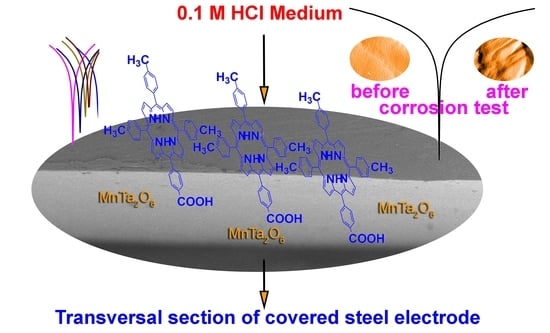
Efficient Decrease in Corrosion of Steel in 0.1 M HCl Medium Realized by a Coating with Thin Layers of MnTa2O6 and Porphyrins Using Suitable Laser-Type Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Pseudo-Binary Oxides and Porphyrins
2.1.1. Obtaining and Characterizing MnTa2O6
2.1.2. Obtaining Porphyrins
2.2. Data on the Selected Steel Substrate
2.3. Apparatus
2.4. Design of Steel Coverings
3. Results and Discussion
3.1. Electrochemical Test
3.2. Microscopic Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olorundaisi, E.; Jamiru, T.; Adegbola, A.T. Mitigating the effect of corrosion and wear in the application of high strength low alloy steels (HSLA) in the petrochemical transportation industry—A review. Mater. Res. Express. 2020, 6, 1265k9. [Google Scholar] [CrossRef]
- Farahbod, F. Investigations to find appropriate range of pH and a new replacement for hydrazine to protect corrosion in steam-tanks of petrochemical industries. Eng. Fail. Anal. 2012, 22, 38–49. [Google Scholar] [CrossRef]
- Conde, A.; Arenas, M.A.; de Damborenea, J.J. Electrodeposition of Zn-Ni coatings as Cd replacement for corrosion protection of high strength steel. Corros. Sci. 2011, 53, 1489–1497. [Google Scholar] [CrossRef]
- Rashmi, S.; Elias, L.; Hegde, A.C. Multilayered Zn-Ni alloy coatings for better corrosion protection of mild steel. Int. J. Eng. Sci. Technol. 2017, 20, 1227–1232. [Google Scholar] [CrossRef] [Green Version]
- Sorkhabi, H.A.; Seifzadeh, D.; Hosseini, M.G. EIS and polarization studies to evaluate the inhibition effect of 3H-phenothiazin-3-one, 7-dimethylamin on carbon steel corrosion in 1 M HCl solution. Corros. Sci. 2008, 50, 3363–3370. [Google Scholar] [CrossRef]
- Ma, C.; Ma, M.G.; Si, C.; Ji, X.X.; Wan, P. Flexible MXene-Based Composites for Wearable Devices. Adv. Funct. Mater. 2021, 31, 2009524. [Google Scholar] [CrossRef]
- Ali, A.; Falih, S.; Yousif, N.; Rezgar, R.; Kamal, I. Modeling and Optimization of Structural Steel Corrosion Inhibition using barely Grass Extract as Green Inhibitor. Am. J. Environ. Sci. 2017, 7, 73–81. [Google Scholar] [CrossRef]
- Tan, Y.; Mocerino, M.; Paterson, T. Organic molecules showing the characteristics of localised corrosion aggravation and inhibition. Corros. Sci. 2011, 53, 2041–2045. [Google Scholar] [CrossRef]
- Lyu, J.; Kashkarov, E.B.; Travitzky, N.; Syrtanov, M.S.; Lider, A.M. Sintering of MAX-phase materials by spark plasma and other methods. J. Mater. Sci. 2021, 56, 1980–2015. [Google Scholar] [CrossRef]
- Epuran, C.; Fratilescu, I.; Anghel, D.; Birdeanu, M.; Orha, C.; Fagadar-Cosma, E. A Comparison of Uric Acid Optical Detection Using as Sensitive Materials an Amino-Substituted Porphyrin and Its Nanomaterials with CuNPs, PtNPs and Pt@CuNPs. Processes 2021, 9, 2072. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mele, G. Stainless steel bipolar plate coated with polyaniline/Zn-porphyrin composites coatings for pro-ton exchange membrane fuel cell. Sci. Rep. 2020, 10, 3277. [Google Scholar] [CrossRef]
- Costa e Silva, R.; Oliveira da Silva, L.; de Andrade Bartolomeu, A.; Brocksom, T.J.; de Oliveira, T.K. Recent applications of porphyrins as photocatalysts in organic synthesis: Batch and continuous flow Approaches. Beilstein J. Org. Chem. 2020, 6, 917–955. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Quraishi, M.A.; Olasunkanmi, L.O.; Fayemi, O.E.; Sasikumar, Y.; Ramaganthan, B.; Bahadur, I.; Obot, I.B.; Adekunle, A.S.; et al. Porphyrins as Corrosion Inhibitors for N80 Steel in 3.5% NaCl Solution: Electrochemical, Quantum Chemical, QSAR and Monte Carlo Simulations Studies. Molecules 2015, 20, 15122–15146. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Chen, S.; Guo, W.; Liu, G.; Ma, H.; Wu, L. Electrochemical and molecular simulation studies on the corrosion inhibition of 5,10,15,20-tetraphenylporphyrin adlayers on iron surface. Appl. Surf. Sci. 2007, 253, 8734–8742. [Google Scholar] [CrossRef]
- Popa, I.; Fagadar-Cosma, E.; Taranu, B.O.; Birdeanu, M.; Fagadar-Cosma, G.; Taranu, I. Corrosion Protection Efficiency of Bilayer Porphyrin-Polyaniline Film Deposited on Carbon Steel. Macromol. Symp. 2015, 352, 16–24. [Google Scholar] [CrossRef]
- Singh, A.; Talha, M.; Xu, X.; Sun, Z.; Lin, Y. Heterocyclic Corrosion Inhibitors for J55 Steel in a Sweet Corrosive Medium. ACS Omega 2017, 2, 8177–8186. [Google Scholar] [CrossRef] [Green Version]
- Deyab, M.A.; Mele, G.; Al-Sabagh, A.M.; Bloise, E.; Lomonaco, D.; Mazzetto, S.E.; Clemente, C.D.S. Synthesis and characteristics of alkyd resin/M-Porphyrins nanocomposite for corrosion protection application. Prog. Org. Coat. 2017, 105, 286–290. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Singh, A.; Liu, W. Investigation of some Porphyrin Derivatives as Inhibitors for Corrosion of N80 Steel at High Temperature and High Pressure in 3.5% NaCl solution containing carbon dioxide. Int. J. Electrochem. Sci. 2018, 13, 11961–11973. [Google Scholar] [CrossRef]
- Hieringer, W.; Flechtner, K.; Kretschmann, A.; Seufert, K.; AuwŠrter, W.; Barth, J.V.; Gšrling, A.; SteinrŸck, H.P.; Gottfried, J.M. The surface trans effect: Influence of axial ligands on the surface chemical bonds of adsorbed metalloporphyrins. J. Am. Chem. Soc. 2011, 133, 6206–6222. [Google Scholar] [CrossRef]
- Lokesh, K.S.; De Keersmaecker, M.; Adriaens, A. Self Assembled Films of Porphyrins with Amine Groups at Different Positions: Influence of Their Orientation on the Corrosion Inhibition and the Electrocatalytic Activity. Molecules 2012, 17, 7824–7842. [Google Scholar] [CrossRef] [Green Version]
- Signh, R.P.; Sharma, K.; Mausam, K. Dispersion and stability of metal oxide nanoparticles in aqueous suspension: A review. Mater. Today Proc. 2020, 26, 2021–2025. [Google Scholar] [CrossRef]
- Birdeanu, M.; Vaida, M.; Fagadar-Cosma, E. Hydrothermal synthesis of ZnTa2O6, ZnNb2O6, MgTa2O6 and MgNb2O6 pseudo-binary oxide nanomaterials with anticorrosive properties. Manuf. Rev. 2020, 7, 39. [Google Scholar] [CrossRef]
- Madern, N.; Charbonnier, V.; Monnier, J.; Zhang, J.; Paul-Boncour, V.; Latroche, M. Investigation of H Sorption and Corrosion Properties of Sm2MnxNi7−x (0 ≤ x < 0.5) Intermetallic Compounds Forming Reversible Hydrides. Energies 2020, 13, 3470. [Google Scholar] [CrossRef]
- Bosch, J.; Martin, U.; Aperador, W.; Bastidas, J.M.; Ress, J.; Bastidas, D.M. Corrosion Behavior of High-Mn Austenitic Fe–Mn–Al–Cr–C Steels in NaCl and NaOH Solutions. Materials 2021, 14, 425. [Google Scholar] [CrossRef]
- Bîrdeanu, A.V.; Birdeanu, M.; Fagadar-Cosma, E. Corrosion protection characteristics of ceramics, porphyrins and hybrid/ceramics porphyrins, deposited as single and sandwich layers, by pulsed laser deposition (PLD). J. Alloys Compd. 2017, 706, 220–226. [Google Scholar] [CrossRef]
- Birdeanu, M.; Epuran, C.; Fratilescu, I.; Fagadar-Cosma, E. Structured Thin Films Based on Synergistic Effects of MnTa2O6 Oxide and bis-Carboxy-phenyl-substituted Porphyrins, Capable to Inhibit Steel Corrosion. Processes 2021, 9, 1890. [Google Scholar] [CrossRef]
- Birdeanu, M.; Vaida, M.; Bîrdeanu, A.V.; Fagadar- Cosma, E. PLD deposited layers of pseudo-binary zinc oxides and zinc-porphyrin for steel corrosion inhibition. Corrosion 2020, 76, 734–741. [Google Scholar] [CrossRef]
- Ryl, J.; Brodowski, M.; Kowalski, M.; Lipinska, W.; Niedzialkowski, P.; Wysocka, J. Corrosion Inhibition Mechanism and Efficiency Differentiation of Dihydroxybenzene Isomers Towards Aluminum Alloy 5754 in Alkaline Media. Materials 2019, 12, 3067. [Google Scholar] [CrossRef] [Green Version]
- Heim, D.; Seufert, K.; AuwŠrter, W.; Aurisicchio, C.; Fabbro, C.; Bonifazi, D.; Barth, J.V. Surface-assisted assembly of discrete porphyrin-based cyclic supramolecules. Nano. Lett. 2010, 10, 122–128. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E.; Hussain, C.M. Recent advancements in corrosion inhibitor systems through carbon allotropes: Past, present, and future. Nano. Select. 2021, 2, 2237. [Google Scholar] [CrossRef]
- Bakar, M.B.; Oelgemöller, M.; Senge, M.O. Lead structures for applications in photodynamic therapy. Part 2: Synthetic studies for photo-triggered release systems of bioconjugate porphyrin photosensitizers. Tetrahedron 2009, 65, 7064–7078. [Google Scholar] [CrossRef]
- Nowak-Krol, A.; Plamont, R.; Canard, G.; Edzang, J.A.; Gryko, D.T.; Balaban, T.S. An Efficient Synthesis of Porphyrins with Different meso Substituents that Avoids Scrambling in Aqueous Media. Chem. Eur. J. 2015, 21, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, Z.; Corner, G. A study of the effect of clinical washing decontamination process on corrosion resistance of Martensitic Stainless Steel 420. Bio-Med. Mater. Eng. 2016, 27, 341–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.Y.; Yao, K.F.; Chen, Y.B.; Wang, M.H.; Shao, Y.; Ge, X.Y. Effects of austenitizing temperature on the microstructure and electrochemical behavior of a martensitic stainless steel. J. Appl. Electrochem. 2015, 45, 375–383. [Google Scholar] [CrossRef]
- Bösing, I.; Cramer, L.; Steinbacher, M.; Zoch, H.W.; Thöming, J.; Baune, M. Influence of heat treatment on the microstructure and corrosion resistance of martensitic stainless steel. AIP Adv. 2019, 9, 065317. [Google Scholar] [CrossRef] [Green Version]
- Kapaklis, V.; Poulopoulos, P.; Karoutsos, V.; Manouras, T.; Politis, C. Growth of thin Ag films produced by radio frequency magnetron sputtering. Thin Solid Films 2006, 510, 138–142. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control, 1st ed.; IChemE Series; Butterworth-Heinemann: Oxford, UK, 2006; p. 377. [Google Scholar]
- Majidi, H.J.; Mirzaee, A.; Jafari, S.M.; Amiri, M.; Shahrousvand, M.; Babaei, A. Fabrication and characterization of graphene oxide-chitosan-zinc oxideternary nano-hybrids for the corrosion inhibition of mild steel. Int. J. Biol. Macromol. 2020, 148, 1190–1200. [Google Scholar] [CrossRef]
- Zakaria, K.; Abbas, M.A.; Bedair, M.A. Herbal expired drug bearing glycosides and polysaccharides moieties as green and cost-effective oilfield corrosion inhibitor: Electrochemical and computational studies. J. Mol. Liq. 2022, 352, 118689. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Ansari, K.R.; Sorour, A.A.; Quraishi, M.A.; Lgaz, H.; Salghi, R. Thiosemicarbazide and thiocarbohydrazide functionalized chitosan as ecofriendly corrosion inhibitors for carbon steel in hydrochloric acid solution. Int. J. Biol. Macromol. 2018, 107, 1747–1757. [Google Scholar] [CrossRef]










| Sample | The Order of Covering the Steel Surface | Deposition Mode |
|---|---|---|
| a | MnTa2O6(h) | Monolayer/PLD |
| b | 5-(4-carboxy-phenyl)-10,15,20-tris (4-methyl-phenyl)-porphyrin | Monolayer/MAPLE |
| c | 5-(4-methyl-benzoate)-10,15,20-tris (4-methyl-phenyl)-porphyrin | Monolayer/MAPLE |
| d | 5-(4-carboxy-phenyl)-10,15,20-tris (4-methyl-phenyl)-porphyrin/MnTa2O6(h) | Sandwich/ MAPLE/PLD |
| e | MnTa2O6(h)/5-(4-carboxy-phenyl)-10,15,20-tris (4-methyl-phenyl)-porphyrin | Sandwich /PLD/MAPLE |
| f | 5-(4-methyl-benzoate)-10,15,20-tris (4-methyl-phenyl)-porphyrin/MnTa2O6(h) | Sandwich MAPLE/PLD |
| g | MnTa2O6(h)/5-(4-methyl-benzoate)-10,15,20-tris (4-methyl-phenyl)-porphyrin | Sandwich PLD/MAPLE |
| Sample | E (I = 0) (mV) | Rp (Ωxcm2) | icorr (mA/cm2) | βa (mV) | βc (mV) | vcorr (mm/Y) | IE (%) |
|---|---|---|---|---|---|---|---|
| OL | −414.1 | 88.53 | 1.2924 | 258.0 | −263.5 | 1.511 | - |
| a | −430.0 | 130.72 | 0.4440 | 84.1 | −85.9 | 0.5192 | 65.64 |
| b | −449.9 | 139.39 | 0.3401 | 79.2 | −81.1 | 0.3977 | 73.68 |
| c | −440.0 | 131.76 | 0.4118 | 82.7 | −84.1 | 0.5067 | 68.13 |
| d | −502.8 | 159.67 | 0.2101 | 71.5 | −72.4 | 0.2458 | 83.74 |
| e | −477.1 | 150.44 | 0.2573 | 75.8 | −77.2 | 0.3010 | 80.09 |
| f | −488.4 | 154.53 | 0.2380 | 73.2 | −74.8 | 0.2784 | 81.58 |
| g | −469.8 | 146.42 | 0.2677 | 77.3 | −79.3 | 0.3131 | 79.28 |
| Sample | Area (pm2) | Sa Before/After (nm) | Sq Before/After (nm) | Sy Before/After (nm) | Particle Dimensions Before/After (nm) |
|---|---|---|---|---|---|
| OL | 1.326 | 1.7956/51.0779 | 2.3321/74.8436 | - | - |
| a | 5.0354/47.8121 | 6.0733/70.1462 | 41.003/199.116 | 81.8/48.3 | |
| b | 5.4929/43.5298 | 7.2987/65.9352 | 51.656/161.129 | 65.8/39.9 | |
| c | 5.2775/46.0276 | 6.9578/68.6247 | 41.598/182.468 | 76.1/42.6 | |
| d | 10.978/30.5807 | 16.134/49.5292 | 99.116/103.435 | 35.5/18.2 | |
| e | 7.3613/38.6299 | 9.5030/52.7320 | 81.798/135.338 | 47.8/30.5 | |
| f | 8.8238/35.3781 | 13.024/50.0736 | 95.753/113.598 | 43.6/24.8 | |
| g | 6.6588/37.0684 | 8.6253/59.6374 | 59.809/153.524 | 57.4/35.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birdeanu, M.; Fratilescu, I.; Epuran, C.; Murariu, A.C.; Socol, G.; Fagadar-Cosma, E. Efficient Decrease in Corrosion of Steel in 0.1 M HCl Medium Realized by a Coating with Thin Layers of MnTa2O6 and Porphyrins Using Suitable Laser-Type Approaches. Nanomaterials 2022, 12, 1118. https://doi.org/10.3390/nano12071118
Birdeanu M, Fratilescu I, Epuran C, Murariu AC, Socol G, Fagadar-Cosma E. Efficient Decrease in Corrosion of Steel in 0.1 M HCl Medium Realized by a Coating with Thin Layers of MnTa2O6 and Porphyrins Using Suitable Laser-Type Approaches. Nanomaterials. 2022; 12(7):1118. https://doi.org/10.3390/nano12071118
Chicago/Turabian StyleBirdeanu, Mihaela, Ion Fratilescu, Camelia Epuran, Alin Constantin Murariu, Gabriel Socol, and Eugenia Fagadar-Cosma. 2022. "Efficient Decrease in Corrosion of Steel in 0.1 M HCl Medium Realized by a Coating with Thin Layers of MnTa2O6 and Porphyrins Using Suitable Laser-Type Approaches" Nanomaterials 12, no. 7: 1118. https://doi.org/10.3390/nano12071118
APA StyleBirdeanu, M., Fratilescu, I., Epuran, C., Murariu, A. C., Socol, G., & Fagadar-Cosma, E. (2022). Efficient Decrease in Corrosion of Steel in 0.1 M HCl Medium Realized by a Coating with Thin Layers of MnTa2O6 and Porphyrins Using Suitable Laser-Type Approaches. Nanomaterials, 12(7), 1118. https://doi.org/10.3390/nano12071118