Nonvolatile Ternary Memristor Based on Fluorene-Benzimidazole Copolymer/Au NP Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Storage Device Preparation Procedure
3. Results and Discussion
3.1. Characterization Test of PF-BBO
3.2. SEM Images of the Device Structure
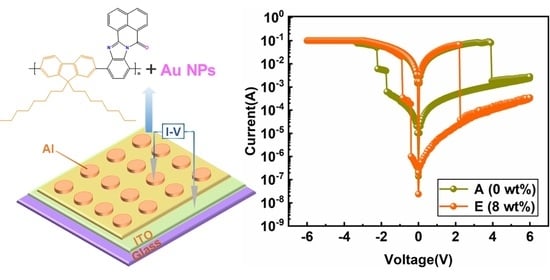
3.3. Storage Performance of the Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, T.-C.; Chang, K.-C.; Tsai, T.-M.; Chu, T.-J.; Sze, S.M. Resistance random access memory. Mater. Today 2016, 19, 254–264. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, D.; Bai, X.; Lu, J.; Ai, C. Ternary Resistance Switching Memory Behavior Based on Graphene Oxide Embedded in a Polystyrene Polymer Layer. Sci. Rep. 2017, 7, 3938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.; Kapur, S.; Saurabh, S.; Grover, A. Resistive Random Access Memory: A Review of Device Challenges. IETE Tech. Rev. 2019, 37, 377–390. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, J.; Guo, Z.; Sun, Z. An overview of materials issues in resistive random access memory. J. Mater. 2015, 1, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Akinaga, H.; Shima, H. Resistive Random Access Memory (ReRAM) Based on Metal Oxides. Proc. IEEE 2010, 98, 2237–2251. [Google Scholar] [CrossRef]
- Mangalam, J.; Agarwal, S.; Resmi, A.N.; Sundararajan, M.; Jinesh, K.B. Resistive switching in polymethyl methacrylate thin films. Org. Electron. 2016, 29, 33–38. [Google Scholar] [CrossRef]
- Yang, X. Demonstration of Ultra-Fast Switching in Nanometallic Resistive Switching Memory Devices. J. Nanosci. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Yi, X.; Shang, J.; Liu, G.; Li, R.W. Organic and hybrid resistive switching materials and devices. Chem. Soc. Rev. 2019, 48, 1531–1565. [Google Scholar] [CrossRef]
- Sun, C.; Lu, S.M.; Jin, F.; Mo, W.Q.; Song, J.L.; Dong, K.F. Investigation of FePt electrode induced influence on resistive switching characteristics of SiO2-based RRAM. J. Mater. Sci. Mater. Electron. 2020, 31, 19989–19996. [Google Scholar] [CrossRef]
- Park, C.J.; Han, S.W.; Shin, M.W. Laser-Assisted Interface Engineering for Functional Interfacial Layer of Al/ZnO/Al Resistive Random Access Memory (RRAM). ACS Appl. Mater. Interfaces 2020, 12, 32131–32142. [Google Scholar] [CrossRef]
- Pooyodying, P.; Son, Y.-H.; Sung, Y.-M.; Ok, J.-W. The effect of sputtering Ar gas pressure on optical and electrical properties of flexible ECD device with WO3 electrode deposited by RF magnetron sputtering on ITO/PET substrate. Opt. Mater. 2022, 123, 111829. [Google Scholar] [CrossRef]
- Lee, J.; Schell, W.; Zhu, X.; Kioupakis, E.; Lu, W.D. Charge Transition of Oxygen Vacancies during Resistive Switching in Oxide-Based RRAM. ACS Appl. Mater. Interfaces 2019, 11, 11579–11586. [Google Scholar] [CrossRef]
- Khurana, G.; Misra, P.; Kumar, N.; Katiyar, R.S. Tunable Power Switching in Nonvolatile Flexible Memory Devices Based on Graphene Oxide Embedded with ZnO Nanorods. J. Phys. Chem. C 2014, 118, 21357–21364. [Google Scholar] [CrossRef]
- Asaad, J.N. Synthesis and characterization of unsaturated polyester/carborundum composites. J. Appl. Polym. Sci. 2013, 129, 1812–1819. [Google Scholar] [CrossRef]
- Ling, Q.-D.; Liaw, D.-J.; Teo, E.Y.-H.; Zhu, C.; Chan, D.S.-H.; Kang, E.-T.; Neoh, K.-G. Polymer memories: Bistable electrical switching and device performance. Polymer 2007, 48, 5182–5201. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, S.; Pal, P.; Tsai, T.-J.; Lin, P.-C.; Wang, Y.-H. A Low Program Voltage Enabled Flash like AlGaN/GaN Stack Layered MIS-HEMTs Using Trap Assisted Technique. ECS J. Solid State Sci. Technol. 2021, 10, 055019. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Hwang, S.; Kim, M.H.; Chang, Y.F.; Park, B.G. Analog Synaptic Behavior of a Silicon Nitride Memristor. ACS Appl. Mater. Interfaces 2017, 9, 40420–40427. [Google Scholar] [CrossRef] [PubMed]
- Nemati-Kande, E.; Karimian, R.; Goodarzi, V.; Ghazizadeh, E. Feasibility of pristine, Al-doped and Ga-doped Boron Nitride nanotubes for detecting SF4 gas: A DFT, NBO and QTAIM investigation. Appl. Surf. Sci. 2020, 510, 145490. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Q.; Zhu, X.; Li, Y.; Zhang, M.; Li, J.; Ma, C.; Li, H.; Lu, J.; Zhang, Q. Recent advances in organic-based materials for resistive memory applications. InfoMat 2020, 2, 995–1033. [Google Scholar] [CrossRef]
- Liu, S.-J.; Lin, Z.-H.; Zhao, Q.; Ma, Y.; Shi, H.-F.; Yi, M.-D.; Ling, Q.-D.; Fan, Q.-L.; Zhu, C.-X.; Kang, E.-T.; et al. Flash-Memory Effect for Polyfluorenes with On-Chain Iridium(III) Complexes. Adv. Funct. Mater. 2011, 21, 979–985. [Google Scholar] [CrossRef]
- Hahm, S.G.; Kang, N.G.; Kwon, W.; Kim, K.; Ko, Y.G.; Ahn, S.; Kang, B.G.; Chang, T.; Lee, J.S.; Ree, M. Programmable Bipolar and Unipolar Nonvolatile Memory Devices Based on Poly(2-(N-carbazolyl)ethyl methacrylate) End-Capped with Fullerene. Adv. Mater. 2012, 24, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Padhy, H.; Huang, J.-H.; Sahu, D.; Patra, D.; Kekuda, D.; Chu, C.-W.; Lin, H.-C. Synthesis and Applications of Low-Bandgap Conjugated Polymers Containing Phenothiazine Donor and Various Benzodiazole Acceptors for Polymer Solar Cells. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4823–4834. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef]
- Mutiso, R.M.; Kikkawa, J.M.; Winey, K.I. Resistive switching in silver/polystyrene/silver nano-gap devices. Appl. Phys. Lett. 2013, 103, 223302. [Google Scholar] [CrossRef]
- Ielmini, D. Resistive switching memories based on metal oxides: Mechanisms, reliability and scaling. Semicond. Sci. Technol. 2016, 31, 063002. [Google Scholar] [CrossRef]
- Lorenzi, P.; Rao, R.; Irrera, F. Role of the electrode metal, waveform geometry, temperature, and postdeposition treatment on SET and RESET of HfO2-based resistive random access memory 1R-cells: Experimental aspects. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2015, 33, 01A107. [Google Scholar] [CrossRef]
- Ye, C.; Wu, J.; He, G.; Zhang, J.; Deng, T.; He, P.; Wang, H. Physical Mechanism and Performance Factors of Metal Oxide Based Resistive Switching Memory: A Review. J. Mater. Sci. Technol. 2016, 32, 1–11. [Google Scholar] [CrossRef]
- Hong, X.; Loy, D.J.; Dananjaya, P.A.; Tan, F.; Ng, C.; Lew, W. Oxide-based RRAM materials for neuromorphic computing. J. Mater. Sci. 2018, 53, 8720–8746. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Wang, D.-Y.; Huang, I.S.; Chen, Y.-T.; Hsu, Y.-H.; Lin, T.-Y.; Meng, H.-F.; Chang, T.-C.; Yang, Y.-J.; Chen, C.-C.; et al. Low operation voltage macromolecular composite memory assisted by graphene nanoflakes. J. Mater. Chem. C 2013, 1, 552–559. [Google Scholar] [CrossRef]
- Liu, G.; Ling, Q.-D.; Hwee Teo, E.-Y.; Zhu, C.-X.; Chan, D.S.-H.; Neoh, K.-G.; Kang, E.-T. Electrical Conductance Tuning and Bistable Switching in Poly(N-vinylcarbazole)-Carbon Nanotube Composite Films. ACS Nano 2009, 3, 1929–1937. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, T.-K.; Zhao, Q.; Huang, W.; Ho, C.-L.; Wong, W.-Y. Ferrocene-containing poly(fluorenylethynylene)s for nonvolatile resistive memory devices. J. Mater. Chem. C 2016, 4, 921–928. [Google Scholar] [CrossRef]
- Yang, Y.; Ouyang, J.; Ma, L.; Tseng, R.J.H.; Chu, C.W. Electrical Switching and Bistability in Organic/Polymeric Thin Films and Memory Devices. Adv. Funct. Mater. 2006, 16, 1001–1014. [Google Scholar] [CrossRef]
- Zahoor, F.; Azni Zulkifli, T.Z.; Khanday, F.A. Resistive Random Access Memory (RRAM): An Overview of Materials, Switching Mechanism, Performance, Multilevel Cell (mlc) Storage, Modeling, and Applications. Nanoscale Res. Lett. 2020, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, C.; Shi, Z.; Li, J.; Qian, Q.; Ling, S.; Zhang, Y.; Zhu, X.; Wu, X.; Zhang, J.; et al. Nonvolatile Ternary Resistive Memory Performance of a Benzothiadiazole-Based Donor-Acceptor Material on ITO-Coated Glass. Coatings 2021, 11, 318. [Google Scholar] [CrossRef]
- Liu, S.-J.; Lin, W.-P.; Yi, M.-D.; Xu, W.-J.; Tang, C.; Zhao, Q.; Ye, S.-H.; Liu, X.-M.; Huang, W. Conjugated polymers with cationic iridium(III) complexes in the side-chain for flash memory devices utilizing switchable through-space charge transfer. J. Mater. Chem. 2012, 22, 22964. [Google Scholar] [CrossRef]
- Bozano, L.D.; Kean, B.W.; Beinhoff, M.; Carter, K.R.; Rice, P.M.; Scott, J.C. Organic Materials and Thin-Film Structures for Cross-Point Memory Cells Based on Trapping in Metallic Nanoparticles. Adv. Funct. Mater. 2005, 15, 1933–1939. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, Y.; Lee, S.K.; Ruan, G.; Kim, T.W.; Fei, H.; Lee, S.H.; Kim, D.Y.; Yoon, J.; Tour, J.M. Flexible Nanoporous WO3−x Nonvolatile Memory Device. ACS Nano 2016, 10, 7598–7603. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, Z.; Cao, X.; Lu, G.; Wang, L.H.; Fan, Q.L.; Huang, W.; Zhang, H. Preparation of MoS2-polyvinylpyrrolidone nanocomposites for flexible nonvolatile rewritable memory devices with reduced graphene oxide electrodes. Small 2012, 8, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Munjal, S.; Khare, N.; Vankar, V.D. Bipolar resistive switching and nonvolatile memory effect in poly(3-hexylthiophene)-carbon nanotube composite films. Carbon 2018, 130, 553–558. [Google Scholar] [CrossRef]
- Kim, S.-J.; Song, J.-M.; Lee, J.-S. Transparent organic thin-film transistors and nonvolatile memory devices fabricated on flexible plastic substrates. J. Mater. Chem. 2011, 21, 14516–14522. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhuang, H.; He, J.; Xia, S.; Li, H.; Li, N.; Xu, Q.; Lu, J. Improved ternary memory performance of donor-acceptor structured molecules through cyano substitution. J. Mater. Chem. C 2015, 3, 6778–6785. [Google Scholar] [CrossRef]
- Hu, Q.; Park, M.R.; Abbas, H.; Kang, T.S.; Yoon, T.-S.; Kang, C.J. Forming-free resistive switching characteristics in tantalum oxide and manganese oxide based crossbar array structure. Microelectron. Eng. 2018, 190, 7–10. [Google Scholar] [CrossRef]
- Krishnan, K.; Tsuruoka, T.; Mannequin, C.; Aono, M. Mechanism for Conducting Filament Growth in Self-Assembled Polymer Thin Films for Redox-Based Atomic Switches. Adv. Mater. 2016, 28, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, S.; Noh, T.W. Resistive switching phenomena: A review of statistical physics approaches. Appl. Phys. Rev. 2015, 2, 031303. [Google Scholar] [CrossRef] [Green Version]
- Gul, F. Carrier transport mechanism and bipolar resistive switching behavior of a nano-scale thin film TiO2 memristor. Ceram. Int. 2018, 44, 11417–11423. [Google Scholar] [CrossRef]
- Wu, J.; Ma, L.; Yang, Y. Single-band Hubbard model for the transport properties in bistable organic/metal nanoparticle/organic devices. Phys. Rev. B 2004, 69, 115321. [Google Scholar] [CrossRef] [Green Version]
- Heng-Tien, L.; Zingway, P.; Yi-Jen, C. Carrier Transport Mechanism in a Nanoparticle-Incorporated Organic Bistable Memory Device. IEEE Electron Device Lett. 2007, 28, 569–571. [Google Scholar] [CrossRef]
- Zhang, T.; Guerin, D.; Alibart, F.; Troadec, D.; Hourlier, D.; Patriarche, G.; Yassin, A.; Ocafrain, M.; Blanchard, P.; Roncali, J.; et al. Physical mechanisms involved in the formation and operation of memory devices based on a monolayer of gold nanoparticle-polythiophene hybrid materials. Nanoscale Adv. 2019, 1, 2718–2726. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Long, S.; Zhang, K.; Liu, X.; Wang, G.; Lian, X.; Liu, Q.; Lv, H.; Wang, M.; Xie, H.; et al. Investigation on the RESET switching mechanism of bipolar Cu/HfO2/Pt RRAM devices with a statistical methodology. J. Phys. D Appl. Phys. 2013, 46, 245107. [Google Scholar] [CrossRef]
- Chen, K.-H.; Kao, M.-C.; Huang, S.-J.; Li, C.-Y.; Cheng, C.-M.; Wu, S.; Wu, Z.-H. Bipolar switching properties and electrical conduction mechanism of manganese oxide RRAM devices. Ceram. Int. 2017, 43, S253–S257. [Google Scholar] [CrossRef]
- Tang, W.; Shi, H.Z.; Xu, G.; Ong, B.S.; Popovic, Z.D.; Deng, J.C.; Zhao, J.; Rao, G.H. Memory Effect and Negative Differential Resistance by Electrode-Induced Two-Dimensional Single-Electron Tunneling in Molecular and Organic Electronic Devices. Adv. Mater. 2005, 17, 2307–2311. [Google Scholar] [CrossRef]
- Jaafar, A.H.; Al Chawa, M.M.; Cheng, F.; Kelly, S.M.; Picos, R.; Tetzlaff, R.; Kemp, N.T. Polymer/TiO2 Nanorod Nanocomposite Optical Memristor Device. J. Phys. Chem. C 2021, 125, 14965–14973. [Google Scholar] [CrossRef]
- Bozano, L.D.; Kean, B.W.; Deline, V.R.; Salem, J.R.; Scott, J.C. Mechanism for bistability in organic memory elements. Appl. Phys. Lett. 2004, 84, 607–609. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Z.; Zhang, C.; Gu, P.; Chen, W.; Li, H.; Lu, J.; Zhang, Q. Thiadizoloquinoxaline-Based N-Heteroacenes as Active Elements for High-Density Data-Storage Device. ACS Appl. Mater. Interfaces 2018, 10, 15971–15979. [Google Scholar] [CrossRef]
- Scott, J.C.; Bozano, L.D. Nonvolatile Memory Elements Based on Organic Materials. Adv. Mater. 2007, 19, 1452–1463. [Google Scholar] [CrossRef]
- Prime, D.; Paul, S.; Josephs-Franks, P.W. Gold nanoparticle charge trapping and relation to organic polymer memory devices. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 4215–4225. [Google Scholar] [CrossRef]
- Tseng, R.J.; Huang, J.; Ouyang, J.; Kaner, R.B.; Yang, Y. Polyaniline Nanofiber/Gold Nanoparticle Nonvolatile Memory. Nano Lett. 2005, 5, 1077–1080. [Google Scholar] [CrossRef] [PubMed]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Du, Y.; Yu, H.; He, Z.; Wang, S.; Wang, C. Nonvolatile Ternary Memristor Based on Fluorene-Benzimidazole Copolymer/Au NP Composites. Nanomaterials 2022, 12, 4117. https://doi.org/10.3390/nano12234117
Gao M, Du Y, Yu H, He Z, Wang S, Wang C. Nonvolatile Ternary Memristor Based on Fluorene-Benzimidazole Copolymer/Au NP Composites. Nanomaterials. 2022; 12(23):4117. https://doi.org/10.3390/nano12234117
Chicago/Turabian StyleGao, Meng, Yanting Du, Haifeng Yu, Zhaohua He, Shuhong Wang, and Cheng Wang. 2022. "Nonvolatile Ternary Memristor Based on Fluorene-Benzimidazole Copolymer/Au NP Composites" Nanomaterials 12, no. 23: 4117. https://doi.org/10.3390/nano12234117
APA StyleGao, M., Du, Y., Yu, H., He, Z., Wang, S., & Wang, C. (2022). Nonvolatile Ternary Memristor Based on Fluorene-Benzimidazole Copolymer/Au NP Composites. Nanomaterials, 12(23), 4117. https://doi.org/10.3390/nano12234117