Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells
Abstract
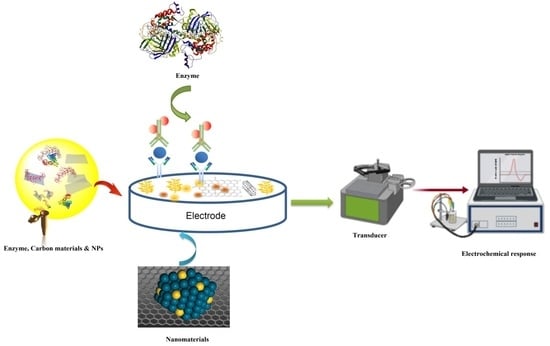
:1. Introduction
2. Classical Methods for H2O2 Detection
2.1. Electrochemical Systems
2.2. Potentiometric
2.3. Amperometric Biosensor
2.4. Calorimetric Biosensors
2.5. Chemiluminescence Material for the Detection of H2O2
2.6. Titrimetry
2.7. Spectroscopy
2.8. Colorimetry
2.9. Chromatography
2.10. Fluorescence
3. Recent Advances
3.1. Current Approaches in the Construction of Biosensors
3.2. Electrochemical Sensing of H2O2 via Metal Nanoparticles
3.3. H2O2 Detection Using Enzymatic Biosensors
3.4. Carbon-Based Material for H2O2 Sensing
3.4.1. Graphene-Based Metal-Free Electrocatalysts
3.4.2. Carbon Composite with Enzymes for H2O2 Detection
3.4.3. Graphene Composite with Metal Nanoparticles for H2O2 Detection
| Graphene Based Material | Sensitivity μA mM−1 cm−2 | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|---|
| Au-PEI/GO | 460.0 | 0.5–1680 | 0.2 | [270] |
| AgNPs-MWCNT-rGO | 0.833 | 100–100,000 | 0.9 | [271] |
| RGO-Au-PTBO | 63.39 | 5.0–1077.1 | 0.2 | [272] |
| Ag-MnOOH-GO | 59.14 | 0.5–17,800 | 0.2 | [273] |
| Au NPs@POM-G | 58.87 | 5.0–18,000 | 1.54 | [274] |
| AgNPs-TWEEN-GO | 0.7459 | 20–23,100 | 8.7 | [41] |
| GO-ATP-Pd | 504.85 | 0.1–10,000 | 0.016 | [275] |
| GN/Au-NPs | - | 0.5–500 | 0.22 | [276] |
| GN-Pt | 0.01 | 2–710 | 0.5 | [277] |
| Ag NWs-graphene | 12.37 | 10.0–34,300 | 1.0 | [278] |
| GR-AuNRs | 389.2 | 30–5000 | 10 | [279] |
| Au@C-Co3O4 | 7553 | - | 0.019 | [237] |
| Au NPs-N-GQDs | 186.22 | 0.25–13,327 | 0.12 | [79] |
| AuNPs-NH2/Cu-MOF/GCE | 1.71 | 5–850 | 1.2 | [265] |
| GO/Au@Pt@Au | - | 0.05–17,500 | 0.02 | [280] |
| NG-hAuPd | 5095.5 | 0.1–20 | 0.02 | [281] |
| PDA-RGO/Ag NP | 0.0111 | 0.5–8000 | 2.07 | [282] |
| AgNPs/GN | - | 100–100,000 | 0.5 | [283] |
| Ag/SG | - | 100–136,500 | 0.14 | [284] |
| Pt/PG | 341.14 | 1–1477 | 0.5 | [285] |
| PDDA-RGO/MnO2/AuNPs | 1132.8 | 5.0–500 | 0.6 | [286] |
| AgNP/rGO | - | 100–80,000 | 7.1 | [287] |
| AgNPs-GO | - | 10–20,000 | 0.5 | [288] |
| RGO-AuNP | 5.3 | 250–22,500 | 6.2 | [289] |
| GNPs/SGS | 27.7 | 20–15,000 | 0.2 | [290] |
| AgNPs-CNT-rGO | - | 10–10,000 | 1.0 | [291] |
| PpyNFs/AgNPs-rGO | 0.7367 | 100–5000 | 1.099 | [292] |
| polystyrene@RGO-Pt | 0.0675 | 0.5–8000 | 0.1 | [41] |
| Graphene/Nafion/Azl/AuNPs | - | 30–5000 | 10 | [268] |
| Pt/GN | 0.0204 | 2.5–6650 | 0.8 | [41] |
| RGO-AuNPs (B) | 9.5 | 25–41,500 | 5.0 | [273] |
| PtAu/G-CNTs | 313.4 | 2.0–8561 | 0.6 | [293] |
| PtAuNPs-CTAB-GR | 0.1654 | 0.005–4.8 | 0.0017 | [294] |
| PtAu/RGO | 4.105 | 0.015–8.73 | 0.008 | [295] |
| Pt/graphene-CNT paper | 1.41 | 0–25.0 | 0.01 | [296] |
| pFeMOF/OMC | 67.54 | 70.5–1830.5 | 0.45 | [238] |
| Pd-PEI/GO | - | 0.5–459 | 0.2 | [297] |
| Pd-NPs/GN | 0.019 | 0.001–2000 | 0.0002 | [298] |
| PdNPGNs | 2.75 | 0.1–1000 | 0.05 | [299] |
| RGO-PMS@AuNPs | 39.2 | 0.5–50,000 | 0.06 | [69] |
| 2Au1Ag-PDA/CFME | 12966 | 0–55 | 0.12 | [300] |
| TiO2NTs/r-GO/AgNPs | 1152 | 15,500–50,000 | 2.2 | [301] |
| PtPb/G | - | 2–2.5 | 0.02 | [267] |
| 3DGA-AuNPs/cytc/GCE | 351.57 | - | [302] | |
| PdPt NCs@SGN/GCE | - | 1–300 | 0.3 | [303] |
| AuNFs/Fe3O4@ZIF-8-MoS2 | - | 5–120 | 0.9 | [304] |
3.4.4. Graphene-Loaded Biomolecules for Selective Detection of H2O2
3.5. Carbon Nanotubes (CNTs)
3.5.1. H2O2 Electrochemical Sensors Based on the Association of CNTs and Hemoproteins
| CNTs H2O2 Biosensors | Sensitivity μA mM−1 cm−2 | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|---|
| ZnO/COOH-MWNTs | - | 1–21 | -- | [323] |
| GCE/MWCNTs-CDs | 0.039 | 3.5–300 | 0.25 | [324] |
| ((APy)6[H2W12O40])/(SWCNT-COOH) | - | - | 0.4 | [325] |
| GCE/CNTs-PAMAM DENs-PtNCs | 987.5 | 3–400 | 0.8 | [326] |
| GCE/C60-MWCNTs CS-IL/MB/CuNP | 0.0243 | 2–4 | 0.055 | [327] |
| 3D PB NPs/G-CNTs | 0.11343 | 1–3161 | 0.095 | [328] |
| CF@N-CNTAs–AuNPs | 142 | 1–4300 | 0.05 | [329] |
| CDs/MWCNTs/GCE | -- | -- | - | [324] |
| OECT/PET/CE-CNTs/PtNPs | - | 0.5–100 | 0.2 | [330] |
| ZNBs/fMWCNTs | - | 0.049–22 | 0.035 | [331] |
| ZnONPs/MWCNTs | - | 1000–200,000 | - | [332] |
| N-CNTs | 30 | - | 0.5 | [333] |
| (GC) (BG-CNPs/GC) | - | - | [334] | |
| GCE/rGONRs/MnO2 | 0.0142 | 0.25–2245 | 0.071 | [335] |
3.5.2. H2O2 Electrochemical Sensors Based on the Association of Metallic Nanoparticles and CNTs
3.5.3. In Vivo Sensing of H2O2 Release from Carcinoma Cells
3.6. MXenes Materials
4. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinkova, P.; Kostelnik, A.; Válek, T.; Pohanka, M. Main streams in the construction of biosensors and their applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Li, P.; Lee, G.-H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.-R.; Park, S. From diagnosis to treatment: Recent advances in patient-friendly biosensors and implantable devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, J.; Liu, J.; Wang, X.; Chen, B. Disease-related detection with electrochemical biosensors: A review. Sensors 2017, 17, 2375. [Google Scholar] [CrossRef]
- Monošík, R.; Stred'anský, M.; Šturdík, E. Application of electrochemical biosensors in clinical diagnosis. J. Clin. Lab. Anal. 2012, 26, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Monosik, R.; Stredansky, M.; Tkac, J.; Sturdik, E. Application of enzyme biosensors in analysis of food and beverages. Food Anal. Methods 2012, 5, 40–53. [Google Scholar] [CrossRef]
- Faridbod, F.; Gupta, V.K.; Zamani, H.A. Electrochemical sensors and biosensors. Int. J. Electrochem. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. The World Health Report: 2004: Changing History; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Steward, B.; Kleihues, P. Colorectal Cancer; World Cancer Report; IACR Press: Lyon, France, 2003. [Google Scholar]
- Iannazzo, D.; Espro, C.; Celesti, C.; Ferlazzo, A.; Neri, G. Smart biosensors for cancer diagnosis based on graphene quantum dots. Cancers 2021, 13, 3194. [Google Scholar] [CrossRef]
- Mahato, K.; Kumar, A.; Maurya, P.K.; Chandra, P. Shifting paradigm of cancer diagnoses in clinically relevant samples based on miniaturized electrochemical nanobiosensors and microfluidic devices. Biosens. Bioelectron. 2018, 100, 411–428. [Google Scholar] [CrossRef]
- Chen, W.; Cai, S.; Ren, Q.-Q.; Wen, W.; Zhao, Y.-D. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martindale, J.L.; Liu, Y.; Holbrook, N.J. The cellular response to oxidative stress: Influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998, 333, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.-I.; Berk, B.C. Fyn and JAK2 mediate Ras activation by reactive oxygen species. J. Biol. Chem. 1999, 274, 21003–21010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, H.; Vayssié, S. Reactive peroxo compounds generated in situ from hydrogen peroxide: Kinetics and catalytic application in oxidation processes. Peroxide Chem. Mech. Prep. Asp. Oxyg. Transf. 2000, 128–138. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J. Bacteriol. 1987, 169, 2967–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.; De, B. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 91–100. [Google Scholar]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Outschoorn, U.E.; Balliet, R.M.; Lin, Z.; Whitaker-Menezes, D.; Howell, A.; Sotgia, F.; Lisanti, M.P. Hereditary ovarian cancer and two-compartment tumor metabolism: Epithelial loss of BRCA1 induces hydrogen peroxide production, driving oxidative stress and NFκB activation in the tumor stroma. Cell Cycle 2012, 11, 4152–4166. [Google Scholar] [CrossRef] [Green Version]
- Brigelius-Flohe, R.; Kipp, A. Glutathione peroxidases in different stages of carcinogenesis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Weinstain, R.; Savariar, E.N.; Felsen, C.N.; Tsien, R.Y. In vivo targeting of hydrogen peroxide by activatable cell-penetrating peptides. J. Am. Chem. Soc. 2014, 136, 874–877. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Xu, P.; Wen, W.; Li, X.; Xu, J. PtW/MoS2 hybrid nanocomposite for electrochemical sensing of H2O2 released from living cells. Biosens. Bioelectron. 2016, 80, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xiao, J.; Duan, X.; Ren, J.; Xiao, F.; Wang, S. Pd nanoparticles decorated N-doped graphene quantum dots@ N-doped carbon hollow nanospheres with high electrochemical sensing performance in cancer detection. ACS Appl. Mater. Interfaces 2016, 8, 22563–22573. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, L.; Li, W.; Zhu, C.; Bao, N.; Gu, H. Detection of cellular H2O2 in living cells based on horseradish peroxidase at the interface of Au nanoparticles decorated graphene oxide. Sens. Actuators B Chem. 2015, 211, 17–24. [Google Scholar] [CrossRef]
- Razmi, H.; Mohammad-Rezaei, R.; Heidari, H. Self-assembled Prussian blue nanoparticles based electrochemical sensor for high sensitive determination of H2O2 in acidic media. Electroanalysis 2009, 21, 2355–2362. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Xu, L.; Hou, H.; You, T. A novel and simple route to prepare a Pt nanoparticle-loaded carbon nanofiber electrode for hydrogen peroxide sensing. Biosens. Bioelectron. 2011, 26, 4585–4590. [Google Scholar] [CrossRef]
- Liu, C.-J.; Yu, S.-L.; Liu, Y.-P.; Dai, X.-J.; Wu, Y.; Li, R.-J.; Tao, J.-C. Synthesis, cytotoxic activity evaluation and HQSAR study of novel isosteviol derivatives as potential anticancer agents. Eur. J. Med. Chem. 2016, 115, 26–40. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Moreira, F.T.; Fernandes, R.; Sales, M.G.F. Novel and simple electrochemical biosensor monitoring attomolar levels of miRNA-155 in breast cancer. Biosens. Bioelectron. 2016, 80, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Yahalom, G.; Weiss, D.; Novikov, I.; Bevers, T.B.; Radvanyi, L.G.; Liu, M.; Piura, B.; Iacobelli, S.; Sandri, M.T.; Cassano, E. An antibody-based blood test utilizing a panel of biomarkers as a new method for improved breast cancer diagnosis. Biomark. Cancer 2013, 5, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dai, Z. Carbon nanomaterial-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luong, J.H. Recent advances in electrochemical biosensing schemes using graphene and graphene-based nanocomposites. Carbon 2015, 84, 519–550. [Google Scholar] [CrossRef]
- Lawal, A.T. Synthesis and utilisation of graphene for fabrication of electrochemical sensors. Talanta 2015, 131, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef]
- Wu, S.; He, Q.; Tan, C.; Wang, Y.; Zhang, H. Graphene-based electrochemical sensors. Small 2013, 9, 1160–1172. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Fan, K.; Wu, J.; Ying, Y. Direct electrochemical reduction of graphene oxide on ionic liquid doped screen-printed electrode and its electrochemical biosensing application. Biosens. Bioelectron. 2011, 28, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, W. Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors. Biosens. Bioelectron. 2017, 89, 249–268. [Google Scholar] [CrossRef]
- Pohanka, M.; Skládal, P. Electrochemical biosensors—Principles and applications. J. Appl. Biomed. 2008, 6, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Vigneshvar, S.; Sudhakumari, C.; Senthilkumaran, B.; Prakash, H. Recent advances in biosensor technology for potential applications–An overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Lazcka, O.; del Campo, F.J.; Munoz, F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- He, F. Development of Capillary-Driven Microfludic Biosensors for Food Safety and Quality Assurance. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2014. [Google Scholar]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Pundir, C.S.; Deswal, R.; Narwal, V. Quantitative analysis of hydrogen peroxide with special emphasis on biosensors. Bioprocess Biosyst. Eng. 2018, 41, 313–329. [Google Scholar] [CrossRef]
- Yunus, S.; Jonas, A.M.; Lakard, B. Potentiometric biosensors. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Parrilla, M.; Cánovas, R.; Andrade, F.J. Enhanced potentiometric detection of hydrogen peroxide using a platinum electrode coated with nafion. Electroanalysis 2017, 29, 223–230. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, Z. Potentiometric determination of hydrogen peroxide at MnO2-doped carbon paste electrode. Talanta 2000, 50, 1157–1162. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, Y.; Zhu, L.; Li, X.; Li, G. An amperometric biosensor for the detection of hydrogen peroxide released from human breast cancer cells. Biosens. Bioelectron. 2013, 41, 815–819. [Google Scholar] [CrossRef]
- Li, J.; Tan, S.N.; Ge, H. Silica sol-gel immobilized amperometric biosensor for hydrogen peroxide. Anal. Chim. Acta 1996, 335, 137–145. [Google Scholar] [CrossRef]
- Wang, B.; Dong, S. Sol–gel-derived amperometric biosensor for hydrogen peroxide based on methylene green incorporated in Nafion film. Talanta 2000, 51, 565–572. [Google Scholar] [CrossRef]
- Tripathi, V.S.; Kandimalla, V.B.; Ju, H. Amperometric biosensor for hydrogen peroxide based on ferrocene-bovine serum albumin and multiwall carbon nanotube modified ormosil composite. Biosens. Bioelectron. 2006, 21, 1529–1535. [Google Scholar] [CrossRef]
- Yu, J.; Ju, H. Amperometric biosensor for hydrogen peroxide based on hemoglobin entrapped in titania sol–gel film. Anal. Chim. Acta 2003, 486, 209–216. [Google Scholar] [CrossRef]
- Povedano, E.; Montiel, V.R.-V.; Gamella, M.; Serafín, V.; Pedrero, M.; Moranova, L.; Bartosik, M.; Montoya, J.J.; Yáñez-Sedeño, P.; Campuzano, S. A novel zinc finger protein–based amperometric biosensor for miRNA determination. Anal. Bioanal. Chem. 2019, 412, 5031–5041. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huan, K.; Deng, D.; Tang, L.; Wang, J.; Luo, L. Facile synthesis of ZnMn2O4@ rGO microspheres for ultrasensitive electrochemical detection of hydrogen peroxide from human breast cancer cells. ACS Appl. Mater. Interfaces 2019, 12, 3430–3437. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ren, Y.; Bai, Z.; Yang, Y.; Chen, Q. Fabrication of hexahedral Au-Pd/graphene nanocomposites biosensor and its application in cancer cell H2O2 detection. Bioelectrochemistry 2019, 128, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Pan, M.; Liu, X.; Li, B.; Liu, J.; Chen, Q. A non-enzymatic sensor based on trimetallic nanoalloy with poly (diallyldimethylammonium chloride)-capped reduced graphene oxide for dynamic monitoring hydrogen peroxide production by cancerous cells. Sensors 2020, 20, 71. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.; Zhang, L.; Cai, H.; Yang, Y.; Zeng, J.; Ma, D.; Gao, Q. Hierarchical mo2c@ MoS2 nanorods as electrochemical sensors for highly sensitive detection of hydrogen peroxide and cancer cells. Sens. Actuators B Chem. 2020, 127863. [Google Scholar] [CrossRef]
- Thiruppathi, M.; Lin, P.-Y.; Chou, Y.-T.; Ho, H.-Y.; Wu, L.-C.; Ho, J.-A.A. Simple aminophenol-based electrochemical probes for non-enzymatic, dual amperometric detection of NADH and hydrogen peroxide. Talanta 2019, 200, 450–457. [Google Scholar] [CrossRef]
- Maji, S.K. Plasmon-enhanced electrochemical biosensing of hydrogen peroxide from cancer cells by gold nanorods. ACS Appl. Nano Mater. 2019, 2, 7162–7169. [Google Scholar] [CrossRef]
- Du, H.; Zhang, X.; Liu, Z.; Qu, F. A supersensitive biosensor based on MoS2 nanosheet arrays for the real-time detection of H2O2 secreted from living cells. Chem. Commun. 2019, 55, 9653–9656. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Zhang, L.; Ge, S.; Liu, H.; Ren, N.; Yan, M.; Yu, J. Based device for colorimetric and photoelectrochemical quantification of the flux of H2O2 releasing from MCF-7 cancer cells. Anal. Chem. 2016, 88, 5369–5377. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Deng, H.-H.; Lin, F.-L.; Xu, X.-W.; Weng, S.-H.; Liu, A.-L.; Lin, X.-H.; Xia, X.-H.; Chen, W. In situ growth of porous platinum nanoparticles on graphene oxide for colorimetric detection of cancer cells. Anal. Chem. 2014, 86, 2711–2718. [Google Scholar] [CrossRef]
- Ge, S.; Liu, W.; Liu, H.; Liu, F.; Yu, J.; Yan, M.; Huang, J. Colorimetric detection of the flux of hydrogen peroxide released from living cells based on the high peroxidase-like catalytic performance of porous PtPd nanorods. Biosens. Bioelectron. 2015, 71, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Shi, H.; He, X.; Wang, K.; He, D.; Yan, L.A.; Xu, F.; Lei, Y.; Tang, J.; Yu, Y. Iodide-responsive Cu–Au nanoparticle-based colorimetric platform for ultrasensitive detection of target cancer cells. Anal. Chem. 2015, 87, 7141–7147. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Patra, C.R.; Arkalgud, J.R.; Boghossian, A.A.; Zhang, J.; Han, J.-H.; Reuel, N.F.; Ahn, J.-H.; Mukhopadhyay, D.; Strano, M.S. Single-molecule detection of H2O2 mediating angiogenic redox signaling on fluorescent single-walled carbon nanotube array. ACS Nano 2011, 5, 7848–7857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maji, S.K.; Sreejith, S.; Mandal, A.K.; Ma, X.; Zhao, Y. Immobilizing gold nanoparticles in mesoporous silica covered reduced graphene oxide: A hybrid material for cancer cell detection through hydrogen peroxide sensing. ACS Appl. Mater. Interfaces 2014, 6, 13648–13656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, L.; Li, Z.; Lin, Y.; Li, J. In situ simultaneous monitoring of ATP and GTP using a graphene oxide nanosheet–based sensing platform in living cells. Nat. Protoc. 2014, 9, 1944. [Google Scholar] [CrossRef]
- McKibbin, P.L.; Kobori, A.; Taniguchi, Y.; Kool, E.T.; David, S.S. Surprising repair activities of nonpolar analogs of 8-oxoG expose features of recognition and catalysis by base excision repair glycosylases. J. Am. Chem. Soc. 2012, 134, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Lin, H.; Lei, C.; Nie, Z.; Huang, Y.; Yao, S. Label-free fluorescence assay for thrombin based on unmodified quantum dots. Biosens. Bioelectron. 2014, 54, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wong, N.T.; Handoko, A.D.; Huang, Y.; Yeo, B.S. Mechanistic insights into the enhanced activity and stability of agglomerated Cu nanocrystals for the electrochemical reduction of carbon dioxide to n-propanol. J. Phys. Chem. Lett. 2016, 7, 20–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lau, C.; Lu, J. Sequential determination of two proteins by temperature-triggered homogeneous chemiluminescent immunoassay. Anal. Chem. 2006, 78, 5920–5924. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, Z.; Lin, J.-M. A review on applications of chemiluminescence detection in food analysis. Anal. Chim. Acta 2010, 670, 1–10. [Google Scholar] [CrossRef]
- Kong, H.; Liu, D.; Zhang, S.; Zhang, X. Protein sensing and cell discrimination using a sensor array based on nanomaterial-assisted chemiluminescence. Anal. Chem. 2011, 83, 1867–1870. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Mohsen, M.G.; Harcourt, E.M.; Kool, E.T. ATP-Releasing Nucleotides: Linking DNA Synthesis to Luciferase Signaling. Angew. Chem. Int. Ed. 2016, 55, 2087–2091. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.-F.; Ozaki, M.; Hisamoto, H.; Luo, Q.; Utsumi, Y.; Hattori, T.; Terabe, S. Microfluidic chip toward cellular ATP and ATP-conjugated metabolic analysis with bioluminescence detection. Anal. Chem. 2005, 77, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, W. In situ growth of surfactant-free gold nanoparticles on nitrogen-doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Jiang, X. A facile one-pot synthesis of copper sulfide-decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal. Chem. 2013, 85, 8095–8101. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Zhang, S.; Wang, S.; Zhang, S.; Zhang, X. Aptamer-Based Plasmonic Sensor Array for Discrimination of Proteins and Cells with the Naked Eye. Anal. Chem. 2013, 85, 6571–6574. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, X.; Lu, Y.; Yang, W. Self-assembled dipeptide–gold nanoparticle hybrid spheres for highly sensitive amperometric hydrogen peroxide biosensors. Biosens. Bioelectron. 2015, 66, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, H. Core-Shell-Type Magnetic Mesoporous Silica Nanocomposites for Bioimaging and Therapeutic Agent Delivery. Adv. Mater. 2015, 27, 576–585. [Google Scholar] [CrossRef]
- Yang, J.; Shen, D.; Zhou, L.; Li, W.; Li, X.; Yao, C.; Wang, R.; El-Toni, A.M.; Zhang, F.; Zhao, D. Spatially confined fabrication of core–shell gold nanocages@mesoporous silica for near-infrared controlled photothermal drug release. Chem. Mater. 2013, 25, 3030–3037. [Google Scholar] [CrossRef]
- Lee, D.; Khaja, S.D.; Velasquez-Castano, J.C.; Dasari, M.; Sun, C.; A Petros, J.; Taylor, W.R.; Murthy, N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Arnous, A.; Petrakis, C.; Makris, D.P.; Kefalas, P. A peroxyoxalate chemiluminescence-based assay for the evaluation of hydrogen peroxide scavenging activity employing 9,10-diphenylanthracene as the fluorophore. J. Pharmacol. Toxicol. Methods 2002, 48, 171–177. [Google Scholar] [CrossRef]
- Koike, R.; Kato, Y.; Motoyoshiya, J.; Nishii, Y.; Aoyama, H. Unprecedented chemiluminescence behaviour during peroxyoxalate chemiluminescence of oxalates with fluorescent or electron-donating aryloxy groups. Luminescence 2006, 21, 164–173. [Google Scholar] [CrossRef]
- Stevani, C.V.; Silva, S.M.; Baader, W.J. Studies on the Mechanism of the Excitation Step in Peroxyoxalate Chemiluminescence. Eur. J. Org. Chem. 2000, 2000, 4037–4046. [Google Scholar] [CrossRef]
- Matsumoto, M. Advanced chemistry of dioxetane-based chemiluminescent substrates originating from bioluminescence. J. Photochem. Photobiol. C 2004, 5, 27–53. [Google Scholar] [CrossRef]
- Lee, D.; Dasari, M.; Erigala, V.; Murthy, N.; Yu, J.; Dickson, R. Detection of hydrogen peroxide with chemiluminescent micelles. Int. J. Nanomed. 2008, 3, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Zhao, W.; Li, N.; Liang, Z.; Xu, X. Chemiluminescence-triggered fluorophore release: Approach for in vivo fluorescence imaging of hydrogen peroxide. Sens. Actuators B Chem. 2019, 281, 296–302. [Google Scholar] [CrossRef]
- Lee, Y.-D.; Lim, C.-K.; Singh, A.; Koh, J.; Kim, J.; Kwon, I.C.; Kim, S. Dye/Peroxalate Aggregated Nanoparticles with Enhanced and Tunable Chemiluminescence for Biomedical Imaging of Hydrogen Peroxide. ACS Nano 2012, 6, 6759–6766. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Deepagan, V.G.; Gil You, D.; Jeon, J.; Yi, G.-R.; Lee, J.Y.; Lee, D.S.; Suh, Y.D.; Park, J.H. Nanoparticles based on quantum dots and a luminol derivative: Implications for in vivo imaging of hydrogen peroxide by chemiluminescence resonance energy transfer. Chem. Commun. 2016, 52, 4132–4135. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, K.; Qin, W.; Tang, B.Z.; Liu, B. Red-Emissive Chemiluminescent Nanoparticles with Aggregation-Induced Emission Characteristics for In Vivo Hydrogen Peroxide Imaging. Part. Part. Syst. Charact. 2014, 31, 1238–1243. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, S.; Cui, X.; Wang, X.; Yang, L. Enzyme-like catalysis of polyoxometalates for chemiluminescence: Application in ultrasensitive detection of H2O2 and blood glucose. Talanta 2019, 205, 120139. [Google Scholar] [CrossRef] [PubMed]
- Klassen, N.V.; Marchington, D.; McGowan, H.C. H2O2 Determination by the I3− Method and by KMnO4 Titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Kieber, R.J.; Helz, G.R. Two-method verification of hydrogen peroxide determinations in natural waters. Anal. Chem. 1986, 58, 2312–2315. [Google Scholar] [CrossRef]
- Putt, K.S.; Pugh, R.B. A High-throughput microtiter plate based method for the determination of peracetic acid and hydrogen peroxide. PLoS ONE 2013, 8, e79218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaribafan, A.; Haghbeen, K.; Fazli, M.; Akhondali, A. Spectrophotometric method for hydrogen peroxide determination through oxidation of organic dyes. Environ. Stud. Persian Gulf 2014, 1, 93–101. [Google Scholar]
- Matsubara, C.; Kudo, K.; Kawashita, T.; Takamura, K. Spectrophotometric determination of hydrogen peroxide with titanium 2-((5-bromopyridyl)azo)-5-(N-propyl-N-sulfopropylamino)phenol reagent and its application to the determination of serum glucose using glucose oxidase. Anal. Chem. 1985, 57, 1107–1109. [Google Scholar] [CrossRef]
- Clapp, P.A.; Evans, D.F.; Sheriff, T.S. Spectrophotometric determination of hydrogen peroxide after extraction with ethyl acetate. Anal. Chim. Acta 1989, 218, 331–334. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Dasgupta, P.; Roy, D.S.; Palchoudhuri, S.; Chatterjee, I.; Ali, S.; Dastidar, S.G. A Sensitive In vitro Spectrophotometric Hydrogen Peroxide Scavenging Assay using 1,10-Phenanthroline. Free Radic. Antioxid. 2016, 6, 124–132. [Google Scholar] [CrossRef]
- Elnemma, E.M. Spectrophotometric Determination of Hydrogen Peroxide by a Hydroquinone-Aniline System Catalyzed by Molybdate. Bull. Korean Chem. Soc. 2004, 25, 127–129. [Google Scholar]
- Zhang, L.-S.; Wong, G.T. Spectrophotometric determination of H2O2 in marine waters with leuco crystal violet. Talanta 1994, 41, 2137–2145. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, R.; Mao, L.; LIU, Z.; HUANG, H. Spectrophotometric determination of hydrogen peroxide using β-CD-Hemin as a mimetic enzyme of peroxidase. Anal. Sci. 1999, 15, 889–894. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Fu, S.; Li, H.; Liu, Y. A novel method for the determination of hydrogen peroxide in bleaching effluents by spectroscopy. BioResources 2013, 8, 3699–3705. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, G. Industrial and engineering chemistry. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Graf, E.; Penniston, J.T. Method for determination of hydrogen peroxide, with its application illustrated by glucose assay. Clin. Chem. 1980, 26, 658–660. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of H2O2 produced by cells in culture J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. MethodsX 2015, 2, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Wei, Y.; Guo, M. Direct Colorimetric Detection of Hydrogen Peroxide Using 4-Nitrophenyl Boronic Acid or Its Pinacol Ester. Am. J. Anal. Chem. 2011, 2, 879–884. [Google Scholar] [CrossRef] [Green Version]
- Nitinaivinij, K.; Parnklang, T.; Thammacharoen, C.; Ekgasit, S.; Wongravee, K. Colorimetric determination of hydrogen peroxide by morphological decomposition of silver nanoprisms coupled with chromaticity analysis. Anal. Methods 2014, 6, 9816–9824. [Google Scholar] [CrossRef]
- Takahashi, A.; Hashimoto, K.; Kumazawa, S.; Nakayama, T. Determination of Hydrogen Peroxide by High-Performance Liquid Chromatography with a Cation-Exchange Resin Gel Column and Electrochemical Detector. Anal. Sci. 1999, 15, 481–483. [Google Scholar] [CrossRef] [Green Version]
- Wada, M.; Inoue, K.; Ihara, A.; Kishikawa, N.; Nakashima, K.; Kuroda, N. Determination of organic peroxides by liquid chromatography with on-line post-column ultraviolet irradiation and peroxyoxalate chemiluminescence detection. J. Chromatogr. A 2003, 987, 189–195. [Google Scholar] [CrossRef]
- Nepomnyashchikh, Y.V.; Borkina, G.G.; Karavaeva, A.V.; Perkel’, A.L. Photometric and Gas-Chromatographic Determination of Hydrogen Peroxide and Peroxybutanoic Acid in Oxidized Butanoic Acid. J. Anal. Chem. 2005, 60, 1024–1028. [Google Scholar] [CrossRef]
- Magara, K.; Ikeda, T.; Sugimoto, T.; Hosoya, S. Quantitative Analysis of Hydrogen Peroxide by High Performance Liquid Chromatography. Jpn. TAPPI J. 2007, 61, 1481–1493. [Google Scholar] [CrossRef] [Green Version]
- Tarno, H.; Qi, H.; Endoh, R.; Kobayashi, M.; Goto, H.; Futai, K. Types of frass produced by the ambrosia beetle Platypus quercivorus during gallery construction, and host suitability of five tree species for the beetle. J. For. Res. 2011, 16, 68–75. [Google Scholar] [CrossRef]
- Wielandt, H. On the eigenvalues of A + B and AB. J. Res. Natl. Bur. Stand. Sect. B Math. Sci. 1973, 77B, 61. [Google Scholar] [CrossRef]
- Xu, K.; Tang, B.; Huang, H.; Yang, G.; Chen, Z.; Li, P.; An, L. Strong red fluorescent probes suitable for detecting hydrogen peroxide generated by mice peritoneal macrophages. Chem. Commun. 2005, 48, 5974–5976. [Google Scholar] [CrossRef]
- Paździoch-Czochra, M.; Wideńska, A. Spectrofluorimetric determination of hydrogen peroxide scavenging activity. Anal. Chim. Acta 2002, 452, 177–184. [Google Scholar] [CrossRef]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-Based Fluorescent Probes for Imaging Cellular Hydrogen Peroxide. J. Am. Chem. Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef] [Green Version]
- Qian, P.; Qin, Y.; Lyu, Y.; Li, Y.; Wang, L.; Wang, S.; Liu, Y. A hierarchical cobalt/carbon nanotube hybrid nanocomplex-based ratiometric fluorescent nanosensor for ultrasensitive detection of hydrogen peroxide and glucose in human serum. Anal. Bioanal. Chem. 2019, 411, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Onoda, M.; Uchiyama, T.; Mawatari, K.-I.; Kaneko, K.; Nakagomi, K. Simple and Rapid Determination of Hydrogen Peroxide Using Phosphine-based Fluorescent Reagents with Sodium Tungstate Dihydrate. Anal. Sci. 2006, 22, 815–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyublinskaya, O.; Antunes, F. Measuring intracellular concentration of hydrogen peroxide with the use of genetically encoded H2O2 biosensor HyPer. Redox Biol. 2019, 24, 101200. [Google Scholar] [CrossRef]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.; Staroverov, D.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef]
- Zheng, M.; Åslund, F.; Storz, G. Activation of the OxyR Transcription Factor by Reversible Disulfide Bond Formation. Science 1998, 279, 1718–1722. [Google Scholar] [CrossRef]
- Markvicheva, K.N.; Bilan, D.; Mishina, N.; Gorokhovatsky, A.Y.; Vinokurov, L.M.; Lukyanov, S.; Belousov, V.V. A genetically encoded sensor for H2O2 with expanded dynamic range. Bioorganic Med. Chem. 2011, 19, 1079–1084. [Google Scholar] [CrossRef]
- Bilan, D.S.; Pase, L.; Joosen, L.; Gorokhovatsky, A.Y.; Ermakova, Y.G.; Gadella, T.W.J.; Grabher, C.; Schultz, C.; Lukyanov, S.; Belousov, V.V. HyPer-3: A Genetically Encoded H2O2 Probe with Improved Performance for Ratiometric and Fluorescence Lifetime Imaging. ACS Chem. Biol. 2013, 8, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Pak, V.V.; Ezerina, D.; Lyublinskaya, O.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Gache, S.A.M.; et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab. 2020, 31, 642–653.e6. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, Y.; Bilan, D.; Matlashov, M.; Mishina, N.; Markvicheva, K.N.; Subach, O.M.; Subach, F.V.; Bogeski, I.; Hoth, M.; Enikolopov, G.; et al. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 2014, 5, 5222. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kim, S.-J.; Mukhopadhyay, P.; Cho, S.; Woo, J.-R.; Storz, G.; Ryu, S.-E. Structural Basis of the Redox Switch in the OxyR Transcription Factor. Cell 2001, 105, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, Y.; Yu, H.; Gao, X.; Shao, S. Mitochondria-Targeted Fluorescent Probe for Imaging Hydrogen Peroxide in Living Cells. Anal. Chem. 2015, 88, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, P.; Hu, X.; Shi, X.; Zhang, W.; Tang, B. Simultaneous fluorescence imaging of hydrogen peroxide in mitochondria and endoplasmic reticulum during apoptosis. Chem. Sci. 2016, 7, 6153–6159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, R.; Liu, P.; Zhang, Y.; Yu, Z.; Chen, X.; Zhou, L.; Nie, B.; Żaczek, A.; Chen, J.; Liu, J. Sensitive Detection of Single-Cell Secreted H2O2 by Integrating a Microfluidic Droplet Sensor and Au Nanoclusters. Anal. Chem. 2018, 90, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, J.; Lv, G.; Liu, W.; Jiang, H.; Liu, X.; Wang, X. Hydrogen Peroxide and Hypochlorite Responsive Fluorescent Nanoprobes for Sensitive Cancer Cell Imaging. Biosensors 2022, 12, 111. [Google Scholar] [CrossRef]
- Hu, L.; Yuan, Y.; Zhang, L.; Zhao, J.; Majeed, S.; Xu, G. Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal. Chim. Acta 2013, 762, 83–86. [Google Scholar] [CrossRef]
- Liu, H.; Gu, C.; Xiong, W.; Zhang, M. A sensitive hydrogen peroxide biosensor using ultra-small CuInS2 nanocrystals as peroxidase mimics. Sens. Actuators B Chem. 2015, 209, 670–676. [Google Scholar] [CrossRef]
- Su, L.; Qin, W.; Zhang, H.; Rahman, Z.U.; Ren, C.; Ma, S.; Chen, X. The peroxidase/catalase-like activities of MFe2O4 (M = Mg, Ni, Cu) MNPs and their application in colorimetric biosensing of glucose. Biosens. Bioelectron. 2015, 63, 384–391. [Google Scholar] [CrossRef]
- Regalado, C.; García-Almendárez, B.E.; Duarte-Vázquez, M.A. Biotechnological applications of peroxidases. Phytochem. Rev. 2004, 3, 243–256. [Google Scholar] [CrossRef]
- Hamid, M. Potential applications of peroxidases. Food Chem. 2009, 115, 1177–1186. [Google Scholar] [CrossRef]
- Chekin, F.; Gorton, L.; Tapsobea, I. Direct and mediated electrochemistry of peroxidase and its electrocatalysis on a variety of screen-printed carbon electrodes: Amperometric hydrogen peroxide and phenols biosensor. Anal. Bioanal. Chem. 2014, 407, 439–446. [Google Scholar] [CrossRef]
- Yang, H.; Liu, B.; Ding, Y.; Li, L.; Ouyang, X. Fabrication of cuprous oxide nanoparticles-graphene nanocomposite for determination of acetaminophen. J. Electroanal. Chem. 2015, 757, 88–93. [Google Scholar] [CrossRef]
- Chulkova, I.; Derina, K.; Taishibekova, Y. The modified electrode for the determination of cholesterol. In Proceedings of the Chemistry and Chemical Technology in the XXI Century: Materials of the XVI International Scientific-Practical Conference of Students and Young Scientists Dedicated to the 115th Anniversary of Professor L.P. Kuleva, Tomsk, Russia, 25–29 May 2015; pp. 195–197. [Google Scholar]
- Jelikić-Stankov, M.D.; Djurdjevic, P.; Stankov, D. Determination of uric acid in human serum by an enzymatic method using N-methyl-N-(4-aminophenyl)-3-methoxyaniline reagent. J. Serb. Chem. Soc. 2003, 68, 691–698. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Guo, Z.; Tan, H.; Zhu, X. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul. 2006, 49, 113–118. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Kakoti, A.; Santhosh, M.; Goswami, P. A novel amperometric alcohol biosensor developed in a 3rd generation bioelectrode platform using peroxidase coupled ferrocene activated alcohol oxidase as biorecognition system. Biosens. Bioelectron. 2014, 55, 120–126. [Google Scholar] [CrossRef]
- Yu, F.; Huang, Y.; Cole, A.J.; Yang, V.C. The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials 2009, 30, 4716–4722. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.; Zhang, L.; Zhao, M.; Wang, Y. Co3O4 nanoparticles as an efficient catalase mimic: Properties, mechanism and its electrocatalytic sensing application for hydrogen peroxide. J. Mol. Catal. A Chem. 2013, 378, 30–37. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, T.; Bapurao G., B.; Jo, J.; Oh, B.-K.; Choi, J.-W. Electrochemical H2O2 biosensor composed of myoglobin on MoS2 nanoparticle-graphene oxide hybrid structure. Biosens. Bioelectron. 2017, 93, 14–20. [Google Scholar] [CrossRef]
- Wen, Z.; Ci, S.; Li, J. Pt Nanoparticles Inserting in Carbon Nanotube Arrays: Nanocomposites for Glucose Biosensors. J. Phys. Chem. C 2009, 113, 13482–13487. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.M.; Dong, S.J. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, S.; Hu, Z.; Liu, S. Nitrogen-doped carbon nanotubes: High electrocatalytic activity toward the oxidation of hydrogen peroxide and its application for biosensing. ACS Nano 2010, 4, 4292–4298. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Rui, Q.; Tian, Y. Detection of Extracellular H2O2 Released from Human Liver Cancer Cells Based on TiO2 Nanoneedles with Enhanced Electron Transfer of Cytochrome c. Anal. Chem. 2009, 81, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Kim, G.; Lee, Y.-E.K.; Xu, H.; Philbert, M.A.; Kopelman, R. Nanoencapsulation method for high selectivity sensing of hydrogen peroxide inside live cells. Anal. Chem. 2010, 82, 2165–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Guo, S.; Liu, Y.; Sun, S. Dumbbell-like PtPd–Fe3O4 nanoparticles for enhanced electrochemical detection of H2O2. Nano Lett. 2012, 12, 4859–4863. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, H.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Lubarsky, G.; Lin, J.; Li, M. Biosensor Based on Ultrasmall MoS2 Nanoparticles for Electrochemical Detection of H2O2 Released by Cells at the Nanomolar Level. Anal. Chem. 2013, 85, 10289–10295. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Yang, J.; Yuan, R.; Xiang, Y. Trimetallic Hybrid Nanoflower-Decorated MoS2 Nanosheet Sensor for Direct in Situ Monitoring of H2O2 Secreted from Live Cancer Cells. Anal. Chem. 2018, 90, 5945–5950. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Ho, J.-A.A. Gold nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal. Chem. 2015, 87, 10362–10367. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, W.; Duan, C.-F.; Dong, Y.-P.; Guo, J.-Z. Synthesis, characterization, and electrochemiluminescence of luminol-reduced gold nanoparticles and their application in a hydrogen peroxide sensor. Chem.–A Eur. J. 2007, 13, 6975–6984. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Lv, X.; Ding, Y.; Zhang, Y.; Jing, J.; Xu, C. One-step synthesis of uniform nanoparticles of porphyrin functionalized ceria with promising peroxidase mimetics for H2O2 and glucose colorimetric detection. Sens. Actuators B Chem. 2017, 240, 726–734. [Google Scholar] [CrossRef]
- Xiong, X.; You, C.; Cao, X.; Pang, L.; Kong, R.; Sun, X. Ni2P nanosheets array as a novel electrochemical catalyst electrode for non-enzymatic H2O2 sensing. Electrochim. Acta 2017, 253, 517–521. [Google Scholar] [CrossRef]
- Chen, L.; Wang, N.; Wang, X.; Ai, S. Protein-directed in situ synthesis of platinum nanoparticles with superior peroxidase-like activity, and their use for photometric determination of hydrogen peroxide. Mikrochim. Acta 2013, 180, 1517–1522. [Google Scholar] [CrossRef]
- Ma, B.; Kong, C.; Hu, X.; Liu, K.; Huang, Q.; Lv, J.; Lu, W.; Zhang, X.; Yang, Z.; Yang, S. A sensitive electrochemical nonenzymatic biosensor for the detection of H2O2 released from living cells based on ultrathin concave Ag nanosheets. Biosens. Bioelectron. 2018, 106, 29–36. [Google Scholar] [CrossRef]
- Asif, M.; Liu, H.; Aziz, A.; Wang, H.; Wang, Z.; Ajmal, M.; Xiao, F.; Liu, H. Core-shell iron oxide-layered double hydroxide: High electrochemical sensing performance of H2O2 biomarker in live cancer cells with plasma therapeutics. Biosens. Bioelectron. 2017, 97, 352–359. [Google Scholar] [CrossRef]
- Li, Z.; Xin, Y.; Wu, W.; Fu, B.; Zhang, Z. Topotactic Conversion of Copper(I) Phosphide Nanowires for Sensitive Electrochemical Detection of H2O2 Release from Living Cells. Anal. Chem. 2016, 88, 7724–7729. [Google Scholar] [CrossRef]
- Su, S.; Han, X.; Lu, Z.; Liu, W.; Zhu, D.; Chao, J.; Fan, C.; Wang, L.; Song, S.; Weng, L.; et al. Facile Synthesis of a MoS2–Prussian Blue Nanocube Nanohybrid-Based Electrochemical Sensing Platform for Hydrogen Peroxide and Carcinoembryonic Antigen Detection. ACS Appl. Mater. Interfaces 2017, 9, 12773–12781. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Xie, Y.; Chen, B.; Zhu, S. Single fluorescent probe responds to H2O2, NO, and H2O2/NO with three different sets of fluorescence signals. J. Am. Chem. Soc. 2012, 134, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Boero, C.; Casulli, M.A.; Olivo, J.; Foglia, L.; Orso, E.; Mazza, M.; Carrara, S.; De Micheli, G. Design, development, and validation of an in-situ biosensor array for metabolite monitoring of cell cultures. Biosens. Bioelectron. 2014, 61, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.-X.; Wang, Y.; Zhang, K.; Lam, T.-L.; Chan, H.L.-W. Monitoring of dopamine release in single cell using ultrasensitive ITO microsensors modified with carbon nanotubes. Biosens. Bioelectron. 2011, 26, 2917–2921. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-W.; Qin, L.-X.; Li, Y.; Nia, R.P.; Long, Y.-T.; Chen, H.-Y. CdSe/ZnS quantum dot–Cytochrome c bioconjugates for selective intracellular O 2–Sensing. Chem. Commun. 2011, 47, 8539–8541. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Liu, S.; Bao, J.; Dai, Z. Pd nanoparticle assemblies—As the substitute of HRP, in their biosensing applications for H2O2 and glucose. Biosens. Bioelectron. 2012, 31, 151–156. [Google Scholar] [CrossRef]
- Wang, Y.; Hasebe, Y. Carbon felt-based bioelectrocatalytic flow-through detectors: Highly sensitive amperometric determination of H2O2 based on a direct electrochemistry of covalently modified horseradish peroxidase using cyanuric chloride as a linking agent. Sens. Actuators B Chem. 2011, 155, 722–729. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Leng, K.; Li, J.; Zheng, F.; Shen, G.; Yu, R. A Sequence-Selective Electrochemical DNA Biosensor Based on HRP-Labeled Probe for Colorectal Cancer DNA Detection. Anal. Lett. 2008, 41, 24–35. [Google Scholar] [CrossRef]
- Crulhas, B.P.; Ramos, N.P.; Castro, G.R.; Pedrosa, V.A. Detection of hydrogen peroxide releasing from prostate cancer cell using a biosensor. J. Solid State Electrochem. 2016, 20, 2427–2433. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Liao, C.; Zhang, L.; Wang, Q.; Tian, Y. Molecular Hydrogel-Stabilized Enzyme with Facilitated Electron Transfer for Determination of H2O2 Released from Live Cells. Anal. Chem. 2014, 86, 4395–4401. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Gao, H.; Duan, H. 2D and 3D graphene materials: Preparation and bioelectrochemical applications. Biosens. Bioelectron. 2015, 65, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Fusco, G.; Mazzei, F.; Tasca, F.; Antiochia, R. Electrochemical Characterization of Graphene and MWCNT Screen-Printed Electrodes Modified with AuNPs for Laccase Biosensor Development. Nanomaterials 2015, 5, 1995–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, C.; Zhang, H.; Wang, L. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef]
- Tan, S.M.; Sofer, Z.; Pumera, M. Biomarkers Detection on Hydrogenated Graphene Surfaces: Towards Applications of Graphane in Biosensing. Electroanalysis 2013, 25, 703–705. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, C.; Du, D.; Zhu, J.; Lin, Y. Graphene-like two-dimensional layered nanomaterials: Applications in biosensors and nanomedicine. Nanoscale 2015, 7, 14217–14231. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Lin, Y.; Connell, J.W. Advances in 2D boron nitride nanostructures: Nanosheets, nanoribbons, nanomeshes, and hybrids with graphene. Nanoscale 2012, 4, 6908–6939. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Du, D.; Lin, Y. Graphene and graphene-like 2D materials for optical biosensing and bioimaging: A review. 2D Mater. 2015, 2, 032004. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Abiko, N.; Anzai, J.-I. Redox Response of Reduced Graphene Oxide-Modified Glassy Carbon Electrodes to Hydrogen Peroxide and Hydrazine. Materials 2013, 6, 1840–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R. High-Yield Organic Dispersions of Unfunctionalized Graphene. Nano Lett. 2009, 9, 3460–3462. [Google Scholar] [CrossRef]
- Lv, W.; Guo, M.; Liang, M.-H.; Jin, F.-M.; Cui, L.; Zhi, L.; Yang, Q.-H. Graphene-DNA hybrids: Self-assembly and electrochemical detection performance. J. Mater. Chem. 2010, 20, 6668–6673. [Google Scholar] [CrossRef]
- Woo, S.; Kim, Y.-R.; Chung, T.D.; Piao, Y.; Kim, H. Synthesis of a graphene–carbon nanotube composite and its electrochemical sensing of hydrogen peroxide. Electrochim. Acta 2012, 59, 509–514. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.-S.; Ren, W.; Cheng, H.-M.; Tang, N.; Wu, W.; Zhong, W.; Du, Y. The doping of reduced graphene oxide with nitrogen and its effect on the quenching of the material’s photoluminescence. Carbon 2012, 50, 5286–5291. [Google Scholar] [CrossRef]
- Yeh, M.-H.; Li, Y.-S.; Chen, G.-L.; Lin, L.-Y.; Li, T.-J.; Chuang, H.-M.; Hsieh, C.-Y.; Lo, S.-C.; Chiang, W.-H.; Ho, K.-C. Facile Synthesis of Boron-doped Graphene Nanosheets with Hierarchical Microstructure at Atmosphere Pressure for Metal-free Electrochemical Detection of Hydrogen Peroxide. Electrochim. Acta 2015, 172, 52–60. [Google Scholar] [CrossRef]
- Yang, G.-H.; Zhou, Y.-H.; Wu, J.-J.; Cao, J.-T.; Li, L.-L.; Liu, H.-Y.; Zhu, J.-J. Microwave-assisted synthesis of nitrogen and boron co-doped graphene and its application for enhanced electrochemical detection of hydrogen peroxide. RSC Adv. 2013, 3, 22597–22604. [Google Scholar] [CrossRef]
- Zor, E.; Saglam, M.E.; Akin, I.; Saf, A.O.; Bingol, H.; Ersoz, M. Green synthesis of reduced graphene oxide/nanopolypyrrole composite: Characterization and H2O2 determination in urine. RSC Adv. 2014, 4, 12457–12466. [Google Scholar] [CrossRef]
- Luo, J.; Chen, Y.; Ma, Q.; Liu, R.; Liu, X. Layer-by-layer assembled ionic-liquid functionalized graphene–polyaniline nanocomposite with enhanced electrochemical sensing properties. J. Mater. Chem. C 2014, 2, 4818–4827. [Google Scholar] [CrossRef]
- Wang, Q.; Li, M.; Szunerits, S.; Boukherroub, R. Environmentally Friendly Reduction of Graphene Oxide Using Tyrosine for Nonenzymatic Amperometric H2O2 Detection. Electroanalysis 2014, 26, 156–163. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Tran, T.H.; Shim, J.-J. Glassy carbon electrode modified with a graphene oxide/poly(o-phenylenediamine) composite for the chemical detection of hydrogen peroxide. Mater. Sci. Eng. C 2014, 44, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.F.Y. Electrocatalytic performance of silica nanoparticles on graphene oxide sheets for hydrogen peroxide sensing. J. Electroanal. Chem. 2013, 690, 8–12. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Li, W.-W.; Wang, J.-Y.; Fang, H.-L.; Fan, D.-H.; Wang, W. Direct electrolytic exfoliation of graphite with hemin and single-walled carbon nanotube: Creating functional hybrid nanomaterial for hydrogen peroxide detection. Anal. Chim. Acta 2015, 884, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Wu, L.; Huang, W.; Hao, Q.; Zhang, Y.; Xia, X. Microwave-assisted synthesis of hemin–graphene/poly(3,4-ethylenedioxythiophene) nanocomposite for a biomimetic hydrogen peroxide biosensor. J. Mater. Chem. B 2014, 2, 4324–4330. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, Y.; Li, C.; Yan, X.; Lu, N.; Liu, H.; Zhang, Z.; Zhang, H. Fabrication of Novel Electrochemical Biosensor Based on Graphene Nanohybrid to Detect H2O2 Released from Living Cells with Ultrahigh Performance. ACS Appl. Mater. Interfaces 2017, 9, 37991–37999. [Google Scholar] [CrossRef]
- Xi, F.; Zhao, D.; Wang, X.; Chen, P. Non-enzymatic detection of hydrogen peroxide using a functionalized three-dimensional graphene electrode. Electrochem. Commun. 2013, 26, 81–84. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, M.; Yang, J.; Wu, G.; Wu, H.; Chen, C.; Liu, A. Metal-free rGO/GO hybrid microelectrode array for sensitive and in-situ hydrogen peroxide sensing. Electrochim. Acta 2019, 326, 134967. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, Z.; Zhang, K.; Peng, S.; Zhang, X.; Liu, W.; Chu, K. Three-dimensional phosphorus-doped graphene as an efficient metal-free electrocatalyst for electrochemical sensing. Sens. Actuators B Chem. 2017, 241, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, S.; Kim, S.J. An enzymatic biosensor for hydrogen peroxide based on one-pot preparation of CeO2-reduced graphene oxide nanocomposite. RSC Adv. 2015, 5, 12937–12943. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Y.; Yang, X.; Li, C. Photoelectrochemical detection of H2O2 based on flower-like CuInS2-graphene hybrid. Electroanalysis 2014, 26, 573–580. [Google Scholar] [CrossRef]
- Song, H.; Ni, Y.; Kokot, S. Investigations of an electrochemical platform based on the layered MoS2–graphene and horseradish peroxidase nanocomposite for direct electrochemistry and electrocatalysis. Biosens. Bioelectron. 2014, 56, 137–143. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Guo, Z.; Hu, Z.; Xue, Z.; Lu, X. Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively. Biosens. Bioelectron. 2017, 87, 101–107. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, Q.; Gong, P.; Liu, B.; Wang, J.; Yang, S. A new enzymatic immobilization carrier based on graphene capsule for hydrogen peroxide biosensors. Electrochim. Acta 2015, 151, 186–194. [Google Scholar] [CrossRef]
- Wu, P.; Cai, Z.; Chen, J.; Zhang, H.; Cai, C. Electrochemical measurement of the flux of hydrogen peroxide releasing from RAW 264.7 macrophage cells based on enzyme-attapulgite clay nanohybrids. Biosens. Bioelectron. 2011, 26, 4012–4017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Yao, D.; Pu, J.; Zhang, Y.; Gao, X.; Sun, Y. Direct electrochemistry of hemoglobin on graphene/Fe3O4 nanocomposite-modified glass carbon electrode and its sensitive detection for hydrogen peroxide. J. Solid State Electrochem. 2013, 17, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Feng, B.; Yang, X.; Yang, P.; Ding, Y.; Chen, Y.; Fei, J. Electrochemical biosensing platform based on carboxymethyl cellulose functionalized reduced graphene oxide and hemoglobin hybrid nanocomposite film. Sens. Actuators B Chem. 2013, 182, 288–293. [Google Scholar] [CrossRef]
- Xie, L.; Xu, Y.; Cao, X. Hydrogen peroxide biosensor based on hemoglobin immobilized at graphene, flower-like zinc oxide, and gold nanoparticles nanocomposite modified glassy carbon electrode. Colloids Surf. B Biointerfaces 2013, 107, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, S.; Tang, M.; Liu, L.; Gao, F.; Wang, Y. Direct electrochemistry of horseradish peroxidase on graphene-modified electrode for electrocatalytic reduction towards H2O2. Electrochim. Acta 2011, 56, 1144–1149. [Google Scholar] [CrossRef]
- Zhang, L.; Han, G.; Liu, Y.; Tang, J.; Tang, W. Immobilizing haemoglobin on gold/graphene–chitosan nanocomposite as efficient hydrogen peroxide biosensor. Sens. Actuators B Chem. 2014, 197, 164–171. [Google Scholar] [CrossRef]
- Liu, H.; Su, X.; Duan, C.; Dong, X.; Zhou, S.; Zhu, Z. Microwave-assisted hydrothermal synthesis of Au NPs–Graphene composites for H2O2 detection. J. Electroanal. Chem. 2014, 731, 36–42. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Chen, S.-M. Simple approach for the immobilization of horseradish peroxidase on poly-l-histidine modified reduced graphene oxide for amperometric determination of dopamine and H2O2. RSC Adv. 2014, 4, 55867–55876. [Google Scholar] [CrossRef]
- Xiong, W.; Qu, Q.; Liu, S. Self-assembly of ultra-small gold nanoparticles on an indium tin oxide electrode for the enzyme-free detection of hydrogen peroxide. Mikrochim. Acta 2014, 181, 983–989. [Google Scholar] [CrossRef]
- Sheng, Q.; Wang, M.; Zheng, J. A novel hydrogen peroxide biosensor based on enzymatically induced deposition of polyaniline on the functionalized graphene–carbon nanotube hybrid materials. Sens. Actuators B Chem. 2011, 160, 1070–1077. [Google Scholar] [CrossRef]
- Zhou, K.; Zhu, Y.; Yang, X.; Luo, J.; Li, C.; Luan, S. A novel hydrogen peroxide biosensor based on Au–graphene–HRP–chitosan biocomposites. Electrochim. Acta 2010, 55, 3055–3060. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Ren, J.; Wang, J.; Wang, E. Mimetic biomembrane–AuNPs–graphene hybrid as matrix for enzyme immobilization and bioelectrocatalysis study. Talanta 2015, 143, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Nandini, S.; Manjunatha, R.; Shanmugam, S.; Melo, J.S.; Suresh, G.S. Electrochemical biosensor for the selective determination of hydrogen peroxide based on the co-deposition of palladium, horseradish peroxidase on functionalized-graphene modified graphite electrode as composite. J. Electroanal. Chem. 2013, 689, 233–242. [Google Scholar] [CrossRef]
- Nalini, S.; Shanmugam, S.; Neelagund, S.E.; Melo, J.S.; Suresh, G.S.; Nandini, S. Amperometric hydrogen peroxide and cholesterol biosensors designed by using hierarchical curtailed silver flowers functionalized graphene and enzymes deposits. J. Solid State Electrochem. 2014, 18, 685–701. [Google Scholar] [CrossRef]
- Huang, K.-J.; Niu, D.-J.; Liu, X.; Wu, Z.-W.; Fan, Y.; Chang, Y.-F.; Wu, Y.-Y. Direct electrochemistry of catalase at amine-functionalized graphene/gold nanoparticles composite film for hydrogen peroxide sensor. Electrochim. Acta 2011, 56, 2947–2953. [Google Scholar] [CrossRef]
- Dinesh, B.; Mani, V.; Saraswathi, R.; Chen, S.-M. Direct electrochemistry of cytochrome c immobilized on a graphene oxide–carbon nanotube composite for picomolar detection of hydrogen peroxide. RSC Adv. 2014, 4, 28229–28237. [Google Scholar] [CrossRef]
- Mani, V.; Dinesh, B.; Chen, S.-M.; Saraswathi, R. Direct electrochemistry of myoglobin at reduced graphene oxide-multiwalled carbon nanotubes-platinum nanoparticles nanocomposite and biosensing towards hydrogen peroxide and nitrite. Biosens. Bioelectron. 2014, 53, 420–427. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Q.; Huang, W.; Zhang, Z.; Xiang, G.; Zhang, C.; Liang, C.; Lian, H.; Peng, J. Green synthesis of porous graphene and its application for sensitive detection of hydrogen peroxide and 2,4-dichlorophenoxyacetic acid. Electrochim. Acta 2019, 295, 615–623. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Zhang, K.; Dong, C.; Subramanian, S.; Butler, D.; Bolotsky, A.; Goodnight, L.; Cheng, Y.; Robinson, J.A. FeSx-graphene heterostructures: Nanofabrication-compatible catalysts for ultra-sensitive electrochemical detection of hydrogen peroxide. Sens. Actuators B Chem. 2019, 285, 631–638. [Google Scholar] [CrossRef]
- Hu, Z.; Dai, Z.; Hu, X.; Yang, B.; Liu, Q.; Gao, C.; Zheng, X.; Yu, Y. A facile preparation of FePt-loaded few-layer MoS2 nanosheets nanocomposites (F-MoS2-FePt NCs) and their application for colorimetric detection of H2O2 in living cells. J. Nanobiotechnol. 2019, 17, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Chen, Y.; Niu, X.; Pan, C.; Chen, H.; Chen, X. High-performance electrochemical biosensor for nonenzymatic H2O2 sensing based on Au@C-Co3O4 heterostructures. Biosens. Bioelectron. 2018, 118, 36–43. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.; Yang, J.; Yin, D.; Guo, L. One-step synthesis of porphyrinic iron-based metal-organic framework/ordered mesoporous carbon for electrochemical detection of hydrogen peroxide in living cells. Sens. Actuators B Chem. 2017, 248, 207–213. [Google Scholar] [CrossRef]
- Shu, Y.; Xu, J.; Chen, J.; Xu, Q.; Xiao, X.; Jin, D.; Pang, H.; Hu, X. Ultrasensitive electrochemical detection of H2O2 in living cells based on ultrathin MnO2 nanosheets. Sens. Actuators B Chem. 2017, 252, 72–78. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Jafari–Asl, M.; Rezaei, B. A novel enzyme-free amperometric sensor for hydrogen peroxide based on Nafion/exfoliated graphene oxide–Co3O4 nanocomposite. Talanta 2013, 103, 322–329. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, A.B.; Saha, N.; Bhadu, G.R.; Adhikary, B. Newly Designed Amperometric Biosensor for Hydrogen Peroxide and Glucose Based on Vanadium Sulfide Nanoparticles. ACS Appl. Nano Mater. 2018, 1, 1339–1347. [Google Scholar] [CrossRef]
- Li, S.-J.; Du, J.-M.; Zhang, J.-P.; Zhang, M.-J.; Chen, J. A glassy carbon electrode modified with a film composed of cobalt oxide nanoparticles and graphene for electrochemical sensing of H2O2. Mikrochim. Acta 2014, 181, 631–638. [Google Scholar] [CrossRef]
- Zheng, L.; Ye, D.; Xiong, L.; Xu, J.; Tao, K.; Zou, Z.; Huang, D.; Kang, X.; Yang, S.; Xia, J. Preparation of cobalt-tetraphenylporphyrin/reduced graphene oxide nanocomposite and its application on hydrogen peroxide biosensor. Anal. Chim. Acta 2013, 768, 69–75. [Google Scholar] [CrossRef]
- Hosu, I.S.; Wang, Q.; Vasilescu, A.; Peteu, S.F.; Raditoiu, V.; Railian, S.; Zaitsev, V.; Turcheniuk, K.; Wang, Q.; Li, M.; et al. Cobalt phthalocyanine tetracarboxylic acid modified reduced graphene oxide: A sensitive matrix for the electrocatalytic detection of peroxynitrite and hydrogen peroxide. RSC Adv. 2015, 5, 1474–1484. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, H.; Yang, X. An amperometric hydrogen peroxide chemical sensor based on graphene-Fe3O4 multilayer films modified ITO electrode. Talanta 2011, 87, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, G.; Wang, G.; Zhao, J.; Hu, M.; Qu, L. A novel nonenzymatic H2O2 sensor based on cobalt hexacyanoferrate nanoparticles and graphene composite modified electrode. Sens. Actuators B Chem. 2015, 208, 593–599. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Thirumalraj, B.; Chen, S.-M.; Karuppiah, C. Electrochemical co-preparation of cobalt sulfide/reduced graphene oxide composite for electrocatalytic activity and determination of H2O2 in biological samples. J. Colloid Interface Sci. 2018, 509, 153–162. [Google Scholar] [CrossRef]
- Karimi, M.A.; Banifatemeh, F.; Hatefi-Mehrjardi, A.; Tavallali, H.; Eshaghia, Z.; Deilamy-Rad, G. A novel rapid synthesis of Fe2O3/graphene nanocomposite using ferrate (VI) and its application as a new kind of nanocomposite modified electrode as electrochemical sensor. Mater. Res. Bull. 2015, 70, 856–864. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, X.; Zheng, J. A non-enzymatic sensor based on Au@Ag nanoparticles with good stability for sensitive detection of H2O2. New J. Chem. 2016, 40, 2115–2120. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, J.; Dong, J.; Cui, Z.; Lu, T.; Zhu, C.; Zhang, D.; Ma, J. Sonochemical fabrication of Fe3O4 nanoparticles on reduced graphene oxide for biosensors. Ultrason. Sonochem. 2013, 20, 872–880. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, Y.; Lu, X.; Zhang, S.; Li, J.; Wei, G.; Su, Z. One-Step Synthesis of Large-Scale Graphene Film Doped with Gold Nanoparticles at Liquid–Air Interface for Electrochemistry and Raman Detection Applications. Langmuir 2014, 30, 8980–8989. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Kong, T.; Yu, X.; Wu, Y.; Zhang, K.; Wang, X. Enhanced nonenzymatic hydrogen peroxide sensing with reduced graphene oxide/ferroferric oxide nanocomposites. Talanta 2012, 89, 417–421. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Zhou, G.; Sui, N.; Gu, Y.; Wan, J. Electrochemical Detection of H2O2 Based on Fe3O4 Nanoparticles with Graphene Oxide and Polyamidoamine Dendrimer. J. Clust. Sci. 2014, 26, 789–798. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Ye, J. Sensitive and Selective Sensing of Hydrogen Peroxide with Iron-Tetrasulfophthalocyanine-Graphene-Nafion Modified Screen-Printed Electrode. Electroanalysis 2012, 24, 1212–1219. [Google Scholar] [CrossRef]
- Palanisamy, S.; Chen, S.-M.; Sarawathi, R. A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sens. Actuators B Chem. 2012, 166–167, 372–377. [Google Scholar] [CrossRef]
- Jiang, B.-B.; Wei, X.-W.; Wu, F.-H.; Wu, K.-L.; Chen, L.; Yuan, G.-Z.; Dong, C.; Ye, Y. A non-enzymatic hydrogen peroxide sensor based on a glassy carbon electrode modified with cuprous oxide and nitrogen-doped graphene in a nafion matrix. Mikrochim. Acta 2014, 181, 1463–1470. [Google Scholar] [CrossRef]
- Xu, F.; Deng, M.; Li, G.; Chen, S.; Wang, L. Electrochemical behavior of cuprous oxide–reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim. Acta 2013, 88, 59–65. [Google Scholar] [CrossRef]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, Z.; Liu, S.; Hao, Q.; Wang, Y.; Li, Q.; Wang, T. A novel nonenzymatic hydrogen peroxide sensor based on MnO2/graphene oxide nanocomposite. Talanta 2010, 82, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xi, J.; Wu, Y.; Liu, H.; Fu, C.; Liu, H.; Xiao, F. High loading MnO2 nanowires on graphene paper: Facile electrochemical synthesis and use as flexible electrode for tracking hydrogen peroxide secretion in live cells. Anal. Chim. Acta 2015, 853, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Y.; Song, J.; Chen, N.; Zhou, J.; Huang, Z.; Ma, Y.; Zhang, L.; Wang, L. MnO2/graphene nanocomposites for nonenzymatic electrochemical detection of hydrogen peroxide. Electroanalysis 2015, 27, 353–359. [Google Scholar] [CrossRef]
- Hassan, M.; Jiang, Y.; Bo, X.; Zhou, M. Sensitive nonenzymatic detection of hydrogen peroxide at nitrogen-doped graphene supported-CoFe nanoparticles. Talanta 2018, 188, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zheng, B.; Gu, Y.; Yan, X.; Zhang, T.; Liu, H.; Xu, H.; Xu, Z.; Li, X.; Zhang, Z. Fabrication of CoNPs-embedded porous carbon composites based on morphochemical imprinting strategy for detection of H2O2 released from living cells. Electrochim. Acta 2019, 321, 134717. [Google Scholar] [CrossRef]
- Benvidi, A.; Nafar, M.T.; Jahanbani, S.; Tezerjani, M.D.; Rezaeinasab, M.; Dalirnasab, S. Developing an electrochemical sensor based on a carbon paste electrode modified with nano-composite of reduced graphene oxide and CuFe2O4 nanoparticles for determination of hydrogen peroxide. Mater. Sci. Eng. C 2017, 75, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Sun, Y.; Jiao, H.; Xu, L.; Lin, M. AuNPs-NH2/Cu-MOF modified glassy carbon electrode as enzyme-free electrochemical sensor detecting H2O2. J. Electroanal. Chem. 2020, 856, 113592. [Google Scholar] [CrossRef]
- Wang, W.; Tang, H.; Wu, Y.; Zhang, Y.; Li, Z. Highly electrocatalytic biosensor based on Hemin@ AuNPs/reduced graphene oxide/chitosan nanohybrids for non-enzymatic ultrasensitive detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2019, 132, 217–223. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, M.; Meng, X.; Xiang, J.; Wang, L.; Ren, Q.; Guo, S. Graphene/Intermetallic PtPb Nanoplates Composites for Boosting Electrochemical Detection of H2O2 Released from Cells. Anal. Chem. 2017, 89, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; He, J.; Pang, P.; Gao, Y.; Hu, Q. Electrochemical behavior of graphene/Nafion/Azure I/Au nanoparticles composites modified glass carbon electrode and its application as nonenzymatic hydrogen peroxide sensor. Electrochim. Acta 2013, 90, 550–555. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Zhang, Y.; Zhang, Z.; Chi, K.; Yuan, H.; Zhao, A.; Ren, J.; Xiao, F.; Wang, S. PtAu alloy nanoflowers on 3D porous ionic liquid functionalized graphene-wrapped activated carbon fiber as a flexible microelectrode for near-cell detection of cancer. NPG Asia Mater. 2016, 8, e337. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Xu, C.; Liu, L.; Shi, Y.; Li, S.; Zhang, R.; Zhang, D. Polyethylenimine-bridged graphene oxide–gold film on glassy carbon electrode and its electrocatalytic activity toward nitrite and hydrogen peroxide. Sens. Actuators B 2014, 198, 55–61. [Google Scholar] [CrossRef]
- Lorestani, F.; Shahnavaz, Z.; Mn, P.; Alias, Y.; Manan, N.S.A. One-step hydrothermal green synthesis of silver nanoparticle-carbon nanotube reduced-graphene oxide composite and its application as hydrogen peroxide sensor. Sens. Actuators B Chem. 2015, 208, 389–398. [Google Scholar] [CrossRef]
- Chang, H.-C.; Wang, X.; Shiu, K.-K.; Zhu, Y.; Wang, J.; Li, Q.; Chen, B.; Jiang, H. Layer-by-layer assembly of graphene, Au and poly (toluidine blue O) films sensor for evaluation of oxidative stress of tumor cells elicited by hydrogen peroxide. Biosens. Bioelectron. 2013, 41, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Shiu, K.-K. Investigation of the optimal weight contents of reduced graphene oxide–gold nanoparticles composites and theirs application in electrochemical biosensors. J. Electroanal. Chem. 2014, 720–721, 84–91. [Google Scholar] [CrossRef]
- Liu, R.; Li, S.; Zhang, G.; Dolbecq, A.; Mialane, P.; Keita, B. Polyoxometalate-Mediated Green Synthesis of Graphene and Metal Nanohybrids: High-Performance Electrocatalysts. J. Clust. Sci. 2014, 25, 711–740. [Google Scholar] [CrossRef]
- Yu, B.; Feng, J.; Liu, S.; Zhang, T. Preparation of reduced graphene oxide decorated with high density Ag nanorods for non-enzymatic hydrogen peroxide detection. RSC Adv. 2013, 3, 14303–14307. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, S.; Zhu, C.; Zhai, Y.; Wang, E. Self-Assembly of Cationic Polyelectrolyte-Functionalized Graphene Nanosheets and Gold Nanoparticles: A Two-Dimensional Heterostructure for Hydrogen Peroxide Sensing. Langmuir 2010, 26, 11277–11282. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sun, Y.; Zhang, Y.; Shi, Y.; Wen, Z.; Li, Z. Graphene–Pt nanocomposite for nonenzymatic detection of hydrogen peroxide with enhanced sensitivity. Electrochem. Commun. 2011, 13, 1131–1134. [Google Scholar] [CrossRef]
- Gao, C.-H.; Zhu, X.-Z.; Zhang, L.; Zhou, D.-Y.; Wang, Z.-K.; Liao, L.-S. Comparative studies on the inorganic and organic p-type dopants in organic light-emitting diodes with enhanced hole injection. Appl. Phys. Lett. 2013, 102, 153301. [Google Scholar] [CrossRef]
- Pang, P.; Yang, Z.; Xiao, S.; Xie, J.; Zhang, Y.; Gao, Y. Nonenzymatic amperometric determination of hydrogen peroxide by graphene and gold nanorods nanocomposite modified electrode. J. Electroanal. Chem. 2014, 727, 27–33. [Google Scholar] [CrossRef]
- Li, X.-R.; Xu, M.-C.; Chen, H.-Y.; Xu, J.-J. Bimetallic Au@Pt@Au core–shell nanoparticles on graphene oxide nanosheets for high-performance H2O2 bi-directional sensing. J. Mater. Chem. B 2015, 3, 4355–4362. [Google Scholar] [CrossRef]
- Yao, L.; Yan, Y.; Lee, J.-M. Synthesis of Porous Pd Nanostructure and Its Application in Enzyme-Free Sensor of Hydrogen Peroxide. ACS Sustain. Chem. Eng. 2017, 5, 1248–1252. [Google Scholar] [CrossRef]
- Fu, L.; Lai, G.; Jia, B.; Yu, A. Preparation and Electrocatalytic Properties of Polydopamine Functionalized Reduced Graphene Oxide-Silver Nanocomposites. Electrocatalysis 2014, 6, 72–76. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Sun, X. Microwave-assisted rapid synthesis of Ag nanoparticles/graphene nanosheet composites and their application for hydrogen peroxide detection. J. Nanopart. Res. 2011, 13, 4539–4548. [Google Scholar] [CrossRef]
- Shan, L.; Liu, H.; Wang, G. Preparation of tungsten-doped BiVO4 and enhanced photocatalytic activity. J. Nanopart. Res. 2015, 17, 181. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.; Zhao, Z.; Guo, L. Highly exposed Pt nanoparticles supported on porous graphene for electrochemical detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2015, 74, 71–77. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Miao, Z.; Ma, M.; Du, X.; Lin, J.; Han, B.; Takahashi, S.; Anzai, J.-I.; Chen, Q. Dual-function amperometric sensors based on poly (diallydimethylammoniun chloride)-functionalized reduced graphene oxide/manganese dioxide/gold nanoparticles nanocomposite. Sens. Actuators B Chem. 2016, 222, 663–673. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Tian, J.; Luo, Y.; Zhang, X.; Sun, X. Aniline as a dispersing and stabilizing agent for reduced graphene oxide and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. J. Colloid Interface Sci. 2011, 363, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kim, K.; Liu, Z.; Feng, H.; Hou, S. Electroless Deposition of Silver Nanoparticles on Graphene Oxide Surface and Its Applications for the Detection of Hydrogen Peroxide. Electroanalysis 2014, 26, 2513–2519. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Zhang, S.; Lu, X.; Li, Q.; Su, Z.; Wei, G. One-pot green synthesis, characterizations, and biosensor application of self-assembled reduced graphene oxide–gold nanoparticle hybrid membranes. J. Mater. Chem. B 2013, 1, 6525–6531. [Google Scholar] [CrossRef]
- Li, S.-J.; Shi, Y.-F.; Liu, L.; Song, L.-X.; Pang, H.; Du, J.-M. Electrostatic self-assembly for preparation of sulfonated graphene/gold nanoparticle hybrids and their application for hydrogen peroxide sensing. Electrochim. Acta 2012, 85, 628–635. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Ji, Y.; Liu, S. Synthesis of Ag nanoparticle–carbon nanotube–reduced graphene oxide hybrids for highly sensitive non-enzymatic hydrogen peroxide detection. RSC Adv. 2015, 5, 39037–39041. [Google Scholar] [CrossRef]
- Nia, P.M.; Lorestani, F.; Meng, W.P.; Alias, Y. A novel non-enzymatic H2O2 sensor based on polypyrrole nanofibers–silver nanoparticles decorated reduced graphene oxide nano composites. Appl. Surf. Sci. 2015, 332, 648–656. [Google Scholar]
- Lu, D.; Zhang, Y.; Lin, S.; Wang, L.; Wang, C. Synthesis of PtAu bimetallic nanoparticles on graphene–carbon nanotube hybrid nanomaterials for nonenzymatic hydrogen peroxide sensor. Talanta 2013, 112, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, J.; Liu, X.; Li, M.; Lu, X. One-pot synthesis of highly dispersed PtAu nanoparticles–CTAB–graphene nanocomposites for nonenzyme hydrogen peroxide sensor. J. Electroanal. Chem. 2015, 751, 1–6. [Google Scholar] [CrossRef]
- Cui, X.; Wu, S.; Li, Y.; Wan, G. Sensing hydrogen peroxide using a glassy carbon electrode modified with in-situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Mikrochim. Acta 2014, 182, 265–272. [Google Scholar] [CrossRef]
- Sun, Y.; He, K.; Zhang, Z.; Zhou, A.; Duan, H. Real-time electrochemical detection of hydrogen peroxide secretion in live cells by Pt nanoparticles decorated graphene–carbon nanotube hybrid paper electrode. Biosens. Bioelectron. 2015, 68, 358–364. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; Liu, L.; Shi, Y.; Wang, H.; Wang, X.; Wang, F.; Yuan, B.; Zhang, D. A novel enzyme-free hydrogen peroxide sensor based on polyethylenimine-grafted graphene oxide-Pd particles modified electrode. J. Electroanal. Chem. 2014, 731, 67–71. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Huang, L.; Wang, J.; Pan, H. Palladium Nanoparticles Embedded into Graphene Nanosheets: Preparation, Characterization, and Nonenzymatic Electrochemical Detection of H2O2. Electroanalysis 2014, 26, 556–564. [Google Scholar] [CrossRef]
- Chen, X.-M.; Cai, Z.-X.; Huang, Z.-Y.; Oyama, M.; Jiang, Y.-Q.; Chen, X. Ultrafine palladium nanoparticles grown on graphene nanosheets for enhanced electrochemical sensing of hydrogen peroxide. Electrochim. Acta 2013, 97, 398–403. [Google Scholar] [CrossRef]
- Sun, W.; Cai, X.; Wang, Z.; Zhao, H.; Lan, M. A novel modification method via in-situ reduction of AuAg bimetallic nanoparticles by polydopamine on carbon fiber microelectrode for H2O2 detection. Microchem. J. 2020, 154, 104595. [Google Scholar] [CrossRef]
- Xie, Y.; Zhan, Y. Electrochemical capacitance of porous reduced graphene oxide/nickel foam. J. Porous Mater. 2015, 22, 403–412. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Hou, J.; Jia, Z.; Zhong, D.; Zhou, S.; Huo, D.; Yang, M.; Hou, C. Electrochemical biointerface based on electrodeposition AuNPs on 3D graphene aerogel: Direct electron transfer of Cytochrome c and hydrogen peroxide sensing. J. Electroanal. Chem. 2019, 842, 16–23. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, D.; Li, C.; Zou, L.; Ye, B. Graphene blended with SnO2 and Pd-Pt nanocages for sensitive non-enzymatic electrochemical detection of H2O2 released from living cells. Anal. Chim. Acta 2018, 1014, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Hu, Y.; Wang, P.; Liu, P.; Chen, Z.; Sun, D. Electrochemical biosensor based on gold nanoflowers-encapsulated magnetic metal-organic framework nanozymes for drug evaluation with in-situ monitoring of H2O2 released from H9C2 cardiac cells. Sens. Actuators B Chem. 2020, 311, 127909. [Google Scholar] [CrossRef]
- Hu, J.; Wisetsuwannaphum, S.; Foord, J.S. Glutamate biosensors based on diamond and graphene platforms. Faraday Discuss. 2014, 172, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Tang, H. Optical determination of glucose and hydrogen peroxide using a nanocomposite prepared from glucose oxidase and magnetite nanoparticles immobilized on graphene oxide. Mikrochim. Acta 2014, 181, 527–534. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S. Graphene Oxide as a Matrix for Enzyme Immobilization. Langmuir 2010, 26, 6083–6085. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Jorio, A. Unusual properties and structure of carbon nanotubes. Annu. Rev. Mater. Res. 2004, 34, 247–278. [Google Scholar] [CrossRef]
- Primo, E.N.; Gutiérrez, F.; Rubianes, M.D.; Ferreyra, N.F.; Rodríguez, M.C.; Pedano, M.L.; Gasnier, A.; Gutierrez, A.; Eguílaz, M.; Dalmasso, P.; et al. Electrochemistry in one dimension: Applications of carbon nanotubes. Electrochem. Carbon Electrodes 2015, 83–120. [Google Scholar] [CrossRef]
- Yu, D.; Wang, P.; Zhao, Y.; Fan, A. Iodophenol blue-enhanced luminol chemiluminescence and its application to hydrogen peroxide and glucose detection. Talanta 2016, 146, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; González-Cortés, A.; Agüí, L.; Pingarrón, J.M. Uncommon carbon nanostructures for the preparation of electrochemical immunosensors. Electroanalysis 2016, 28, 1679–1691. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, Y.; Zhang, R.; Wang, Y.; He, P.; Fang, Y. Direct Electrochemistry and Electrocatalysis of the Hemoglobin Immobilized on Diazonium-Functionalized Aligned Carbon Nanotubes Electrode. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1672–1677. [Google Scholar] [CrossRef]
- Esplandiu, M.J.; Pacios, M.; Cyganek, L.; Bartroli, J.; Del Valle, M. Enhancing the electrochemical response of myoglobin with carbon nanotube electrodes. Nanotechnology 2009, 20, 355502. [Google Scholar] [CrossRef]
- Zhang, H.; Ruan, J.; Liu, W.; Jiang, X.; Du, T.; Jiang, H.; Alberto, P.; Gottschalk, K.-E.; Wang, X. Monitoring dynamic release of intracellular hydrogen peroxide through a microelectrode based enzymatic biosensor. Anal. Bioanal. Chem. 2018, 410, 4509–4517. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, F.; Liu, G. Direct electrochemistry and electrocatalysis of heme proteins on SWCNTs-CTAB modified electrodes. Talanta 2009, 77, 1343–1350. [Google Scholar] [CrossRef]
- Chen, L.; Lu, G. Novel amperometric biosensor based on composite film assembled by polyelectrolyte-surfactant polymer, carbon nanotubes and hemoglobin. Sens. Actuators B Chem. 2007, 121, 423–429. [Google Scholar] [CrossRef]
- Zong, S.; Cao, Y.; Ju, H. Amperometric biosensor for hydrogen peroxide based on myoglobin doped multiwalled carbon nanotube enhanced grafted collagen matrix. Anal. Lett. 2007, 40, 1556–1568. [Google Scholar] [CrossRef]
- Nagaraju, D.; Pandey, R.K.; Lakshminarayanan, V. Electrocatalytic studies of Cytochrome c functionalized single walled carbon nanotubes on self-assembled monolayer of 4-ATP on gold. J. Electroanal. Chem. 2009, 627, 63–68. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Yin, B.; Xu, Y. Multilayer Assembly of Hemoglobin and Colloidal Gold Nanoparticles on Multiwall Carbon Nanotubes/Chitosan Composite for Detecting Hydrogen Peroxide. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 2141–2147. [Google Scholar] [CrossRef]
- Ren, Q.-Q.; Wu, J.; Zhang, W.-C.; Wang, C.; Qin, X.; Liu, G.-C.; Li, Z.-X.; Yu, Y. Real-time in vitro detection of cellular H2O2 under camptothecin stress using horseradish peroxidase, ionic liquid, and carbon nanotube-modified carbon fiber ultramicroelectrode. Sens. Actuators B Chem. 2017, 245, 615–621. [Google Scholar] [CrossRef]
- Pandey, R.R.; Guo, Y.; Gao, Y.; Chusuei, C.C. A Prussian blue ZnO carbon nanotube composite for chronoamperometrically assaying H2O2 in BT20 and 4T1 breast cancer cells. Anal. Chem. 2019, 91, 10573–10581. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Sun, C.; Jiang, X. Carbon dots-decorated multiwalled carbon nanotubes nanocomposites as a high-performance electrochemical sensor for detection of H2O2 in living cells. Anal. Bioanal. Chem. 2016, 408, 4705–4714. [Google Scholar] [CrossRef]
- Sahraoui, Y.; Chaliaa, S.; Maaref, A.; Haddad, A.; Bessueille, F.; Jaffrezic-Renault, N. Synergistic effect of polyoxometalate and single walled carbon nanotubes on peroxidase-like mimics and highly sensitive electrochemical detection of hydrogen peroxide. Electroanalysis 2020, 32, 683–689. [Google Scholar] [CrossRef]
- Liu, J.-X.; Ding, S.-N. Non-enzymatic amperometric determination of cellular hydrogen peroxide using dendrimer-encapsulated Pt nanoclusters/carbon nanotubes hybrid composites modified glassy carbon electrode. Sens. Actuators B Chem. 2017, 251, 200–207. [Google Scholar] [CrossRef]
- Roushani, M.; Bakyas, K.; Dizajdizi, B.Z. Development of sensitive amperometric hydrogen peroxide sensor using a CuNPs/MB/MWCNT-C60-Cs-IL nanocomposite modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 64, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, Y.; Jiang, L.; Chen, S.; Ji, Z.; Hou, C.; Huo, D.; Yang, M. 3D carbon nanotubes spaced graphene aerogel incorporated with prussian blue nanoparticles for real-time detection of H2O2 released from living cells. J. Electrochem. Soc. 2020, 167, 047511. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Sun, Y.; Wang, L.; Dong, X.; Ren, J.; He, W.; Xiao, F. Flexible nanohybrid microelectrode based on carbon fiber wrapped by gold nanoparticles decorated nitrogen doped carbon nanotube arrays: In situ electrochemical detection in live cancer cells. Biosens. Bioelectron. 2018, 100, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, Q.; Liu, Y.; He, T.; Liu, J.; Huang, S.; Tang, H.; Ma, M. Organic electrochemical transistor for in situ detection of H2O2 released from adherent cells and its application in evaluating the in vitro cytotoxicity of nanomaterial. Anal. Chem. 2019, 92, 908–915. [Google Scholar] [CrossRef]
- Tavakkoli, H.; Akhond, M.; Ghorbankhani, G.A.; Absalan, G. Electrochemical sensing of hydrogen peroxide using a glassy carbon electrode modified with multiwalled carbon nanotubes and zein nanoparticle composites: Application to HepG2 cancer cell detection. Mikrochim. Acta 2020, 187, 105. [Google Scholar] [CrossRef]
- Wayu, M.B.; Spidle, R.T.; Devkota, T.; Deb, A.K.; Delong, R.K.; Ghosh, K.C.; Wanekaya, A.K.; Chusuei, C.C. Morphology of hydrothermally synthesized ZnO nanoparticles tethered to carbon nanotubes affects electrocatalytic activity for H2O2 detection. Electrochim. Acta 2013, 97, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Goran, J.M.; Phan, E.N.H.; Favela, C.A.; Stevenson, K.J. H2O2 Detection at carbon nanotubes and nitrogen-doped carbon nanotubes: Oxidation, reduction, or disproportionation? Anal. Chem. 2015, 87, 5989–5996. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Liu, T.; Hei, Y.; Hassan, M.; Zhang, S.; Lin, J.; Wang, T.; Bo, X.; Wang, H.-L.; et al. The biomass of ground cherry husks derived carbon nanoplates for electrochemical sensing. Sens. Actuators B Chem. 2018, 255, 3248–3256. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Li, C.-K.; Yu, J.-G.; Chen, X.-Q. MnO2/reduced graphene oxide nanoribbons: Facile hydrothermal preparation and their application in amperometric detection of hydrogen peroxide. Sens. Actuators B Chem. 2017, 239, 544–552. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, J.; Wang, J.; Xu, J.; Hayat, T.; Alharbi, N.S. Direct electrochemistry of cytochrome c immobilized on one dimensional Au nanoparticles functionalized magnetic N-doped carbon nanotubes and its application for the detection of H2O2. Sens. Actuators B Chem. 2019, 282, 85–95. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Mikrochim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Chou, T.-C.; Wu, K.-Y.; Hsu, F.-X.; Lee, C.-K. Pt-MWCNT modified carbon electrode strip for rapid and quantitative detection of H2O2 in food. J. Food Drug Anal. 2018, 26, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Tabrizi, M.A.; Shamsipur, M.; Saber, R.; Sarkar, S. Flow injection amperometric sandwich-type aptasensor for the determination of human leukemic lymphoblast cancer cells using MWCNTs-Pdnano/PTCA/aptamer as labeled aptamer for the signal amplification. Anal. Chim. Acta 2017, 985, 61–68. [Google Scholar] [CrossRef]
- Werner, E. Determination of cellular H2O2 production. Sci. Signal. 2003, 2003, PL3. [Google Scholar] [CrossRef]
- Ma, Z.; Jiang, M.; Zhu, Q.; Luo, Y.; Chen, G.; Pan, M.; Xie, T.; Huang, X.; Chen, D. A porous hollow fiber sensor for detection of cellular hydrogen peroxide release based on cell-in-lumen configuration. Sens. Actuators B Chem. 2020, 321, 128516. [Google Scholar] [CrossRef]
- Zhao, Y.; Huo, D.; Bao, J.; Yang, M.; Chen, M.; Hou, J.; Fa, H.; Hou, C. Biosensor based on 3D graphene-supported Fe3O4 quantum dots as biomimetic enzyme for in situ detection of H2O2 released from living cells. Sens. Actuators B Chem. 2017, 244, 1037–1044. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, Y.; Wang, Q.; Xiao, J.; Chi, K.; Duan, X.; Chen, J.; Tang, C.; Sun, Y.; Xiao, F.; et al. Multi-element doping design of high-efficient carbocatalyst for electrochemical sensing of cancer cells. Sens. Actuators B Chem. 2018, 273, 108–117. [Google Scholar] [CrossRef]
- Zhao, A.; She, J.; Manoj, D.; Wang, T.; Sun, Y.; Zhang, Y.; Xiao, F. Functionalized graphene fiber modified by dual nanoenzyme: Towards high-performance flexible nanohybrid microelectrode for electrochemical sensing in live cancer cells. Sens. Actuators B Chem. 2020, 310, 127861. [Google Scholar] [CrossRef]
- Yang, Y.; Ohnoutek, L.; Ajmal, S.; Zheng, X.; Feng, Y.; Li, K.; Wang, T.; Deng, Y.; Liu, Y.; Xu, D.; et al. “Hot edges” in an inverse opal structure enable efficient CO2 electrochemical reduction and sensitive in situ Raman characterization. J. Mater. Chem. A 2019, 7, 11836–11846. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Chen, J.; Tong, P.; Huang, L.; Yu, Z.; Tang, D. Ti3C2 MXene nanosheet-based capacitance immunoassay with tyramine-enzyme repeats to detect prostate-specific antigen on interdigitated micro-comb electrode. Electrochim. Acta 2019, 319, 375–381. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Xu, W.; Pan, G.; Zhou, D.; Zhu, J.; Wang, H.; Bai, X.; Dong, B.; Song, H. Ratiometric photoluminescence sensing based on Ti3C2MXene quantum dots as an intracellular pH sensor. Nanoscale 2018, 10, 1111–1118. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Insights into 2D MXenes for versatile biomedical applications: Current advances and challenges ahead. Adv. Sci. 2018, 5, 1800518. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Lin, H.; Xu, G.; Liu, Z.; Wu, R.; Chen, Y. Biocompatible 2D titanium carbide (MXenes) composite nanosheets for pH-responsive MRI-guided tumor hyperthermia. Chem. Mater. 2017, 29, 8637–8652. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Zhang, R.; Zhang, Y.; Wu, L.; Lu, W.; Li, J.; Li, Y.; Zhang, H. MXene-Enabled Electrochemical Microfluidic Biosensor: Applications toward Multicomponent Continuous Monitoring in Whole Blood. Adv. Funct. Mater. 2019, 29, 1807326. [Google Scholar] [CrossRef]
- Guan, Q.; Ma, J.; Yang, W.; Zhang, R.; Zhang, X.; Dong, X.; Fan, Y.; Cai, L.; Cao, Y.; Zhang, Y.; et al. Highly fluorescent Ti3C2 MXene quantum dots for macrophage labeling and Cu2+ ion sensing. Nanoscale 2019, 11, 14123–14133. [Google Scholar] [CrossRef]







| Carbon Material | Sensitivity μA mM−1 cm−2 | Liner Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|---|
| CR-GO | - | 0.05–1500 | 0.05 | [155] |
| Graphene-MWCNT | 32.91 | 20–2100 | 9.4 | [196] |
| rGO/nPPy | 47.69 | 0.1–4 | 0.034 | [201] |
| IL-GR-s-PANI | 280.0 | 0.5–2000 | 0.06 | [202] |
| rGO/Tyrosine | 69.07 | 100–2100 | 80 | [203] |
| Poly(o-Phenylenediamine)/GO | 16.2 | 2.5–25 | 0.84 | [204] |
| BGNs | 266.7 | 1000–20,000 | 3.8 | [199] |
| NB-G | - | 0.5–5000 | 0.05 | [200] |
| GSnano/CS | 18.78 | 5.22–10,430 | 2.6 | [205] |
| GN-HN-SWCNT | 0.015 | 0.2–400 | 0.05 | [206] |
| H-GNs/PEDOT | 235 | 0.1–10 | 0.08 | [207] |
| NS-GQD/G | - | 0.4–33 | 0.026 | [208] |
| Functionalized 3D Graphene | 169.7 | 0.4–660 | 0.08 | [209] |
| rGO/GO hybrid MEA | - | 0.18–9.6 | - | [210] |
| 3D-G/GCE | - | 0.2–41,200 | 0.17 | [211] |
| Graphene-Based Materials | Sensitivity μA mM−1 cm−2 | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|---|
| GE/Fe3O4/Hb GCE | 0.3837 | 100–1700 | 6.00 | [218] |
| rGO-CMC/Hb | - | 0.083–13.94 | 0.08 | [219] |
| Hb/AuNPs/ZnO/Gr | - | 6.0–1130 | 0.8 | [220] |
| HRP/graphene | - | 0.33–14.0 | 0.11 | [221] |
| Hb/Au/GR-CS | 3.47 × 105 | 2.0–935 | 0.35 | [222] |
| Hb/Au NPs-Gr | - | 0.1–70 | 0.03 | [223] |
| HRP/P-L-His-rGO | 2.6 × 105 | 0.2–5000 | 0.05 | [224] |
| GS-PSS/GRCAPS | - | 10–12,000 | 3.3 | [216] |
| HRP/AuNP/ThGP | 0.086 | 0.5–1800 | 0.01 | [225] |
| PANI/HRP/GE-CNT/AuPt NPs | 370 | 0.5–100 | 0.17 | [226] |
| Au/graphene/HRP/CS | - | 5.0–5130 | 1.7 | [227] |
| MP11/DMPG-AuNPs/PDDA-G | 243.7 | 20–280 | 2.6 | [228] |
| (HRP-Pd)/f-graphene | 92.82 | 25–3500 | 0.05 | [229] |
| HRP-f-graphene-Ag | 143.5 | 25–19,350 | 5.0 | [230] |
| HRP/CeO2-rGO | 4.65 | 0.1–500 | 0.021 | [212] |
| HRP-MoS2-Gr | 679.7 | 0.2–1103 | 0.049 | [214] |
| Catalase/AuNPs/graphene-NH2 | 13.4 | 0.3–600 | 0.05 | [231] |
| Cyt c/GO-MWCNT/Au NP | 0.533 | 1 × 10−5–1.4 × 10−4 | 27.7 × 10−6 | [232] |
| RGO-MWCNT-Pt/Mb | 1.990 | 1 × 10−5–1.9 × 10−4 | 16 × 10−6 | [233] |
| PPY-He-RGO | - | 0.1–10 | 0.13 | [41] |
| HRP/PGN/GCE | - | 8.0 ×10−11–6.64 × 10−7 | 2.6 × 10−5 | [215] |
| PGR/catalase/GCE | - | 1.0 × 10−7–7.7 × 10−6 | 1.5 × 10−3 | [234] |
| FeSx/graphene | - | - | 5 × 10−4 | [235] |
| F-MoS2-FePt NCs | - | 8–300 | 2.24 | [236] |
| Graphene-Based Materials | Sensitivity μA mM−1 cm−2 | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|---|
| Nafion/EGO/Co3O4 | 560 | 1–100 | 0.3 | [240] |
| CoHCFNPs/GR | 0.0007 | 0.6–379.5 | 0.1 | [41] |
| VS2 NPs/GCE | 41.96 2 | 0.5–2.5 | 0.224 | [241] |
| CoOxNPs/ERGO | 148.6 | 5–1000 | 0.2 | [242] |
| CoTPP/RGO | 0.0013 | 0.1–4600 | 0.02 | [243] |
| rGO/CoPc-COOH | 14.5 | 100–12,000 | 60 | [244] |
| (PDDA-G/Fe3O4)n | 61.2 | 20–6250 | 2.5 | [245] |
| Fe3O4/GO-PAMAM | 1.385 | 20–1000 | 2.0 | [246] |
| CoS/RGO | 2.519 | 0.1 to 2542.4 | 0.042 | [247] |
| rGO-Fe2O3 | 0.085 | 50–9000 | 6.0 | [248] |
| Fe3O4/rGO | 387.6 | 1–20,000 | 0.17 | [249] |
| Ni2P NA/TM | 690.7 | 0.001–20 | 0.2 | [166] |
| Fe3O4/RGO | 22.27 | 0.5–3000 | 0.18 | [41] |
| Fe3O4/RGO | 0.0468 | 4.0–1000 | 2.0 | [250] |
| PB/TiO2-GR | 480.97 | 0.04–2000 | 0.0086 | [251] |
| RGO/Fe3O4 | 688.0 | 100–6000 | 3.2 | [252] |
| Cu-MOF-GN | 57.73 | 10–11,180 | 2.0 | [33] |
| PFECS/rGO | 117.142 | 10–190 | 1.253 | [253] |
| FeTSPc-GR-Nafion | 36.93 | 0.2–5000 | 0.08 | [254] |
| RGO/ZnO | 13.49 | 0.02–22.48 | 0.02 | [255] |
| Cu2O/N-graphene | 26.67 | 5.0–3570 | 0.8 | [256] |
| Cu2O-rGO | 0.0207 | 30–12,800 | 21.7 | [257] |
| CuS/RGO | 0.035 | 5–1500 | 0.27 | [80] |
| Cu2O/GNs | - | 300–7800 | 20.8 | [258] |
| GO/MnO2 | 38.2 | 5.0–600 | 0.8 | [259] |
| MnO2-ERGO | 59.0 | 100–45,400 | 10 | [260] |
| MnO2 nanosheet/graphene | - | 10–900 | 2.0 | [261] |
| CoFe/NGR | 435.7 | - | 0.28 | [262] |
| Co@NCS | - | 0.5–7500 | 0.08 | [263] |
| H2O2 Biosensors | Sensitivity μA mM−1 cm−2 | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|---|
| GN-HN-SWCNT | 0.015 | 0.2–400 | 0.05 | [206] |
| Cyt c/GO-MWCNT/Au NP | 0.533 | 1 × 10−5–1.4 × 10−4 | 27.7 × 10−6 | [232] |
| HRP/P-L-His-rGO | 2.6 × 105 | 0.2–5000 | 0.05 | [224] |
| HRP/AuNP/ThGP | 0.086 | 0.5–1800 | 0.01 | [225] |
| RGO-MWCNT-Pt/Mb | 1.990 | 1 × 10−5–1.9 × 10−4 | 16 × 10−6 | [233] |
| CoTPP/RGO | 0.0013 | 0.1–4600 | 0.02 | [243] |
| GN-Pt | 0.01 | 2–710 | 0.5 | [277] |
| PDA-RGO/Ag NP | 0.0111 | 0.5–8000 | 2.07 | [282] |
| Pt/GN | 0.0204 | 2.5–6650 | 0.8 | [41] |
| PtAuNPs-CTAB-GR | 0.1654 | 0.005–4.8 | 0.0017 | [294] |
| Pd-NPs/GN | 0.019 | 0.001–2000 | 0.0002 | [298] |
| GCE/MWCNTs-CDs | 0.039 | 3.5–300 | 0.25 | [324] |
| GCE/C60-MWCNTs CS-IL/MB/CuNP | 0.0243 | 2–4 | 0.055 | [327] |
| GCE/rGONRs/MnO2 | 0.0142 | 0.25–2245 | 0.071 | [335] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, T.; Iqbal, A.; Halim, S.A.; Uddin, J.; Khan, A.; El Deeb, S.; Al-Harrasi, A. Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells. Nanomaterials 2022, 12, 1475. https://doi.org/10.3390/nano12091475
Ahmad T, Iqbal A, Halim SA, Uddin J, Khan A, El Deeb S, Al-Harrasi A. Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells. Nanomaterials. 2022; 12(9):1475. https://doi.org/10.3390/nano12091475
Chicago/Turabian StyleAhmad, Touqeer, Ayesha Iqbal, Sobia Ahsan Halim, Jalal Uddin, Ajmal Khan, Sami El Deeb, and Ahmed Al-Harrasi. 2022. "Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells" Nanomaterials 12, no. 9: 1475. https://doi.org/10.3390/nano12091475
APA StyleAhmad, T., Iqbal, A., Halim, S. A., Uddin, J., Khan, A., El Deeb, S., & Al-Harrasi, A. (2022). Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells. Nanomaterials, 12(9), 1475. https://doi.org/10.3390/nano12091475