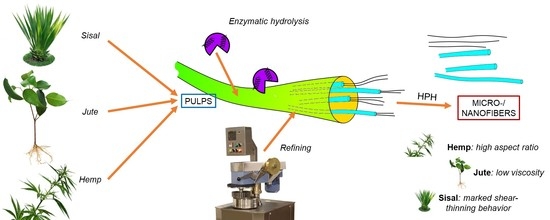
Micro- and Nanofibrillated Cellulose from Annual Plant-Sourced Fibers: Comparison between Enzymatic Hydrolysis and Mechanical Refining
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatments
2.3. Characterization of Pretreated Pulps
2.4. Fibrillation
2.5. Characterization of Micro- and Nanofibrillated Cellulose
3. Results and Discussion
3.1. Pretreated Pulps from Jute, Sisal, and Hemp
3.2. Mechanical Micro- and Nanofibers
3.3. Enzymatic Micro- and Nanofibers
3.4. Effect of Fibrillation Energy on Rheological Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pulp | Pretreatment | Holocellulose (wt.%) | Hemicellulose (wt.%) | Klason Lignin (wt.%) | Soluble Lignin (wt.%) | Extractives (wt.%) | Ashes (wt.%) |
|---|---|---|---|---|---|---|---|
| Jute | Untreated | 81 | 11 | 0.7 | 6.2 | 0.8 | 0.9 |
| Mechanical | 82 | 9.0 | 0.8 | 6.8 | 0.7 | 1.0 | |
| Enzymatic, 80 mg/kg | 80 | 12 | 0.8 | 6.8 | 0.3 | 0.8 | |
| Enzymatic, 240 mg/kg | 82 | 9.3 | 0.6 | 6.7 | 0.3 | 0.9 | |
| Sisal | Untreated | 88 | 13 | 0.7 | 4.6 | 0.9 | 1.0 |
| Mechanical | 87 | 13 | 0.8 | 4.9 | 1.1 | 1.0 | |
| Enzymatic, 80 mg/kg | 89 | 16 | 1.0 | 5.1 | 1.2 | 0.7 | |
| Enzymatic, 240 mg/kg | 88 | 16 | 1.3 | 4.7 | 1.3 | 0.7 | |
| Hemp | Untreated | 87 | 6.6 | 1.3 | 3.3 | 0.8 | 0.6 |
| Mechanical | 87 | 6.8 | 0.9 | 3.1 | 1.2 | 0.7 | |
| Enzymatic, 80 mg/kg | 89 | 5.5 | 1.2 | 3.5 | 0.6 | 0.6 | |
| Enzymatic, 240 mg/kg | 88 | 6.7 | 1.3 | 3.0 | 0.5 | 0.6 |
| Cycles | Cationic Demand (µeq/g) | Carboxyl Content (µeq/g) | C (%) | |
|---|---|---|---|---|
| Jute | 3 | 155 | 47 | 0.95 |
| 3 + 1 | 167 | 0.96 | ||
| 3 + 3 | 182 | 0.97 | ||
| 3 + 3 + 1 | 198 | 0.97 | ||
| 3 + 3 + 3 | 209 | 0.98 | ||
| Sisal | 3 | 131 | 42 | 1.02 |
| 3 + 1 | 145 | 1.02 | ||
| 3 + 3 | 163 | 1.02 | ||
| 3 + 3 + 1 | 181 | 1.03 | ||
| 3 + 3 + 3 | 199 | 1.04 | ||
| Hemp | 3 | 134 | 54 | 0.93 |
| 3 + 1 | 149 | 0.93 | ||
| 3 + 3 | 168 | 0.94 | ||
| 3 + 3 + 1 | 187 | 0.95 | ||
| 3 + 3 + 3 | 205 | 0.95 |
| Dose of Enzyme (mg/kg) | Cycles | Jute | Sisal | Hemp | |||
|---|---|---|---|---|---|---|---|
| Cationic Demand (µeq/g) | C (%) | Cationic Demand (µeq/g) | C (%) | Cationic Demand (µeq/g) | C (%) | ||
| 80 | 3 | 186 | 1.04 | 168 | 1.03 | 151 | 1.02 |
| 3 + 1 | 201 | 1.04 | 179 | 1.03 | 167 | 1.03 | |
| 3 + 3 | 208 | 1.04 | 194 | 1.04 | 184 | 1.05 | |
| 3 + 3 + 1 | 215 | 1.05 | 207 | 1.04 | 201 | 1.05 | |
| 3 + 3 + 3 | 220 | 1.05 | 214 | 1.04 | 210 | 1.06 | |
| 240 | 3 | 204 | 0.95 | 176 | 1.12 | 162 | 0.96 |
| 3 + 1 | 211 | 0.96 | 193 | 1.14 | 189 | 0.97 | |
| 3 + 3 | 223 | 0.98 | 203 | 1.14 | 200 | 0.98 | |
| 3 + 3 + 1 | 226 | 0.98 | 212 | 1.15 | 208 | 0.99 | |
| 3 + 3 + 3 | 233 | 0.99 | 226 | 1.16 | 221 | 1.01 | |
| Number of Passes at P (bar) | Energy Consumption (kWh/kg) | |||
|---|---|---|---|---|
| 300 | 600 | 900 | Non-Cumulative | Cumulative |
| 1 | 0 | 0 | 2.13 | 2.13 |
| 2 | 0 | 0 | 1.80 | 3.93 |
| 3 | 0 | 0 | 1.74 | 5.67 |
| 3 | 1 | 0 | 2.06 | 7.73 |
| 3 | 2 | 0 | 1.94 | 9.67 |
| 3 | 3 | 0 | 2.00 | 11.67 |
| 3 | 3 | 1 | 2.92 | 14.59 |
| 3 | 3 | 2 | 2.42 | 17.01 |
| 3 | 3 | 3 | 2.71 | 19.72 |
| Jute | Sisal | Hemp | ||||
|---|---|---|---|---|---|---|
| Cycles | k (Pa·s−n) | n | k (Pa·s−n) | n | k (Pa·s−n) | n |
| 3 | 0.53 | 0.45 | 0.21 | 0.57 | 0.13 | 0.49 |
| 3 + 1 | 0.75 | 0.38 | 0.27 | 0.56 | 0.19 | 0.46 |
| 3 + 3 | 1.09 | 0.37 | 0.37 | 0.51 | 0.22 | 0.44 |
| 3 + 3 + 1 | 1.23 | 0.32 | 0.38 | 0.43 | 0.33 | 0.36 |
| 3 + 3 + 3 | 2.18 | 0.23 | 0.52 | 0.39 | 0.57 | 0.30 |
| Jute | Sisal | Hemp | ||||
|---|---|---|---|---|---|---|
| Cycles | k (Pa·s−n) | n | k (Pa·s−n) | n | k (Pa·s−n) | n |
| 3 | 1.46 | 0.18 | 0.15 | 0.55 | 0.23 | 0.42 |
| 3 + 1 | 1.88 | 0.18 | 0.20 | 0.51 | 0.28 | 0.33 |
| 3 + 3 | 2.92 | 0.14 | 0.29 | 0.50 | 0.59 | 0.29 |
| 3 + 3 + 1 | 3.58 | 0.14 | 0.36 | 0.46 | 0.72 | 0.29 |
| 3 + 3 + 3 | 4.94 | 0.13 | 0.35 | 0.47 | 1.12 | 0.26 |
References
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in Cellulose Nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Sharma, M.; Aguado, R.; Murtinho, D.; Valente, A.J.M.; Mendes De Sousa, A.P.; Ferreira, P.J.T. A Review on Cationic Starch and Nanocellulose as Paper Coating Components. Int. J. Biol. Macromol. 2020, 162, 578–598. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent Trends and Applications in the Food Industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Noremylia, M.B.; Hassan, M.Z.; Ismail, Z. Recent Advancement in Isolation, Processing, Characterization and Applications of Emerging Nanocellulose: A Review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from Jute Fibers and Their Nanocomposites with Natural Rubber: Preparation and Characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Yang, K.; Chen, W.; Zhao, Y.; Ding, L.; Du, B.; Zhang, S.; Yang, W. Enhancing Dielectric Strength of Thermally Conductive Epoxy Composites by Preventing Interfacial Charge Accumulation Using Micron-Sized Diamond. Compos. Sci. Technol. 2022, 221, 109178. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an Agricultural Waste to a Valuable Pharmaceutical Ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef]
- Gröndahl, J.; Karisalmi, K.; Vapaavuori, J. Micro- and Nanocelluloses from Non-Wood Waste Sources; Processes and Use in Industrial Applications. Soft Matter 2021, 17, 9842–9858. [Google Scholar] [CrossRef] [PubMed]
- Serra-Parareda, F.; Tarrés, Q.; Sanchez-Salvador, J.L.; Campano, C.; Pèlach, M.À.; Mutjé, P.; Negro, C.; Delgado-Aguilar, M. Tuning Morphology and Structure of Non-Woody Nanocellulose: Ranging between Nanofibers and Nanocrystals. Ind. Crops Prod. 2021, 171, 113877. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yashchenko, O.V. Preparation and Application of Nanocellulose from Non-Wood Plants to Improve the Quality of Paper and Cardboard. Appl. Nanosci. 2020, 10, 2705–2716. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Yan, M.; Wang, K.; Jiang, J. Screw Extrusion Pretreatment for High-Yield Lignocellulose Nanofibrils (LCNF) Production from Wood Biomass and Non-Wood Biomass. Carbohydr. Polym. 2022, 277, 118897. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Chen, G. A Comparative Study on the Starch-Based Biocomposite Films Reinforced by Nanocellulose Prepared from Different Non-Wood Fibers. Cellulose 2019, 26, 2425–2435. [Google Scholar] [CrossRef]
- Si, R.; Chen, Y.; Wang, D.; Yu, D.; Ding, Q.; Li, R.; Wu, C. Nanoarchitectonics for High Adsorption Capacity Carboxymethyl Cellulose Nanofibrils-Based Adsorbents for Efficient Cu2+ Removal. Nanomaterials 2022, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aguilar, M.; González, I.; Tarrés, Q.; Alcalà, M.; Pèlach, M.À.; Mutjé, P. Approaching a Low-Cost Production of Cellulose Nanofibers for Papermaking Applications. BioResources 2015, 10, 5330–5344. [Google Scholar] [CrossRef]
- Tarrés, Q.; Aguado, R.; Pèlach, M.À.; Mutjé, P.; Delgado-Aguilar, M. Electrospray Deposition of Cellulose Nanofibers on Paper: Overcoming the Limitations of Conventional Coating. Nanomaterials 2022, 12, 79. [Google Scholar] [CrossRef]
- De Assis, C.A.; Iglesias, M.C.; Bilodeau, M.; Johnson, D.; Phillips, R.; Peresin, M.S.; Bilek, E.M.T.; Rojas, O.J.; Venditti, R.; Gonzalez, R. Cellulose Micro- and Nanofibrils (CMNF) Manufacturing—Financial and Risk Assessment. Biofuels Bioprod. Biorefin. 2018, 12, 251–264. [Google Scholar] [CrossRef]
- Balea, A.; Fuente, E.; Monte, M.C.; Merayo, N.; Campano, C.; Negro, C.; Blanco, A. Industrial Application of Nanocelluloses in Papermaking: A Review of Challenges, Technical Solutions, and Market Perspectives. Molecules 2020, 25, 526. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Salvador, J.L.; Campano, C.; Negro, C.; Monte, M.C.; Blanco, A. Increasing the Possibilities of TEMPO-Mediated Oxidation in the Production of Cellulose Nanofibers by Reducing the Reaction Time and Reusing the Reaction Medium. Adv. Sustain. Syst. 2021, 5, 2000277. [Google Scholar] [CrossRef]
- Ribeiro, R.S.A.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N. Production of Nanocellulose by Enzymatic Hydrolysis: Trends and Challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T.; et al. Transparent and Conductive Paper from Nanocellulose Fibers. Energy Environ. Sci. 2013, 6, 513–518. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra-Parareda, F.; Aguado, R.; Arfelis, S.; Xifré, R.; Fullana-i-Palmer, P.; Delgado-Aguilar, M. Techno-Economic and Environmental Evaluation of a Market Pulp Reinforced with Micro-/Nanofibers as a Strengthening Agent in Packaging Paper. J. Clean. Prod. 2022, 347, 131265. [Google Scholar] [CrossRef]
- Winter, A.; Gindl-Altmutter, W.; Mandlez, D.; Bauer, W.; Eckhart, R.; Leitner, J.; Veigel, S. Reinforcement Effect of Pulp Fines and Microfibrillated Cellulose in Highly Densified Binderless Paperboards. J. Clean. Prod. 2021, 281, 125258. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Aguado, R.; Tarrés, Q.; Mutjé, P.; Delgado-Aguilar, M. Chemical-Free Production of Lignocellulosic Micro-and Nanofibers from High-Yield Pulps: Synergies, Performance, and Feasibility. J. Clean. Prod. 2021, 313, 127914. [Google Scholar] [CrossRef]
- Lourenço, A.F.; Gamelas, J.A.F.; Sarmento, P.; Ferreira, P.J.T. Cellulose Micro and Nanofibrils as Coating Agent for Improved Printability in Office Papers. Cellulose 2020, 27, 6001–6010. [Google Scholar] [CrossRef]
- Pande, H. Non-Wood Fibre and Global Fibre Supply. Available online: https://www.fao.org/3/w7990e/w7990e08.htm (accessed on 21 January 2022).
- Aguado, R.; Moral, A.; Tijero, A. Cationic Fibers from Crop Residues: Making Waste More Appealing for Papermaking. J. Clean. Prod. 2018, 174, 1503–1512. [Google Scholar] [CrossRef]
- CEPI Key Statistics 2020. Available online: https://www.cepi.org/wp-content/uploads/2021/07/Key-Stats-2020-FINAL.pdf (accessed on 8 January 2022).
- Zhang, J.; Wang, Y.; Gou, Q.; Zhou, W.; Liu, Y.; Xu, J.; Liu, Y.; Zhou, W.; Gong, Z. Consolidated Bioprocessing of Cassava Starch into Microbial Lipid for Biodiesel Production by the Amylolytic Yeast Lipomyces Starkeyi. Ind. Crops Prod. 2022, 177, 114534. [Google Scholar] [CrossRef]
- EUR-LEX. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives; Official Journal of the European Union, 312; European Union: Brussels, Belgium, 2008. [Google Scholar]
- Serra-Parareda, F.; Aguado, R.; Tarrés, Q.; Mutjé, P.; Delgado-Aguilar, M. Potentiometric Back Titration as a Robust and Simple Method for Specific Surface Area Estimation of Lignocellulosic Fibers. Cellulose 2021, 28, 10815–10825. [Google Scholar] [CrossRef]
- ISO. ISO TC/6: Paper, Board and Pulps; International Standardization Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Tarrés, Q.; Saguer, E.; Pèlach, M.A.; Alcalà, M.; Delgado-Aguilar, M.; Mutjé, P. The Feasibility of Incorporating Cellulose Micro/Nanofibers in Papermaking Processes: The Relevance of Enzymatic Hydrolysis. Cellulose 2016, 23, 1433–1445. [Google Scholar] [CrossRef]
- TAPPI. TAPPI Standards, Technical Information Papers, and Useful Methods; Technical Association of the Pulp & Paper Industry: New York, NY, USA, 1019. [Google Scholar]
- Aguado, R.; Moral, A.; López, P.; Mutjé, P.; Tijero, A. Morphological Analysis of Pulps from Orange Tree Trimmings and Its Relation to Mechanical Properties. Measurement 2016, 93, 319–326. [Google Scholar] [CrossRef]
- Twagirayezu, F.J. On Generalized Quantum Scattering Theory: Rayleigh Scattering and Thomson Scattering with a Minimal Length. Phys. Lett. A 2021, 400, 127323. [Google Scholar] [CrossRef]
- Varanasi, S.; He, R.; Batchelor, W. Estimation of Cellulose Nanofibre Aspect Ratio from Measurements of Fibre Suspension Gel Point. Cellulose 2013, 20, 1885–1896. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Monte, M.C.; Negro, C.; Batchelor, W.; Garnier, G.; Blanco, A. Simplification of Gel Point Characterization of Cellulose Nano and Microfiber Suspensions. Cellulose 2021, 28, 6995–7006. [Google Scholar] [CrossRef]
- Jan, R.-L.; Ho, C.-H.; Wang, J.-J.; Tseng, S.-H.; Chang, Y.-S. Associations between Sjögren Syndrome, Sociodemographic Factors, Comorbid Conditions, and Scleritis in a Taiwanese Population-Based Study. J. Pers. Med. 2022, 12, 105. [Google Scholar] [CrossRef]
- Basedow, A.M.; Ebert, K.H.; Hunger, H. Effects of Mechanical Stress on the Reactivity of Polymers: Shear Degradation of Polyacrylamide and Dextran. Macromol. Chem. Phys. 1979, 180, 411–427. [Google Scholar] [CrossRef]
- Li, B.; Bandekar, R.; Zha, Q.; Alsaggaf, A.; Ni, Y. Fiber Quality Analysis: OpTest Fiber Quality Analyzer versus L&W Fiber Tester. Ind. Eng. Chem. Res. 2011, 50, 12572–12578. [Google Scholar] [CrossRef]
- Arantes, V.; Gourlay, K.; Saddler, J.N. The Enzymatic Hydrolysis of Pretreated Pulp Fibers Predominantly Involves “Peeling/Erosion” Modes of Action. Biotechnol. Biofuels 2014, 7, 87. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, P. Chapter 9—Paper Bioprocessing. In Biermann’s Handbook of Pulp and Paper, 3rd ed.; Bajpai, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 187–205. ISBN 978-0-12-814238-7. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, C.; Li, Z.; Li, J. The Fiber Charge Measurement Depending on the Poly-DADMAC Accessibility to Cellulose Fibers. Cellulose 2016, 23, 163–173. [Google Scholar] [CrossRef]
- Balea, A.; Fuente, E.; Tarrés, Q.; Pèlach, M.À.; Mutjé, P.; Delgado-Aguilar, M.; Blanco, A.; Negro, C. Influence of Pretreatment and Mechanical Nanofibrillation Energy on Properties of Nanofibers from Aspen Cellulose. Cellulose 2021, 28, 9187–9206. [Google Scholar] [CrossRef]
- AZO Materials Changing the Properties of Particles to Control Their Rheology. Available online: https://www.azom.com/article.aspx?ArticleID=12304 (accessed on 8 April 2022).
- Wang, T.; Wang, X.; Luo, Z.; Ni, M.; Cen, K. Mechanisms of Viscosity Increase for Nanocolloidal Dispersions. J. Nanosci. Nanotechnol. 2011, 11, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; De La Cruz, J.A.; Ding, L.; Wang, B.; Feng, X.; Mao, Z.; Xu, H.; Sui, X. Rheology of Regenerated Cellulose Suspension and Influence of Sodium Alginate. Int. J. Biol. Macromol. 2020, 148, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Mazhari Mousavi, S.M.; Afra, E.; Tajvidi, M.; Bousfield, D.W.; Dehghani-Firouzabadi, M. Cellulose Nanofiber/Carboxymethyl Cellulose Blends as an Efficient Coating to Improve the Structure and Barrier Properties of Paperboard. Cellulose 2017, 24, 3001–3014. [Google Scholar] [CrossRef]






| Pulp | Pretreatment | Arithmetic Length (µm) | Diameter (µm) | Coarseness (mg/m) | Fines (%) |
|---|---|---|---|---|---|
| Jute | Untreated | 566 | 24.6 | 0.249 | 26.3 |
| Mechanical | 440 | 23.2 | 0.141 | 50.3 | |
| Enzymatic, 80 mg/kg | 492 | 24.1 | 0.177 | 29.5 | |
| Enzymatic, 240 mg/kg | 175 | 21.8 | 0.160 | 37.3 | |
| Sisal | Untreated | 449 | 21.6 | 0.353 | 21.6 |
| Mechanical | 246 | 21.3 | 0.190 | 45.3 | |
| Enzymatic, 80 mg/kg | 287 | 21.1 | 0.265 | 25.3 | |
| Enzymatic, 240 mg/kg | 246 | 21.1 | 0.314 | 39.3 | |
| Hemp | Untreated | 551 | 25.4 | 0.370 | 27.8 |
| Mechanical | 279 | 21.8 | 0.349 | 40.2 | |
| Enzymatic, 80 mg/kg | 309 | 22.8 | 0.355 | 32.8 | |
| Enzymatic, 240 mg/kg | 248 | 24.1 | 0.305 | 44.1 |
| Cycles | SSA (m2/g) | Aspect Ratio | |
|---|---|---|---|
| Jute | 3 | 16.7 | 144 |
| 3 + 1 | 18.6 | 147 | |
| 3 + 3 | 20.9 | 156 | |
| 3 + 3 + 1 | 23.4 | 163 | |
| 3 + 3 + 3 | 25.1 | 164 | |
| Sisal | 3 | 13.8 | 117 |
| 3 + 1 | 16.0 | 123 | |
| 3 + 3 | 18.8 | 139 | |
| 3 + 3 + 1 | 21.5 | 139 | |
| 3 + 3 + 3 | 24.3 | 152 | |
| Hemp | 3 | 12.4 | 188 |
| 3 + 1 | 14.7 | 211 | |
| 3 + 3 | 17.7 | 193 | |
| 3 + 3 + 1 | 20.6 | 228 | |
| 3 + 3 + 3 | 23.4 | 217 |
| Dose of Enzyme (mg/kg) | Cycles | Jute | Sisal | Hemp | |||
|---|---|---|---|---|---|---|---|
| SSA (m2/g) | Aspect Ratio | SSA (m2/g) | Aspect Ratio | SSA (m2/g) | Aspect Ratio | ||
| 80 | 3 | 21.5 | 80 | 19.5 | 64 | 15.0 | 83 |
| 3 + 1 | 23.9 | 92 | 21.2 | 64 | 17.5 | 86 | |
| 3 + 3 | 25.0 | 90 | 23.6 | 67 | 20.2 | 87 | |
| 3 + 3 + 1 | 26.0 | 89 | 25.6 | 74 | 22.8 | 97 | |
| 3 + 3 + 3 | 26.8 | 97 | 26.7 | 78 | 24.2 | 103 | |
| 240 | 3 | 24.3 | 89 | 20.8 | 64 | 16.7 | 81 |
| 3 + 1 | 25.4 | 86 | 23.4 | 64 | 20.9 | 84 | |
| 3 + 3 | 27.3 | 79 | 25.0 | 66 | 22.6 | 89 | |
| 3 + 3 + 1 | 27.7 | 82 | 26.4 | 61 | 23.9 | 99 | |
| 3 + 3 + 3 | 28.8 | 86 | 28.5 | 63 | 25.9 | 103 | |
| Cycles | Energy Consumption (kWh/kg) | Jute | Sisal | Hemp | |||
|---|---|---|---|---|---|---|---|
| k (Pa·s−n) | n | k (Pa·s−n) | n | k (Pa·s−n) | n | ||
| 3 | 155 | 1.44 | 0.17 | 1.63 | 0.21 | 1.06 | 0.40 |
| 3 + 1 | 167 | 2.36 | 0.15 | 1.98 | 0.11 | 1.42 | 0.39 |
| 3 + 3 | 182 | 2.66 | 0.15 | 3.28 | 0.07 | 1.64 | 0.33 |
| 3 + 3 + 1 | 198 | 4.02 | 0.11 | 3.31 | 0.07 | 2.80 | 0.33 |
| 3 + 3 + 3 | 209 | 4.22 | 0.10 | 3.49 | 0.06 | 3.06 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado, R.; Tarrés, Q.; Pèlach, M.À.; Mutjé, P.; de la Fuente, E.; Sanchez-Salvador, J.L.; Negro, C.; Delgado-Aguilar, M. Micro- and Nanofibrillated Cellulose from Annual Plant-Sourced Fibers: Comparison between Enzymatic Hydrolysis and Mechanical Refining. Nanomaterials 2022, 12, 1612. https://doi.org/10.3390/nano12091612
Aguado R, Tarrés Q, Pèlach MÀ, Mutjé P, de la Fuente E, Sanchez-Salvador JL, Negro C, Delgado-Aguilar M. Micro- and Nanofibrillated Cellulose from Annual Plant-Sourced Fibers: Comparison between Enzymatic Hydrolysis and Mechanical Refining. Nanomaterials. 2022; 12(9):1612. https://doi.org/10.3390/nano12091612
Chicago/Turabian StyleAguado, Roberto, Quim Tarrés, Maria Àngels Pèlach, Pere Mutjé, Elena de la Fuente, José L. Sanchez-Salvador, Carlos Negro, and Marc Delgado-Aguilar. 2022. "Micro- and Nanofibrillated Cellulose from Annual Plant-Sourced Fibers: Comparison between Enzymatic Hydrolysis and Mechanical Refining" Nanomaterials 12, no. 9: 1612. https://doi.org/10.3390/nano12091612
APA StyleAguado, R., Tarrés, Q., Pèlach, M. À., Mutjé, P., de la Fuente, E., Sanchez-Salvador, J. L., Negro, C., & Delgado-Aguilar, M. (2022). Micro- and Nanofibrillated Cellulose from Annual Plant-Sourced Fibers: Comparison between Enzymatic Hydrolysis and Mechanical Refining. Nanomaterials, 12(9), 1612. https://doi.org/10.3390/nano12091612