Carbon Dot Emission Enhancement in Covalent Complexes with Plasmonic Metal Nanoparticles
Abstract
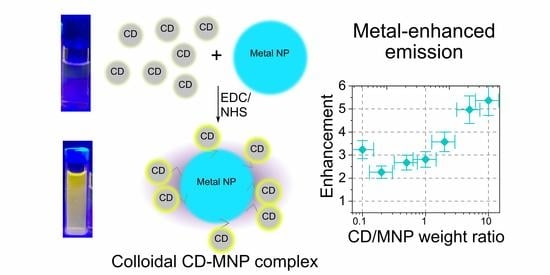
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Experimental Setup
3. Results
3.1. Dynamic Interaction of CDs and MNPs
3.2. Covalent Complexes Based on CDs and MNPs
3.3. Optical Properties of CD–MNP Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ragazzon, G.; Cadranel, A.; Ushakova, E.V.; Wang, Y.; Guldi, D.M.; Rogach, A.L.; Kotov, N.A.; Prato, M. Optical Processes in Carbon Nanocolloids. Chem 2021, 7, 606–628. [Google Scholar] [CrossRef]
- Gong, J.; An, X.; Yan, X. A Novel Rapid and Green Synthesis of Highly Luminescent Carbon Dots with Good Biocompatibility for Cell Imaging. New J. Chem. 2014, 38, 1376–1379. [Google Scholar] [CrossRef]
- Jhonsi, M.A.; Ananth, D.A.; Nambirajan, G.; Sivasudha, T.; Yamini, R.; Bera, S.; Kathiravan, A. Antimicrobial Activity, Cytotoxicity and DNA Binding Studies of Carbon Dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Cailotto, S.; Mazzaro, R.; Enrichi, F.; Vomiero, A.; Selva, M.; Cattaruzza, E.; Cristofori, D.; Amadio, E.; Perosa, A. Design of Carbon Dots for Metal-Free Photoredox Catalysis. ACS Appl. Mater. Interfaces 2018, 10, 40560–40567. [Google Scholar] [CrossRef]
- Javed, N.; O’Carroll, D.M. Carbon Dots and Stability of Their Optical Properties. Part. Part. Syst. Charact. 2021, 38, 2000271. [Google Scholar] [CrossRef]
- He, C.; Xu, P.; Zhang, X.; Long, W. The Synthetic Strategies, Photoluminescence Mechanisms and Promising Applications of Carbon Dots: Current State and Future Perspective. Carbon 2022, 186, 91–127. [Google Scholar] [CrossRef]
- Li, D.; Ushakova, E.V.; Rogach, A.L.; Qu, S. Optical Properties of Carbon Dots in the Deep-Red to Near-Infrared Region Are Attractive for Biomedical Applications. Small 2021, 17, 2102325. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Bose, M.; Das, T.K.; Mondal, S.; Das, A.K.; Das, N.C. Sonochemical Green Reduction to Prepare Ag Nanoparticles Decorated Graphene Sheets for Catalytic Performance and Antibacterial Application. Ultrason. Sonochem. 2017, 39, 577–588. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of Carbon Dots in Optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef]
- Adamczyk, Z. Electrostatic Interactions. In Encyclopedia of Colloid and Interface Science; Tadros, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; p. 362. ISBN 978-3-642-20665-8. [Google Scholar]
- Yan, M.; Qu, L.; Fan, J.; Ren, Y. Electrostatic Complexation of Polyelectrolyte and Magnetic Nanoparticles: From Wild Clustering to Controllable Magnetic Wires. Nanoscale Res. Lett. 2014, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Henderson, M.J.; Wang, K.; Tuo, X.; Leng, Y.; Xiong, K.; Liu, Y.; Ren, Y.; Courtois, J.; Yan, M. Colloidal Assembly of Magnetic Nanoparticles and Polyelectrolytes by Arrested Electrostatic Interaction. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 107–116. [Google Scholar] [CrossRef]
- Kundelev, E.V.; Tepliakov, N.V.; Leonov, M.Y.; Maslov, V.G.; Baranov, A.V.; Fedorov, A.V.; Rukhlenko, I.D.; Rogach, A.L. Toward Bright Red-Emissive Carbon Dots through Controlling Interaction among Surface Emission Centers. J. Phys. Chem. Lett. 2020, 11, 8121–8127. [Google Scholar] [CrossRef]
- Das, A.; Arefina, I.A.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; Parfenov, P.S.; Kuznetsova, V.A.; Ismagilov, A.O.; Litvin, A.P.; Fedorov, A.V.; et al. Chiral Carbon Dots Based on L/D-Cysteine Produced via Room Temperature Surface Modification and One-Pot Carbonization. Nanoscale 2021, 13, 8058–8066. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Wu, H.; Song, X.; Guo, X.; Zhang, D.; Ma, X.; Tan, M. A Mitochondria-Targeted Fluorescent Probe Based on TPP-Conjugated Carbon Dots for Both One- and Two-Photon Fluorescence Cell Imaging. RSC Adv. 2014, 4, 49960–49963. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, K.; Sun, S.; Qian, S.; Wang, Y.; Lin, H. A Conjugated Carbon-Dot–Tyrosinase Bioprobe for Highly Selective and Sensitive Detection of Dopamine. Analyst 2019, 144, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Amat, D.; Peng, Z.; Vanni, S.; Raskin, S.; de Angulo, G.; Othman, A.M.; Graham, R.M.; Leblanc, R.M. Transferrin Conjugated Nontoxic Carbon Dots for Doxorubicin Delivery to Target Pediatric Brain Tumor Cells. Nanoscale 2016, 8, 16662–16669. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple Conjugated Carbon Dots as a Nano-Drug Delivery Model for Glioblastoma Brain Tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Kundelev, E.V.; Khavlyuk, P.D.; Xiong, Y.; Leonov, M.Y.; Zhu, W.; Baranov, A.V.; Fedorov, A.V.; Rogach, A.L.; Rukhlenko, I.D. Sp2-Sp3-Hybridized Atomic Domains Determine Optical Features of Carbon Dots. ACS Nano 2019, 13, 10737–10744. [Google Scholar] [CrossRef]
- Yan, F.; Bai, Z.; Zu, F.; Zhang, Y.; Sun, X.; Ma, T.; Chen, L. Yellow-Emissive Carbon Dots with a Large Stokes Shift Are Viable Fluorescent Probes for Detection and Cellular Imaging of Silver Ions and Glutathione. Microchim. Acta 2019, 186, 113. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Dimos, K. Carbon Quantum Dots: Surface Passivation and Functionalization. Curr. Org. Chem. 2016, 20, 682–695. [Google Scholar] [CrossRef]
- Purcell, E.M. Spontaneous Emission Probabilities at Radio Frequencies. In Confined Electrons and Photons: New Physics and Applications; Burstein, E., Weisbuch, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; p. 839. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Kim, D.H. Surface Plasmon Resonance Mediated Photoluminescence Properties of Nanostructured Multicomponent Fluorophore Systems. Nanoscale 2014, 6, 4966–4984. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; O’Donoghue, M.B.; Huang, Y.F.; Kang, H.; Phillips, J.A.; Chen, X.; Estevez, M.C.; Yang, C.J.; Tan, W. A Surface Energy Transfer Nanoruler for Measuring Binding Site Distances on Live Cell Surfaces. J. Am. Chem. Soc. 2010, 132, 16559–16570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krivenkov, V.; Dyagileva, D.; Samokhvalov, P.; Nabiev, I.; Rakovich, Y. Effect of Spectral Overlap and Separation Distance on Exciton and Biexciton Quantum Yields and Radiative and Nonradiative Recombination Rates in Quantum Dots Near Plasmon Nanoparticles. Ann. Phys. 2020, 532, 2000236. [Google Scholar] [CrossRef]
- Larkin, I.A.; Stockman, M.I.; Achermann, M.; Klimov, V.I. Dipolar Emitters at Nanoscale Proximity of Metal Surfaces: Giant Enhancement of Relaxation in Microscopic Theory. Phys. Rev. B 2004, 69, 121403. [Google Scholar] [CrossRef]
- Jeong, Y.; Kook, Y.M.; Lee, K.; Koh, W.G. Metal Enhanced Fluorescence (MEF) for Biosensors: General Approaches and a Review of Recent Developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Ehrat, F.; Wang, Y.; Milowska, K.Z.; Reckmeier, C.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Carbon Dots: A Unique Fluorescent Cocktail of Polycyclic Aromatic Hydrocarbons. Nano Lett. 2015, 15, 6030–6035. [Google Scholar] [CrossRef]
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y.; et al. Near-Infrared Excitation/Emission and Multiphoton-Induced Fluorescence of Carbon Dots. Adv. Mater. 2018, 30, 1705913. [Google Scholar] [CrossRef]
- Vedernikova, A.A.; Miruschenko, M.D.; Arefina, I.A.; Babaev, A.A.; Stepanidenko, E.A.; Cherevkov, S.A.; Spiridonov, I.G.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; et al. Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: PH and Solvent Polarity Measurement. Nanomaterials 2022, 12, 3314. [Google Scholar] [CrossRef]
- Dubavik, A.; Lesnyak, V.; Gaponik, N.; Eychmüller, A. One-Phase Synthesis of Gold Nanoparticles with Varied Solubility. Langmuir 2011, 27, 10224–10227. [Google Scholar] [CrossRef]
- Oliva, J.M.; Ríos De La Rosa, J.M.; Sayagués, M.J.; Sánchez-Alcázar, J.A.; Merkling, P.J.; Zaderenko, A.P. Solvent-Assisted in Situ Synthesis of Cysteamine-Capped Silver Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 9, 015001. [Google Scholar] [CrossRef]
- Wai, J.L.; New, S.Y. Cysteamine-Coated Gold Nanoparticles for Bimodal Colorimetric Detection with Inverse Sensitivity: A Proof-of-Concept with Lysozyme. RSC Adv. 2020, 10, 1088–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rioux, D.; Vallières, S.; Besner, S.; Muñoz, P.; Mazur, E.; Meunier, M.; Rioux, D.; Vallières, S.; Besner, S.; Meunier, M.; et al. An Analytic Model for the Dielectric Function of Au, Ag, and Their Alloys. Adv. Opt. Mater. 2014, 2, 176–182. [Google Scholar] [CrossRef]
- Khavlyuk, P.D.; Stepanidenko, E.A.; Bondarenko, D.P.; Danilov, D.V.; Koroleva, A.V.; Baranov, A.V.; Maslov, V.G.; Kasak, P.; Fedorov, A.V.; Ushakova, E.V.; et al. The Influence of Thermal Treatment Conditions (Solvothermal: Versus Microwave) and Solvent Polarity on the Morphology and Emission of Phloroglucinol-Based Nitrogen-Doped Carbon Dots. Nanoscale 2021, 13, 3070–3078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, R.X.; Chen, X.; He, X.X.; Han, A.L.; Fang, G.Z.; Liu, J.F.; Wang, S. Study of Efficiency of Coupling Peptides with Gold Nanoparticles. Chin. J. Anal. Chem. 2017, 45, 662–667. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Tait, K.; Liao, X.; Schiferl, D.; Zha, C.; Downs, R.T.; Qian, J.; Zhu, Y.; Shen, T. A Quenchable Superhard Carbon Phase Synthesized by Cold Compression of Carbon Nanotubes. Proc. Natl. Acad. Sci. USA 2004, 101, 13699–13702. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.L.; Wang, J.; Liu, H.Z. FTIR Spectroscopic Investigation of Effects of Temperature and Concentration on PEO-PPO-PEO Block Copolymer Properties in Aqueous Solutions. Macromolecules 2002, 35, 6426–6431. [Google Scholar] [CrossRef]
- Krasnok, A.E.; Slobozhanyuk, A.P.; Simovski, C.R.; Tretyakov, S.A.; Poddubny, A.N.; Miroshnichenko, A.E.; Kivshar, Y.S.; Belov, P.A. An Antenna Model for the Purcell Effect. Sci. Rep. 2015, 5, 12956. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction Coefficient of Gold Nanoparticles with Different Sizes and Different Capping Ligands. Colloids Surf. B Biointerfaces 2007, 58, 3–7. [Google Scholar] [CrossRef]
- Wimuktiwan, P.; Shiowatana, J.; Siripinyanond, A. Investigation of Silver Nanoparticles and Plasma Protein Association Using Flow Field-Flow Fractionation Coupled with Inductively Coupled Plasma Mass Spectrometry (FlFFF-ICP-MS). J. Anal. At. Spectrom. 2014, 30, 245–253. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arefina, I.A.; Kurshanov, D.A.; Vedernikova, A.A.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; Sergeev, A.A.; Fedorov, A.V.; Ushakova, E.V.; Rogach, A.L. Carbon Dot Emission Enhancement in Covalent Complexes with Plasmonic Metal Nanoparticles. Nanomaterials 2023, 13, 223. https://doi.org/10.3390/nano13020223
Arefina IA, Kurshanov DA, Vedernikova AA, Danilov DV, Koroleva AV, Zhizhin EV, Sergeev AA, Fedorov AV, Ushakova EV, Rogach AL. Carbon Dot Emission Enhancement in Covalent Complexes with Plasmonic Metal Nanoparticles. Nanomaterials. 2023; 13(2):223. https://doi.org/10.3390/nano13020223
Chicago/Turabian StyleArefina, Irina A., Danil A. Kurshanov, Anna A. Vedernikova, Denis V. Danilov, Aleksandra V. Koroleva, Evgeniy V. Zhizhin, Aleksandr A. Sergeev, Anatoly V. Fedorov, Elena V. Ushakova, and Andrey L. Rogach. 2023. "Carbon Dot Emission Enhancement in Covalent Complexes with Plasmonic Metal Nanoparticles" Nanomaterials 13, no. 2: 223. https://doi.org/10.3390/nano13020223
APA StyleArefina, I. A., Kurshanov, D. A., Vedernikova, A. A., Danilov, D. V., Koroleva, A. V., Zhizhin, E. V., Sergeev, A. A., Fedorov, A. V., Ushakova, E. V., & Rogach, A. L. (2023). Carbon Dot Emission Enhancement in Covalent Complexes with Plasmonic Metal Nanoparticles. Nanomaterials, 13(2), 223. https://doi.org/10.3390/nano13020223