Butanediammonium Salt Additives for Increasing Functional and Operando Stability of Light-Harvesting Materials in Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
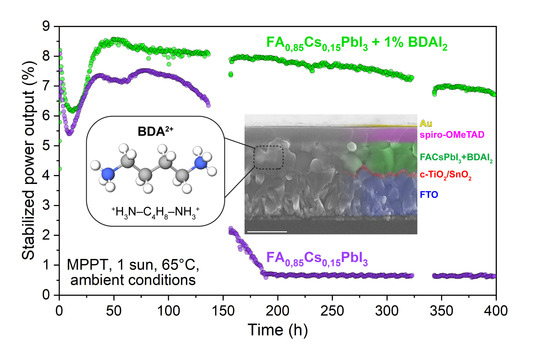
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NREL Best Research-Cell Efficiencies. NREL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 12 November 2022).
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus Statement for Stability Assessment and Reporting for Perovskite Photovoltaics Based on ISOS Procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Poorkazem, K.; Kelly, T.L. Compositional Engineering To Improve the Stability of Lead Halide Perovskites: A Comparative Study of Cationic and Anionic Dopants. ACS Appl. Energy Mater. 2018, 1, 181–190. [Google Scholar] [CrossRef]
- Rakstys, K.; Igci, C.; Nazeeruddin, M.K. Efficiency vs. Stability: Dopant-Free Hole Transporting Materials towards Stabilized Perovskite Solar Cells. Chem. Sci. 2019, 10, 6748–6769. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, Z.; Zhang, W.; Jiang, Z.; Chen, W.; Chen, R.; Huang, Y.; Yang, Z.; Zhang, Y.; Han, L.; et al. Barrier Designs in Perovskite Solar Cells for Long-Term Stability. Adv. Energy Mater. 2020, 10, 1–49. [Google Scholar] [CrossRef]
- Ahmad, S.; Fu, P.; Yu, S.; Yang, Q.; Liu, X.; Wang, X.; Wang, X.; Guo, X.; Li, C. Dion-Jacobson Phase 2D Layered Perovskites for Solar Cells with Ultrahigh Stability. Joule 2019, 3, 794–806. [Google Scholar] [CrossRef] [Green Version]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year Stable Perovskite Solar Cells by 2D/3D Interface Engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, H.; Qi, W.; Zhou, X.; Li, J.; Cheng, J.; Zhao, Y.; Li, Y.; Zhang, X. Passivation of Defects in Perovskite Solar Cell: From a Chemistry Point of View. Nano Energy 2020, 77, 105237. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Li, S.; Liu, S.; Wang, Q.; Mei, A.; Rong, Y.; Han, H.; Hu, Y. Progress in Multifunctional Molecules for Perovskite Solar Cells. Sol. RRL 2020, 4, 1–17. [Google Scholar] [CrossRef]
- Alharbi, E.A.; Alyamani, A.Y.; Kubicki, D.J.; Uhl, A.R.; Walder, B.J.; Alanazi, A.Q.; Luo, J.; Burgos-Caminal, A.; Albadri, A.; Albrithen, H.; et al. Atomic-Level Passivation Mechanism of Ammonium Salts Enabling Highly Efficient Perovskite Solar Cells. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, J.; Li, Z.; Liu, D.; Qin, M.; Cheung, S.H.; Lu, X.; Lei, D.; So, S.K.; Zhu, Z.; et al. Modulation of Defects and Interfaces through Alkylammonium Interlayer for Efficient Inverted Perovskite Solar Cells. Joule 2020, 4, 1248–1262. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden–Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Gharibzadeh, S.; Abdollahi Nejand, B.; Jakoby, M.; Abzieher, T.; Hauschild, D.; Moghadamzadeh, S.; Schwenzer, J.A.; Brenner, P.; Schmager, R.; Haghighirad, A.A.; et al. Record Open-Circuit Voltage Wide-Bandgap Perovskite Solar Cells Utilizing 2D/3D Perovskite Heterostructure. Adv. Energy Mater. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Sidhik, S.; Wang, Y.; de Siena, M.; Asadpour, R.; Torma, A.; Terlier, T.; Ho, K.; Li, W.; Puthirath, A.B.; Shuai, X.; et al. Deterministic Fabrication of 3D/2D Perovskite Bilayer Stacks for Durable and Efficient Solar Cells. Science 2022, 1430, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, F.; Albaqami, M.D.; Poli, I.; Petrozza, A. Thermal- and Light-Induced Evolution of the 2D/3D Interface in Lead-Halide Perovskite Films. ACS Appl. Mater. Interfaces 2022, 14, 34180–34188. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, A.A.; Szostak, R.; Drigo, N.; Queloz, V.I.E.; Marchezi, P.E.; Germino, J.C.; Tolentino, H.C.N.; Nazeeruddin, M.K.; Nogueira, A.F.; Grancini, G. In Situ Analysis Reveals the Role of 2D Perovskite in Preventing Thermal-Induced Degradation in 2D/3D Perovskite Interfaces. Nano Lett. 2020, 20, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Q.; Yang, Z.; Rudd, P.N.; Shao, Y.; Dai, X.; Wei, H.; Zhao, J.; Fang, Y.; Wang, Q.; Liu, Y.; et al. Bilateral Alkylamine for Suppressing Charge Recombination and Improving Stability in Blade-Coated Perovskite Solar Cells. Sci. Adv. 2019, 5, eaav8925. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Shen, D.; Ng, T.-W.; Lo, M.-F.; Lee, C.-S. 2D Perovskites with Short Interlayer Distance for High-Performance Solar Cell Application. Adv. Mater. 2018, 30, 1800710. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Ahmad, S.; Tu, D.; Liu, X.; Jia, G.; Guo, X.; Li, C. Dion-Jacobson 2D-3D Perovskite Solar Cells with Improved Efficiency and Stability. Nano Energy 2020, 75, 104892. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, G.; Su, Y.; Sheng, W.; Gong, L.; Zhang, J.; Tan, L.; Chen, Y. Diammonium Molecular Configuration-Induced Regulation of Crystal Orientation and Carrier Dynamics for Highly Efficient and Stable 2D/3D Perovskite Solar Cells. Angew. Chemie-Int. Ed. 2022, 61. [Google Scholar] [CrossRef]
- Zheng, Y.; Niu, T.; Qiu, J.; Chao, L.; Li, B.; Yang, Y.; Li, Q.; Lin, C.; Gao, X.; Zhang, C.; et al. Oriented and Uniform Distribution of Dion–Jacobson Phase Perovskites Controlled by Quantum Well Barrier Thickness. Sol. RRL 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Guan, X.; Xiao, W.; Chen, R.; Zhou, J.; Ren, F.; Wang, J.; Chen, W.; Li, S.; Qiu, L.; et al. Effective Passivation with Size-Matched Alkyldiammonium Iodide for High-Performance Inverted Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 1–12. [Google Scholar] [CrossRef]
- Ahmad, S.; Lu, R.; Liu, Y.; Liu, X.; Yang, Q.; Guo, X.; Li, C. Cesium-Doped Dion-Jacobson 2D Perovskites for Highly Stable Photovoltaics with an 18.3% Efficiency. Nano Energy 2022, 103, 107822. [Google Scholar] [CrossRef]
- Zhang, F.; Park, S.Y.; Yao, C.; Lu, H.; Dunfield, S.P.; Xiao, C.; Uličná, S.; Zhao, X.; Hill, L.D.; Chen, X.; et al. Metastable Dion-Jacobson 2D Structure Enables Efficient and Stable Perovskite Solar Cells. Science 2022, 375, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Fateev, S.A.; Petrov, A.A.; Ordinartsev, A.A.; Grishko, A.Y.; Goodilin, E.A.; Tarasov, A.B. Universal Strategy of 3D and 2D Hybrid Perovskites Single Crystal Growth via In Situ Solvent Conversion. Chem. Mater. 2020, 32, 9805–9812. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.P.; Wolff, C.M.; Stolterfoht, M.; Phung, N.; Albrecht, S.; Neher, D.; Abate, A. How to Make over 20% Efficient Perovskite Solar Cells in Regular (n-i-p) and Inverted (p-i-n) Architectures. Chem. Mater. 2018, 30, 4193–4201. [Google Scholar] [CrossRef]
- Belich, N.A.; Petrov, A.A.; Ivlev, P.A.; Udalova, N.N.; Pustovalova, A.A.; Goodilin, E.A.; Tarasov, A.B. How to Stabilize Standard Perovskite Solar Cells to Withstand Operating Conditions under an Ambient Environment for More than 1000 Hours Using Simple and Universal Encapsulation. J. Energy Chem. 2022. Just accepted. [Google Scholar]
- Udalova, N.N.; Fateev, S.A.; Nemygina, E.M.; Zanetta, A.; Grancini, G.; Goodilin, E.A.; Tarasov, A.B. Nonmonotonic Photostability of BA2MAn-1PbnI3 N+1 Homologous Layered Perovskites. ACS Appl. Mater. Interfaces 2022, 14, 961–970. [Google Scholar] [CrossRef]
- Motti, S.G.; Meggiolaro, D.; Barker, A.J.; Mosconi, E.; Perini, C.A.R.; Ball, J.M.; Gandini, M.; Kim, M.; De Angelis, F.; Petrozza, A. Controlling Competing Photochemical Reactions Stabilizes Perovskite Solar Cells. Nat. Photonics 2019, 13, 532–539. [Google Scholar] [CrossRef]
- Wang, H.; Chan, C.C.S.; Chu, M.; Xie, J.; Zhao, S.; Guo, X.; Miao, Q.; Wong, K.S.; Yan, K.; Xu, J. Interlayer Cross-Linked 2D Perovskite Solar Cell with Uniform Phase Distribution and Increased Exciton Coupling. Sol. RRL 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Hoffman, J.; Ke, W.; Chen, M.; Tsai, H.; Nie, W.; Mohite, A.D.; Kepenekian, M.; Katan, C.; Even, J.; et al. Two-Dimensional Halide Perovskites Incorporating Straight Chain Symmetric Diammonium Ions, (NH3CmH2mNH3)(CH3NH3)N−1PbnI3n+1 (m = 4–9; n = 1–4). J. Am. Chem. Soc. 2018, 140, 12226–12238. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, S.; Xu, K.; Qiao, H.W.; Xie, J.; Lin, Z.; Ge, B.; He, J.; Chen, M.; Zhang, J.; et al. Diammonium-Cesium Lead Halide Perovskite with Phase-Segregated Interpenetrating Morphology for Photovoltaics. J. Phys. Chem. Lett. 2020, 11, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhou, Y.; Yin, X.; Li, X.; Han, J.; Tai, M.; Zhou, Y.; Li, J.; Hao, F.; Lin, H. Role of Alkyl Chain Length in Diaminoalkane Linked 2D Ruddlesden-Popper Halide Perovskites. CrystEngComm 2018, 20, 6704–6712. [Google Scholar] [CrossRef]
- Marchenko, E.I.; Fateev, S.A.; Petrov, A.A.; Korolev, V.V.; Mitrofanov, A.; Petrov, A.V.; Goodilin, E.A.; Tarasov, A.B. Database of Two-Dimensional Hybrid Perovskite Materials: Open-Access Collection of Crystal Structures, Band Gaps, and Atomic Partial Charges Predicted by Machine Learning. Chem. Mater. 2020, 32, 7383–7388. [Google Scholar] [CrossRef]
- Lemmerer, A.; Billing, D.G. Lead Halide Inorganic-Organic Hybrids Incorporating Diammonium Cations. CrystEngComm 2012, 14, 1954–1966. [Google Scholar] [CrossRef]
- Belich, N.A.; Petrov, A.A.; Rudnev, P.O.; Stepanov, N.M.; Turkevych, I.; Goodilin, E.A.; Tarasov, A.B. From Metallic Lead Films to Perovskite Solar Cells through Lead Conversion with Polyhalide Solutions. ACS Appl. Mater. Interfaces 2020, 12, 20456–20461. [Google Scholar] [CrossRef]
- Petrov, A.A.; Belich, N.A.; Grishko, A.Y.; Stepanov, N.M.; Dorofeev, S.G.; Maksimov, E.G.; Shevelkov, A.V.; Zakeeruddin, S.M.; Graetzel, M.; Tarasov, A.B.; et al. A New Formation Strategy of Hybrid Perovskites via Room Temperature Reactive Polyiodide Melts. Mater. Horiz. 2017, 4, 625–632. [Google Scholar] [CrossRef]
- Zhao, T.; Chueh, C.C.; Chen, Q.; Rajagopal, A.; Jen, A.K.Y. Defect Passivation of Organic-Inorganic Hybrid Perovskites by Diammonium Iodide toward High-Performance Photovoltaic Devices. ACS Energy Lett. 2016, 1, 757–763. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Li, B.; Han, X.; Jin, Z.; Wang, Y.; Zhang, Q.; Rui, Y. Interfacial Modification via a 1,4-Butanediamine-Based 2D Capping Layer for Perovskite Solar Cells with Enhanced Stability and Efficiency. ACS Appl. Mater. Interfaces 2022, 14, 22879–22888. [Google Scholar] [CrossRef]
- Li, W.; Gu, X.; Shan, C.; Lai, X.; Sun, X.W.; Kyaw, A.K.K. Efficient and Stable Mesoscopic Perovskite Solar Cell in High Humidity by Localized Dion-Jacobson 2D-3D Heterostructures. Nano Energy 2022, 91, 106666. [Google Scholar] [CrossRef]
- Leijtens, T.; Bush, K.; Cheacharoen, R.; Beal, R.; Bowring, A.; McGehee, M.D. Towards Enabling Stable Lead Halide Perovskite Solar Cells; Interplay between Structural, Environmental, and Thermal Stability. J. Mater. Chem. A 2017, 5, 11483–11500. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and Challenges of Perovskite Solar Cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.W.; Liu, L.; Zhang, D.; De Marco, N.; Lee, J.W.; Lin, O.; Chen, Q.; Yang, Y. The Emergence of the Mixed Perovskites and Their Applications as Solar Cells. Adv. Energy Mater. 2017, 7, 1–24. [Google Scholar] [CrossRef]
- Ono, L.K.; Juarez-Perez, E.J.; Qi, Y. Progress on Perovskite Materials and Solar Cells with Mixed Cations and Halide Anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zheng, X.; Yao, F.; Ma, J.; Tao, C.; Fang, G. Methylammonium, Formamidinium and Ethylenediamine Mixed Triple-Cation Perovskite Solar Cells with High Efficiency and Remarkable Stability. J. Mater. Chem. A 2018, 6, 17625–17632. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, L.; Li, W.; Li, F.; Pai, N.K.; Scully, A.D.; Tsai, C.M.; Bach, U.; Simonov, A.N.; Cheng, Y.B.; et al. Diammonium and Monoammonium Mixed-Organic-Cation Perovskites for High Performance Solar Cells with Improved Stability. Adv. Energy Mater. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Wolff, C.M.; Márquez, J.A.; Zhang, S.; Hages, C.J.; Rothhardt, D.; Albrecht, S.; Burn, P.L.; Meredith, P.; Unold, T.; et al. Visualization and Suppression of Interfacial Recombination for High-Efficiency Large-Area Pin Perovskite Solar Cells. Nat. Energy 2018, 3, 847–854. [Google Scholar] [CrossRef]
- Uller Rothmann, M.; Kim, J.S.; Borchert, J.; Lohmann, K.B.; O’Leary, C.M.; Sheader, A.A.; Clark, L.; Snaith, H.J.; Johnston, M.B.; Nellist, P.D.; et al. Atomic-Scale Microstructure of Metal Halide Perovskite. Science 2020, 370, eabb5940. [Google Scholar] [CrossRef]
- Chen, J.; Seo, J.Y.; Park, N.G. Simultaneous Improvement of Photovoltaic Performance and Stability by In Situ Formation of 2D Perovskite at (FAPbI3)0.88(CsPbBr3)0.12/CuSCN Interface. Adv. Energy Mater. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.Y.; Noh, J.H.; Seo, J. Efficient, Stable and Scalable Perovskite Solar Cells Using Poly(3-Hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, Z.; Zhang, X.; Lu, J.; Niu, X.; He, Z.; Zhu, C.; Xiao, M.; Song, Q.; Wei, X.; et al. Initializing Film Homogeneity to Retard Phase Segregation for Stable Perovskite Solar Cells. Science 2022, 754, 747–754. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, J. Ion Migration in Organometal Trihalide Perovskite and Its Impact on Photovoltaic Efficiency and Stability. Acc. Chem. Res. 2016, 49, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, J.; Luo, W.; Wehbe, N.; Seitkhan, A.; Babics, M.; Kang, J.; De Bastiani, M.; Aydin, E.; Allen, T.G.; et al. Potential-Induced Degradation in Perovskite/Silicon Tandem Photovoltaic Modules. Cell Reports Phys. Sci. 2022, 3, 101026. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udalova, N.N.; Moskalenko, A.K.; Belich, N.A.; Ivlev, P.A.; Tutantsev, A.S.; Goodilin, E.A.; Tarasov, A.B. Butanediammonium Salt Additives for Increasing Functional and Operando Stability of Light-Harvesting Materials in Perovskite Solar Cells. Nanomaterials 2022, 12, 4357. https://doi.org/10.3390/nano12244357
Udalova NN, Moskalenko AK, Belich NA, Ivlev PA, Tutantsev AS, Goodilin EA, Tarasov AB. Butanediammonium Salt Additives for Increasing Functional and Operando Stability of Light-Harvesting Materials in Perovskite Solar Cells. Nanomaterials. 2022; 12(24):4357. https://doi.org/10.3390/nano12244357
Chicago/Turabian StyleUdalova, Natalia N., Aleksandra K. Moskalenko, Nikolai A. Belich, Pavel A. Ivlev, Andrey S. Tutantsev, Eugene A. Goodilin, and Alexey B. Tarasov. 2022. "Butanediammonium Salt Additives for Increasing Functional and Operando Stability of Light-Harvesting Materials in Perovskite Solar Cells" Nanomaterials 12, no. 24: 4357. https://doi.org/10.3390/nano12244357
APA StyleUdalova, N. N., Moskalenko, A. K., Belich, N. A., Ivlev, P. A., Tutantsev, A. S., Goodilin, E. A., & Tarasov, A. B. (2022). Butanediammonium Salt Additives for Increasing Functional and Operando Stability of Light-Harvesting Materials in Perovskite Solar Cells. Nanomaterials, 12(24), 4357. https://doi.org/10.3390/nano12244357