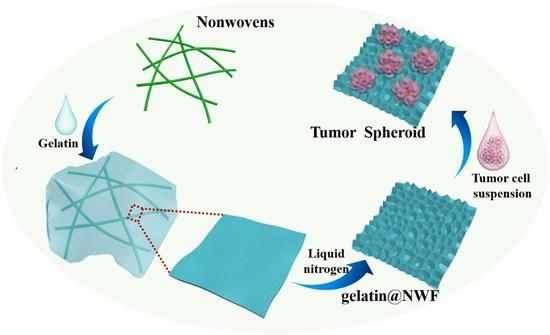
Lyophilized Gelatin@non-Woven Scaffold to Promote Spheroids Formation and Enrich Cancer Stem Cell Incidence
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials and Reagents
2.2. Gelatin@NWF Hybrid Scaffold Fabricated by Lyophilizing
2.3. Characterization of the Lyophilized Gelatin@NWF Scaffold
2.3.1. Morphology
2.3.2. Porosity
2.3.3. Swelling
2.3.4. Mechanical Strength
EAB % = (L1 − L0)/L0 × 100
2.4. Cell Compatibility of Gelatin@NWF Scaffold
2.4.1. MTT Cell Growth Assay
2.4.2. Crystal Violet Staining
2.5. Biomarker Levels of Cells Grown on Gelatin@NWF Scaffold
2.5.1. Quantitative Real-Time PCR
2.5.2. Western Blot
2.6. Migrating Capability of Cells Grown on Gelatin@NWF Scaffold
2.7. The Effect of Chemotherapy Drug DOX on Cells Grown on Gelatin@NWF Scaffold
2.8. Statistical Analysis
3. Result and Discussion
3.1. Tailored Honeycomb-like Micro-Pores Formed by Lyophilizing Gelatin@NWF in Liquid Nitrogen
3.2. Tailored Honeycomb-like Micro-Pores Modulate Cell Aggregating on Gelatin@NWF Scaffolds
3.3. Elevated Cancer Stem Cell-Related Biomarker in Tumor Spheres Grown on Gelatin@NWF Scaffold
3.4. Increased DOX Resistance and Cell Motility of Cells Grown on Gelatin@NWF Scaffold
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sams-Dodd, F. Target-based drug discovery: Is something wrong? Drug Discov. Today 2005, 10, 139–147. [Google Scholar] [CrossRef]
- Edwards, A.M.; Arrowsmith, C.H.; Bountra, C.; Bunnage, M.E.; Feldmann, M.; Knight, J.C.; Young, L.T. Preclinical target validation using patient-derived cells. Nat. Rev. Drug Discov. 2015, 14, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A.; Yingjun, S. Mouse xenograft models vs. GEM models for human cancer therapeutics. Dis. Model. Mech. 2008, 1, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Rodenhizer, D.; Dean, T.; D’Arcangelo, E.; McGuigan, A.P. The current landscape of 3D in vitro tumor models: What cancer hallmarks are accessible for drug discovery? Adv. Healthc. Mater. 2018, 8, 1701174. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, L.; Kassis, J.; Virador, V. 3D in vitro tissue models and their potential for drug screening. Expert Opin. Drug Dis. 2013, 8, 1455–1466. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Chang, T.T.; Hughes-Fulford, M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng. Part A 2009, 15, 559–567. [Google Scholar] [CrossRef]
- Abbott, A. Cell culture: Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, R.B.; Qorri, B.; Sambi, M.; Baluch, N.; Kumar, S.; Das, B.; Cheng, H.L.M. 3D Multicellular Stem-Like Human Breast Tumor Spheroids Enhance Tumorigenicity of Orthotopic Xenografts in Athymic Nude Rat Model. Cancers 2021, 13, 2784. [Google Scholar] [CrossRef] [PubMed]
- Sumkhemthong, S.; Chamni, S.; Ecoy, G.U.; Taweecheep, P.; Suwanborirux, K.; Prompetchara, E.; Chaotham, C. Jorunnamycin A Suppresses Stem-Like Phenotypes and Sensitizes Cisplatin-Induced Apoptosis in Cancer Stem-Like Cell-Enriched Spheroids of Human Lung Cancer Cells. Mar. Drugs 2021, 19, 261. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Gotou, S.; Ito, K.; Kohashi, S.; Goto, Y.; Yoshiura, Y. Micropatterned culture of hepg2 spheroids using microwell chip with honeycomb-patterned polymer film. J. Biosci. Bioeng. 2014, 118, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Yu, S.J.; Choi, Y.; Lee, H.R.; Lee, E.; Lee, E. Polymer thin film–induced tumor spheroids acquire cancer stem cell–like properties. Cancer Res. 2018, 78, 6890–6902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beier, D.; Hau, P.; Proescholdt, M.; Lohmeier, A.; Wischhusen, J.; Oefner, P.J.; Aigner, L.; Brawanski, A.; Bogdahn, U.; Beier, C.P. CD133 (+) and CD133 (−) glioblastomaderived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007, 67, 4010–4015. [Google Scholar] [CrossRef] [Green Version]
- Rajasekhar, V.K.; Studer, L.; Gerald, W.; Socci, N.D.; Scher, H.I. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-κB signalling. Nat. Commun. 2011, 2, 162. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2012, 116, 206–226. [Google Scholar] [CrossRef] [Green Version]
- Benien, P.; Swami, A. 3D tumor models: History, advances and future perspectives. Future Oncol. 2014, 1, 1311–1327. [Google Scholar] [CrossRef]
- Chatzinikolaidou, M. Cell spheroids: The new frontiers in in vitro models for cancer drug validation. Drug Discov. Today 2016, 21, 1553–1560. [Google Scholar] [CrossRef]
- Carvalho, M.P.; Costa, E.C.; Miguel, S.P.; Correia, I.J. Tumor spheroid assembly on hyaluronic acid-based structures: A review. Carbohy. Poly. 2016, 150, 139–148. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloid. Surface B 2017, 159, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Abbasian, M.; Mousavi, E.; Arab-Bafrani, Z. Multi-cellular tumor spheroids formation of colorectal cancer cells on Gelatin/PLCL and Collagen/PLCL nanofibrous scaffolds. Eur. Polym. J. 2019, 115, 115–124. [Google Scholar] [CrossRef]
- Xu, K.; Ganapathy, K.; Andl, T.; Wang, Z.; Florczyk, S.J. 3d porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials 2019, 217, 119311. [Google Scholar] [CrossRef]
- Lan, H.; Li, P.; Wang, H.; Wang, M.; Jiang, C.; Hou, Y.; Niu, Q.J. Construction of a gelatin scaffold with water channels for preparing a high performance nanofiltration membrane. Sep. Purif. Technol. 2021, 264, 118391. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 1, 96–115. [Google Scholar] [CrossRef]
- Verma, P.; Verma, V.; Ray, P.; Ray, A.R. Agar–gelatin hybrid sponge-induced three-dimensional in vitro ‘liver-like’ HepG2 spheroids for the evaluation of drug cytotoxicity. J. Tissue Eng. Regen. Med. 2009, 5, 368–376. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, J.J.; Wang, L.X.; Lu, Z.; Wang, F.; Xia, Q.; Yu, L. Freeze-drying prepared ready-to-use gelatin@polypropylene nonwoven hybrid sheet for stacking 3D cell culture. Cellulose 2019, 26, 6755–6768. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, J.; Youn, I.J.; Lee, W.H. Modified liquid displacement method for determination of pore size distribution in porous membranes. J. Membr. Sci. 1997, 130, 149–156. [Google Scholar] [CrossRef]
- Mandal, B.B.; Priya, A.S.; Kundu, S.C. Novel silk sericin/gelatin 3D scaffolds and 2D films: Fabrication and characterization for potential tissue engineering applications. Acta Biomater. 2009, 5, 3007–3020. [Google Scholar] [CrossRef]
- Fu, J.J.; Lv, X.H.; Wang, L.X.; He, X.; Li, Y.; Yu, L.; Li, C.M. Cutting and Bonding Parafilm® to Fast Prototyping Flexible Hanging Drop Chips for 3D Spheroid Cultures. Cell. Mol. Bioeng. 2021, 14, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, X.B.; Wang, L.X.; Lv, X.H.; Lu, Z.; Wang, F.; Li, C.M. One-Step Dip-Coating-Fabricated Core–Shell Silk Fibroin Rice Paper Fibrous Scaffolds for 3D Tumor Spheroid Formation. ACS Appl. Bio. Mater. 2020, 3, 7462–7471. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Zhou, Y.; Shi, X.X.; Kang, Y.J.; Lu, Z.S.; Li, Y.; Yu, L. Spontaneous formation of tumor spheroid on a hydrophilic filter paper for cancer stem cell enrichment. Colloid Surface B 2019, 174, 426–434. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Wittwer, C.T. The MIQE Guidelines: Minimum information for publication of q uantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linn, D.E.; Yang, X.; Sun, F.; Xie, Y.; Chen, H.; Jiang, R.; Qiu, Y. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer 2010, 1, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, A.Y.; Torisawa, Y.S.; Tung, Y.C.; Sud, S.; Taichman, R.S.; Pienta, K.J.; Takayama, S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 2009, 16, 3020–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hainline, K.M.; Gu, F.; Handley, J.F.; Tian, Y.F.; Wu, Y.; de Wet, L.; Collier, J.H. Self-assembling peptide gels for 3D prostate cancer spheroid culture. Macromol. Biosci. 2019, 1, 1800249. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Dorantes, M.; Cruz-Hernandez, C.D.; Cortés-Ramírez, S.A.; Cruz-Burgos, J.M.; Reyes-Grajeda, J.P.; Peralta-Zaragoza, O.; Losada-Garcia, A. Prostate cancer spheroids: A three-dimensional model for studying tumor heterogeneity. Cancer Cell Signal 2019, 2174, 13–17. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 3, 274–281. [Google Scholar] [CrossRef]
- Malayer, S.K.; Ghourchian, H.; Azarian, M. Genotoxicity of noscapine nanosuspension on DU145 human prostate cancer (spheroid cell model). Adv. Cell Sci. Mut. 2019, 1, 1–7. [Google Scholar]
- Jia, X.; Li, X.; Xu, Y.; Shu, Z.; Mou, W.; Liu, Y. Sox2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J. Mol. Cell Biol. 2011, 4, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.K.; Dallaglio, Y.; Chen, W.A.; Robinson, S.E.; Robinson, M.D.; McCarter, J.; Wang, R.; Gonzalez, D.C.; Thompson, D.A.; Norris, D.R.; et al. Fujita, ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells 2012, 30, 2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Chen, F.; Chai, H.; Gao, L.; Lv, X.; Yu, L. Lyophilized Gelatin@non-Woven Scaffold to Promote Spheroids Formation and Enrich Cancer Stem Cell Incidence. Nanomaterials 2022, 12, 808. https://doi.org/10.3390/nano12050808
Fu J, Chen F, Chai H, Gao L, Lv X, Yu L. Lyophilized Gelatin@non-Woven Scaffold to Promote Spheroids Formation and Enrich Cancer Stem Cell Incidence. Nanomaterials. 2022; 12(5):808. https://doi.org/10.3390/nano12050808
Chicago/Turabian StyleFu, Jingjing, Feng Chen, Huihui Chai, Lixia Gao, Xiaohui Lv, and Ling Yu. 2022. "Lyophilized Gelatin@non-Woven Scaffold to Promote Spheroids Formation and Enrich Cancer Stem Cell Incidence" Nanomaterials 12, no. 5: 808. https://doi.org/10.3390/nano12050808
APA StyleFu, J., Chen, F., Chai, H., Gao, L., Lv, X., & Yu, L. (2022). Lyophilized Gelatin@non-Woven Scaffold to Promote Spheroids Formation and Enrich Cancer Stem Cell Incidence. Nanomaterials, 12(5), 808. https://doi.org/10.3390/nano12050808