO₂ nanowires in vivo and in vitro. Arch Toxicol. 2013, 87, 1219–1230. [Google Scholar] [CrossRef]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-Dependent Retention of Carbon Nanotubes in the Pleural Space of Mice Initiates Sustained Inflammation and Progressive Fibrosis on the Parietal Pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Rao, W.; Deng, Z.S.; Liu, J. Areview of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit. Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable Gold Nanovesicles with an Ultrastrong Plasmonic Coupling Effect for Photoacoustic Imaging and Photothermal Therapy. Angew. Chem. 2013, 125, 14208–14214. [Google Scholar] [CrossRef]
- Larson, T.A.; Bankson, J.; Aaron, J.; Sokolov, K. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology 2007, 18, 325101. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, L.; Cao, J.; Wang, K.; Ge, Y.; Ma, W.; Qi, X.; Shen, S. Effect of magnetic nanoparticles size on rheumatoid arthritis targeting and photothermal therapy. Colloids Surf. B Biointerfaces 2018, 170, 224–232. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Zhang, L.H.; Li, J.; Wu, L.; Zhang, H.; Yang, B.; Zhu, M.; Jiang, J. Anti-inflammatory effects of magnetically targeted mesenchymal stem cells on laser-induced skin injuries in rats. Int. J. Nanomed. 2020, 15, 5645–5659. [Google Scholar] [CrossRef]
- Tang, T.; Valenzuela, A.; Petit, F.; Chow, S.; Leung, K.; Gorin, F.; Louie, A.Y.; Dhenain, M. In Vivo MRI of Functionalized Iron Oxide Nanoparticles for Brain Inflammation. Contrast Media Mol. Imaging 2018, 26, 3476476. [Google Scholar] [CrossRef]
- Van Ede, A.E.; Laan, R.F.J.M.; Blom, H.J.; De Abreu, R.A.; van de Putte, L.B.A. Methotrexate in rheumatoid arthritis: An updatewith focus on mechanisms involved in toxicity. Semin. Arthritis Rheum. 1998, 27, 277–292. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, H.J.; Ha, Y.J.; Park, Y.N.; Lee, S.K.; Park, Y.B.; Yoo, K.H. Targeted Chemo-Photothermal Treatments of Rheumatoid Arthritis Using Gold Half-Shell Multifunctional Nanoparticles. ACS Nano 2013, 7, 50–57. [Google Scholar] [CrossRef]
- Chen, H.; Dorrigan, A.; Saad, S.; Hare, D.J.; Cortie, M.B.; Valenzuela, S.M. In Vivo Study of Spherical Gold Nanoparticles: Inflammatory Effects and Distribution in Mice. PLoS ONE 2013, 8, 58208. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Mendonça-Martins, I.; Santos, M.F.A.; Raposo, L.R.; Mendes, R.; Marques, J.; Romão, C.C.; Romão, M.J.; Santos-Silva, T.; Baptista, P.V. Improving the Anti-inflammatory Response via Gold Nanoparticle Vectorization of CO-Releasing Molecules. ACS Biomater. Sci. Eng. 2020, 6, 1090–1101. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.K.; Seethalakshmi, S.; Gopal, V. Evaluation of In-vitro Anti-Inflammatory Activity of Silver Nanoparticles Synthesised using Piper Nigrum Extract. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar]
- Hebeish, A.; El-Rafie, M.H.; EL-Sheikh, M.A.; Seleem, A.A.; El-Naggar, M.E. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int. J. Biol. Macromol. 2014, 65, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; He, J.; Qiao, Y.; Qi, Y.; Zhang, H.; Santos, H.A.; Zhong, D.; Li, W.; Hua, S.; Wang, W.; et al. Mild temperature photothermal assisted anti-bacterial and anti-inflammatory nanosystem for synergistic treatment of post-cataract surgery endophthalmitis. Theranostics 2020, 10, 8541–8557. [Google Scholar] [CrossRef]
- Walsh, J.T. Basic Interactions of Light with Tissue. In Optical-Thermal Response of Laser-Irradiated Tissue; Springer: Dordrecht, The Netherlands, 2010; pp. 13–26. [Google Scholar]
- Hanf, R.; Fey, S.; Schmitt, M.; Hermann, G.; Dietzek, B.; Popp, J. Catalytic Efficiency of a Photoenzyme—An Adaptation to Natural Light Conditions. ChemPhysChem. 2012, 13, 2013–2015. [Google Scholar] [CrossRef]
- Wang, T.; Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K. Mechanophysical Cues in Extracellular Matrix Regulation of Cell Behavior. ChemBioChem 2020, 21, 1254–1264. [Google Scholar] [CrossRef]
- Stolik, S.; Delgado, J.A.; Pérez, A.; Anasagasti, L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J. Photochem. Photobiol. B: Biol. 2000, 57, 90–93. [Google Scholar] [CrossRef]
- Robinson, J.T.; Welsher, K.; Tabakman, S.M.; Sherlock, S.P.; Wang, H.; Luong, R.; Dai, H. High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 2010, 3, 779–793. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Zou, R.; Wang, Q.; Zhang, B.; An, L.; Yin, F.; Hua, Y.; Hu, J. Self-assembled WO3−x hierarchical nanostructures for photothermal therapy with a 915 nm laser rather than the common 980 nm laser. Dalton Trans. 2014, 43, 6244–6250. [Google Scholar] [CrossRef]
- Jaque, D.; Martínez Maestro, L.; Del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Chakravarty, P.; Marches, R.; Zimmerman, N.S.; Swafford, A.D.-E.; Bajaj, P.; Musselman, I.H.; Pantano, P.; Draper, R.K.; Vitetta, E.S. Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proc. Natl. Acad. Sci. USA 2008, 105, 8697–8702. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal Therapy, Part III: Ablation Techniques. Crit. Rev. Biomed. Eng. 2007, 35, 37–121. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal Therapy, Part 2: Hyperthermia Techniques. Crit. Rev. Biomed. Eng. 2006, 34, 491–542. [Google Scholar] [CrossRef]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef]
- Mierke, C.T. Cell–Cell and Cell–Matrix Adhesion Strength, Local Cell Stiffness and Forces; IOP Publishing Ltd.: Bristol, UK, 2015; pp. 4–49. [Google Scholar]
- Salih, V.; Thomas, D. Fundamentals of cell and matrix biology for tissue engineering. In Standardisation in Cell and Tissue Engineering; Woodhead Publishing: Oxford, UK, 2013; pp. 3–17. [Google Scholar]
- LaPointe, V.L.S.; de Boer, J.; Engler, A.J. Chapter 4—Cellular Signaling. Tissue Eng. 2014, 111–148. [Google Scholar]
- Yi, D.K.; Nanda, S.S.; Kim, K.; Tamil Selvan, S. Recent progress in nanotechnology for stem cell differentiation, labeling, tracking and therapy. J. Mater. Chem. B 2017, 5, 9429–9451. [Google Scholar] [CrossRef]
- Chen, C.S. Mechanotransduction—A field pulling together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef]
- Tseng, P.; Judy, J.W.; Di Carlo, D. Magnetic nanoparticle-mediated massively parallel mechanical modulation of single-cell behavior. Nat. Methods 2012, 9, 1113–1119. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Zhou, H.; Zheng, K.; Wang, D.; Jia, M.; Xu, P.; Ma, K.; Cui, C.; Wang, L. CD47-targeted bismuth selenide nanoparticles actualize improved photothermal therapy by increasing macrophage phagocytosis of cancer cells. Colloids Surf. B Biointerfaces 2019, 184, 110546. [Google Scholar] [CrossRef]
- Tseng, H.Y.; Chen, W.F.; Chu, C.K. On-substrate fabrication of a bio-conjugated Au nanoring solution for photothermal therapy application. Nanotechnology 2013, 24, 065102. [Google Scholar] [CrossRef]
- Huachao Chen, J.T.; Weijiang, H.; Guo, Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef]
- Riethmüller, M.; Burger, N.; Bauer, G. Singlet oxygen treatment of tumor cells triggers extracellular singlet oxygen generation, catalase inactivation and reactivation of intercellular apoptosis-inducing signaling. Redox Biol. 2015, 6, 157–168. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Sun, Y. Co-delivery of bee venom melittin and a photosensitizer with an organic-inorganic hybrid nanocarrier for photodynamic therapy and immunotherapy. ACS Nano 2019, 13, 12638–12652. [Google Scholar] [CrossRef]
- Doix, B.; Trempolec, N.; Riant, O.; Feron, O. Low Photosensitizer Dose and Early Radiotherapy Enhance Antitumor Immune Response of Photodynamic Therapy-Based Dendritic Cell Vaccination. Front. Oncol. 2019, 9, 811. [Google Scholar] [CrossRef]
- Zhu, Y.; Xue, J.; Chen, W. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J. Control Release 2020, 322, 300–311. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, X.; Liu, R.; Zhao, D.; Guo, C.; Zhu, A.; Wen, M.; Liu, Z.; Qu, G.; Meng, H. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Ali, H.R.; Rankin, C.R.; El-Sayed, M.A. Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy. Biomaterials 2016, 102, 1–8. [Google Scholar] [CrossRef]
- Mao, F.; Liu, Y.; Ma, L.; Liu, L.; Jiang, A.; Zhai, X.; Zhou, J. Green synthesis of ultra-small VOx nanodots for acidic-activated HSP60 inhibition and therapeutic enhancement. Biomaterials 2019, 194, 94–104. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Cheng, X. Versatile biomimetic cantharidin-tellurium nanoparticles enhance photothermal therapy by inhibiting the heat shock response for combined tumor therapy. Acta. Biomater. 2020, 110, 208–220. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Ning, X.; Xue, P.; Liu, Y. pH-activated heat shock protein inhibition and radical generation enhanced NIR luminescence imaging-guided photothermal tumour ablation. Int. J. Pharm. 2019, 566, 40–45. [Google Scholar] [CrossRef]








| Materials | In Vivo/In Vitro | Dose/Concentration | Laser Power and Wavelength | Cell(s) | Total Treatment Time/Laser Irradiation Time | Activity | Ref. |
|---|---|---|---|---|---|---|---|
| Si-AuNRs | In vitro | 83 µg/mL | NIR Laser 160 mW, 671 nm Wavelength | MDA-MB-231 | 48 h | Enhance the activities of HSPs. Folding the proteins in cell growth and survival | [29] |
| Gold nanorods–magnetic NPs | In vitro | 5 mg·mL−1–35 mg mL−1 | 671 nm DPSS Laser, 130 mW | E. coli | 12 min (laser irradiation time) | Bactericidal, bacteriostatic | [31] |
| Chit-AgNTs | In vitro | 0.17 µgmL−1–1.71 µgmL−1 | 720–930 nm, CW laser | NCI-H460 cancer cells | 24 h | Cell-membrane destruction by photothermal effect | [33] |
| Fe3O4-ICG@IRM | In vivo | 20 mg/kg in mice | 0.5–2.0 W/cm2 | ID8 tumor in C57BL/6 mice | 18 days | Vacuolar necrotic cells, apoptotic tumor cells | [34] |
| IONF@CuS NPs | In vitro | 10 µL | 0.3 W/cm2 | hMSCs | 21 days | Potential photothermal properties with no adverse biological response | [35] |
| Fe3O4@Dex-PGEA | In vivo | 100 µL | 1 W/cm2 | Breast cancer cells | 10 days | Growth reduction in the solid tumor tissue | [36] |
| PEG-SWCNTs | In vivo | ~120 mg/mL, 100 µL | 808 nm Wavelength, 76 W/cm3 | KB tumor cells | 60 days | Destruction of the solid tumor | [37] |
| Ppy NPs | In vivo | 0.072–2.3 mg/mL | 1 W/cm2 | U87 tumor cells | 18 days | Prominent photothermal efficiency with excellent biosafety | [38] |
| Graphene nanocomposite | In vitro | 0–20% | 808 nm, 800 mW | Neural stem cells | 7 days | Potential photothermal properties and enhanced cell proliferation | [39] |
| Materials | Methods | Size | Animals | Site of Action | Route of Administration | Toxicity | Ref. |
|---|---|---|---|---|---|---|---|
| AuNPs | Percent mortality | 15–100 nm | Mouse and zebrafish | Size-dependent distribution | Intravenous (mouse), embryo | No toxicity observed | [38,39] |
| AgNPs | Histopathology | 42 nm | Mouse | Whole body distribution | Oral | Organ toxicity and inflammatory responses | [75] |
| TiO2 | Morphometric | 19–21 nm | Mouse | Placenta | Intratracheal | Pulmonary toxicity, pulmonary emphysema | [76] |
| Nano-copper | Biochemistry analysis | ~23.5 nm | Mouse | Plasma | Oral | Accumulation of alkalescent substance | [77] |
| Silica nanoparticles | Immunohistochemistry | 50–200 nm | Mouse | Tissue distribution | Intravenous | Inflammatory responses over the size ~100 nm | [78] |
| PLGA | Histopathology | 200–350 nm | Mouse | Histopathology assay | Oral | No toxicity | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.I.; Nanda, S.S.; Selvan, S.T.; Yi, D.K. Recent Insights into NIR-Light-Responsive Materials for Photothermal Cell Treatments. Nanomaterials 2022, 12, 3318. https://doi.org/10.3390/nano12193318
Hossain MI, Nanda SS, Selvan ST, Yi DK. Recent Insights into NIR-Light-Responsive Materials for Photothermal Cell Treatments. Nanomaterials. 2022; 12(19):3318. https://doi.org/10.3390/nano12193318
Chicago/Turabian StyleHossain, Md Imran, Sitansu Sekhar Nanda, Subramanian Tamil Selvan, and Dong Kee Yi. 2022. "Recent Insights into NIR-Light-Responsive Materials for Photothermal Cell Treatments" Nanomaterials 12, no. 19: 3318. https://doi.org/10.3390/nano12193318
APA StyleHossain, M. I., Nanda, S. S., Selvan, S. T., & Yi, D. K. (2022). Recent Insights into NIR-Light-Responsive Materials for Photothermal Cell Treatments. Nanomaterials, 12(19), 3318. https://doi.org/10.3390/nano12193318