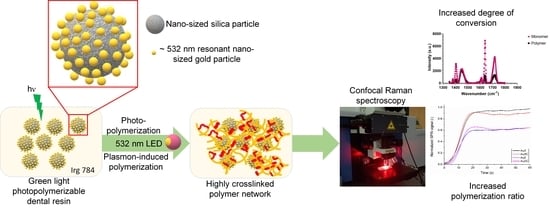
Plasmonic Effect of Gold-Patchy Silica Nanoparticles on Green Light-Photopolymerizable Dental Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Patchy SiO2 NPs
2.2. Synthesis of Patchy SiO2 NPs Containing Nanocomposite
2.3. Instruments for Patchy SiO2 Particles Characterization
2.4. UV-Vis Spectrometry Measurements of Nanocomposites
2.5. Specimen Preparation and Measurements of Diametral Tensile Strength (DTS)
2.6. Differential Scanning Calorimetry (DSC) Measurements of Nanocomposites
2.7. Raman Spectroscopy Measurements of Nanocomposites
2.8. Surface Plasmon Resonance Imaging (SPRi) Measurements of Nanocomposites
2.9. Statistical Analysis
3. Results
3.1. Parameters of Patchy SiO2 Particles: Size and Gold Content
3.2. Results of UV-Vis Spectrometry Measurements
3.3. Results of Diametral Tensile Strength Measurements (DTS)
3.4. Results of Differential Scanning Calorimetry (DSC) Measurements
3.5. Results of Raman Spectroscopy Measurements
3.6. Results of Surface Plasmon Resonance Imaging (SPRi) Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A Review of Dental Composites: Challenges, Chemistry Aspects, Filler Influences, and Future Insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Musanje, L.; Darvell, B.W. Curing-Light Attenuation in Filled-Resin Restorative Materials. Dent. Mater. 2006, 22, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Ruyter, I.E.; Øysæd, H. Conversion in Different Depths of Ultraviolet and Visible Light Activated Composite Materials. Acta Odontol. Scand. 1982, 40, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional Fillers for Dental Resin Composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef]
- Roy, M. Recent Progress on the Application of the Polyaniline-Pd Catalysts for C-C Cross-Coupling Bond Forming Reactions: Trend and Future Analysis. Curr. Green Chem. 2022, 9, 74–93. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, Y.; Zhu, X.; Yu, L. Polyaniline-Supported Nano Metal-Catalyzed Coupling Reactions: Opportunities and Challenges. Chin. Chem. Lett. 2023, 34, 107728. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Motahharifar, N.; Ghorbannezhad, F.; Soheili Bidgoli, N.S.; Baran, T.; Varma, R.S. Recent Advances in Polymer Supported Palladium Complexes as (Nano)Catalysts for Sonogashira Coupling Reaction. Mol. Catal. 2020, 480, 110645. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; Girard, C. Heat Generation in Plasmonic Nanostructures: Influence of Morphology. Appl. Phys. Lett. 2009, 94, 153109. [Google Scholar] [CrossRef]
- De Sio, L.; Placido, T.; Comparelli, R.; Lucia Curri, M.; Striccoli, M.; Tabiryan, N.; Bunning, T.J. Next-Generation Thermo-Plasmonic Technologies and Plasmonic Nanoparticles in Optoelectronics. Prog. Quantum Electron. 2015, 41, 23–70. [Google Scholar] [CrossRef]
- Asmussen, S.V.; Arenas, G.F.; Vallo, C.I. Enhanced Degree of Polymerization of Methacrylate and Epoxy Resins by Plasmonic Heating of Embedded Silver Nanoparticles. Prog. Org. Coat. 2015, 88, 220–227. [Google Scholar] [CrossRef]
- Dong, J.; Firestone, G.E.; Bochinski, J.R.; Clarke, L.I.; Gorga, R.E. In Situ Curing of Liquid Epoxy via Gold-Nanoparticle Mediated Photothermal Heating. Nanotechnology 2017, 28, 065601. [Google Scholar] [CrossRef]
- Bukovinszky, K.; Szalóki, M.; Csarnovics, I.; Bonyár, A.; Petrik, P.; Kalas, B.; Daróczi, L.; Kéki, S.; Kökényesi, S.; Hegedűs, C. Optimization of Plasmonic Gold Nanoparticle Concentration in Green LED Light Active Dental Photopolymer. Polymers 2021, 13, 275. [Google Scholar] [CrossRef]
- Dobrowolska, P.; Krajewska, A.; Gajda-Rączka, M.; Bartosewicz, B.; Nyga, P.; Jankiewicz, B. Application of Turkevich Method for Gold Nanoparticles Synthesis to Fabrication of SiO2@Au and TiO2@Au Core-Shell Nanostructures. Materials 2015, 8, 2849–2862. [Google Scholar] [CrossRef]
- Bukovinszky, K.; Szalóki, M.; Csarnovics, I.; Szabó, I.; Kéki, S.; Nagy, M.; Hegedus, C. Green LED as an Effective Light Source for Curing Acrylate-Based Dental Resins in Combination with Irgacure 784. Adv. Condens. Matter Phys. 2018, 2018, 8265305. [Google Scholar] [CrossRef]
- Soares, L.E.S.; Martin, A.A.; Pinheiro, A.L.B. Degree of Conversion of Composite Resin: A Raman Study. J. Clin. Laser Med. Surg. 2003, 21, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Bonyár, A.; Sántha, H.; Ring, B.; Harsányi, G. A Custom-Developed SPRi Instrument for Biosensor Research. In IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2011; Volume 37, pp. 1050–1053. [Google Scholar]
- Barszczewska-Rybarek, I.M. A Guide through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E.; Sądej, M. Photocurable Methacrylate/Nanosilica Composites. Polimery 2008, 53, 321–323. [Google Scholar] [CrossRef]
- Bonyár, A.; Szalóki, M.; Borók, A.; Rigó, I.; Kámán, J.; Zangana, S.; Veres, M.; Rácz, P.; Aladi, M.; Kedves, M.Á.; et al. The Effect of Femtosecond Laser Irradiation and Plasmon Field on the Degree of Conversion of a UDMA-TEGDMA Copolymer Nanocomposite Doped with Gold Nanorods. Int. J. Mol. Sci. 2022, 23, 13575. [Google Scholar] [CrossRef]
- Alarcon, R.T.; Gaglieri, C.; de Oliveira, A.R.; Bannach, G. Use of DSC in Degree of Conversion of Dimethacrylate Polymers: Easier and Faster than MIR Technique. J. Therm. Anal. Calorim. 2018, 132, 1423–1427. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Hayakawa, T.; Nemoto, K. Analysis of Photopolymerization Behavior of UDMA/TEGDMA Resin Mixture and Its Composite by Differential Scanning Calorimetry. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 310–315. [Google Scholar] [CrossRef]
- Wu, K.; Chen, J.; McBride, J.R.; Lian, T. Efficient Hot-Electron Transfer by a Plasmon-Induced Interfacial Charge-Transfer Transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cushing, S.K.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-Induced Resonance Energy Transfer for Solar Energy Conversion. Nat. Photonics 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Minamimoto, H.; Toda, T.; Futashima, R.; Li, X.; Suzuki, K.; Yasuda, S.; Murakoshi, K. Visualization of Active Sites for Plasmon-Induced Electron Transfer Reactions Using Photoelectrochemical Polymerization of Pyrrole. J. Phys. Chem. C 2016, 120, 16051–16058. [Google Scholar] [CrossRef]
- Ding, T.; Mertens, J.; Lombardi, A.; Scherman, O.A.; Baumberg, J.J. Light-Directed Tuning of Plasmon Resonances via Plasmon-Induced Polymerization Using Hot Electrons. ACS Photonics 2017, 4, 1453–1458. [Google Scholar] [CrossRef]
- Fasciani, C.; Alejo, C.J.B.; Grenier, M.; Netto-Ferreira, J.C.; Scaiano, J.C. High-Temperature Organic Reactions at Room Temperature Using Plasmon Excitation: Decomposition of Dicumyl Peroxide. Org. Lett. 2011, 13, 204–207. [Google Scholar] [CrossRef]
- Adleman, J.R.; Boyd, D.A.; Goodwin, D.G.; Psaltis, D. Heterogenous Catalysis Mediated by Plasmon Heating. Nano Lett. 2009, 9, 4417–4423. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Pacioni, N.L.; Larson, D.; Scaiano, J.C. Plasmon-Mediated Photopolymerization Maps Plasmon Fields for Silver Nanoparticles. J. Am. Chem. Soc. 2011, 133, 9160–9163. [Google Scholar] [CrossRef]
- Ueno, K.; Juodkazis, S.; Shibuya, T.; Yokota, Y.; Mizeikis, V.; Sasaki, K.; Misawa, H. Nanoparticle Plasmon-Assisted Two-Photon Polymerization Induced by Incoherent Excitation Source. J. Am. Chem. Soc. 2008, 130, 6928–6929. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhang, S.; Scherman, O.A.; Baumberg, J.J.; Ding, T.; Xu, H. Plasmon-Directed Polymerization: Regulating Polymer Growth with Light. Nano Res. 2018, 11, 6384–6390. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Skouta, R.; Vazquez, H.; Widawsky, J.R.; Schneebeli, S.; Chen, W.; Hybertsen, M.S.; Breslow, R.; Venkataraman, L. In Situ Formation of Highly Conducting Covalent Au-C Contacts for Single-Molecule Junctions. Nat. Nanotechnol. 2011, 6, 353–357. [Google Scholar] [CrossRef]
- Batra, A.; Kladnik, G.; Gorjizadeh, N.; Meisner, J.; Steigerwald, M.; Nuckolls, C.; Quek, S.Y.; Cvetko, D.; Morgante, A.; Venkataraman, L. Trimethyltin-Mediated Covalent Gold-Carbon Bond Formation. J. Am. Chem. Soc. 2014, 136, 12556–12559. [Google Scholar] [CrossRef] [PubMed]
- Burunkova, J.A.; Alkhalil, D.; Svjazhina, D.S.; Bonyár, A.; Csarnovics, I.; Kokenyesi, S. Influence of Gold Nanoparticles in Polymer Nanocomposite on Space-Temporal-Irradiation Dependent Diffraction Grating Recording. Polymer 2021, 214, 123240. [Google Scholar] [CrossRef]
- Burunkova, J.; Ohoueu, M.J.; Csarnovics, I.; Veres, M.; Bonyár, A.; Kokenyesi, S. Peculiarities of Interaction of Gold Nanoparticles with Photoinitiators in Polymer Nanocomposites for Holographic Recording. J. Photochem. Photobiol. A Chem. 2018, 359, 111–120. [Google Scholar] [CrossRef]
- Gellé, A.; Moores, A. Plasmonic Nanoparticles: Photocatalysts with a Bright Future. Curr. Opin. Green Sustain. Chem. 2019, 15, 60–66. [Google Scholar] [CrossRef]
- Cho, J.D.; Ju, H.T.; Hong, J.W. Photocuring Kinetics of UV-Initiated Free-Radical Photopolymerizations with and without Silica Nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 658–670. [Google Scholar] [CrossRef]
- Randolph, L.D.; Palin, W.M.; Leloup, G.; Leprince, J.G. Filler Characteristics of Modern Dental Resin Composites and Their Influence on Physico-Mechanical Properties. Dent. Mater. 2016, 32, 1586–1599. [Google Scholar] [CrossRef]









| Patchy Silica-Filled Nanocomposite | Silica-Filled Nanocomposite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Au1 | Au2 | Au3 | Au4 | Au5 | Au1 CTRL | Au2 CTRL | Au3 CTRL | Au4 CTRL | Au5 CTRL | |
| filler particles (wt%) | 0.0225 | 0.0451 | 0.0901 | 0.1801 | 0.3563 | 0.0225 | 0.0451 | 0.0901 | 0.1801 | 0.3563 |
| Patchy Silica-Filled Nanocomposite | Silica-Filled Nanocomposite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Au1 | Au2 | Au3 | Au4 | Au5 | Au1 CTRL | Au2 CTRL | Au3 CTRL | Au4 CTRL | Au5 CTRL | |
| filler particles (wt%) | 0.0225 | 0.0451 | 0.0901 | 0.1801 | 0.3563 | 0.0225 | 0.0451 | 0.0901 | 0.1801 | 0.3563 |
| Au NPs (wt%) | 0.0052 | 0.0104 | 0.0208 | 0.0416 | 0.0823 | 0 | 0 | 0 | 0 | 0 |
| Patchy Silica-Filled Nanocomposite | Silica-Filled Nanocomposite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Au1 | Au2 | Au3 | Au4 | Au5 | Au1 CTRL | Au2 CTRL | Au3 CTRL | Au4 CTRL | Au5 CTRL | |
| DC (%) | 73.40 * | 75.80 * | 77.50 * | 74.40 * | 72.80 * | 71.30 | 73.30 | 74.60 | 73.40 | 71.10 |
| SD | 0.70 | 0.70 | 0.80 | 0.70 | 0.60 | 0.50 | 0.60 | 0.50 | 0.60 | 0.50 |
| Patchy Silica-Filled Nanocomposite | Silica-Filled Nanocomposite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Au1 | Au2 | Au3 | Au4 | Au5 | Au1 CTRL | Au2 CTRL | Au3 CTRL | Au4 CTRL | Au5 CTRL | |
| n | 0.62 | 0.71 | 1.00 | 0.78 | 0.60 | 0.57 | 0.79 | 0.91 | 0.79 | 0.64 |
| SD | 0.093 | 0.022 | 0.013 | 0.070 | 0.042 | 0.095 | 0.090 | 0.047 | 0.107 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalóki, M.; Csarnovics, I.; Bonyár, A.; Ungor, D.; Csapó, E.; Sápi, A.; Hegedűs, C. Plasmonic Effect of Gold-Patchy Silica Nanoparticles on Green Light-Photopolymerizable Dental Resin. Nanomaterials 2023, 13, 2554. https://doi.org/10.3390/nano13182554
Szalóki M, Csarnovics I, Bonyár A, Ungor D, Csapó E, Sápi A, Hegedűs C. Plasmonic Effect of Gold-Patchy Silica Nanoparticles on Green Light-Photopolymerizable Dental Resin. Nanomaterials. 2023; 13(18):2554. https://doi.org/10.3390/nano13182554
Chicago/Turabian StyleSzalóki, Melinda, István Csarnovics, Attila Bonyár, Ditta Ungor, Edit Csapó, András Sápi, and Csaba Hegedűs. 2023. "Plasmonic Effect of Gold-Patchy Silica Nanoparticles on Green Light-Photopolymerizable Dental Resin" Nanomaterials 13, no. 18: 2554. https://doi.org/10.3390/nano13182554
APA StyleSzalóki, M., Csarnovics, I., Bonyár, A., Ungor, D., Csapó, E., Sápi, A., & Hegedűs, C. (2023). Plasmonic Effect of Gold-Patchy Silica Nanoparticles on Green Light-Photopolymerizable Dental Resin. Nanomaterials, 13(18), 2554. https://doi.org/10.3390/nano13182554