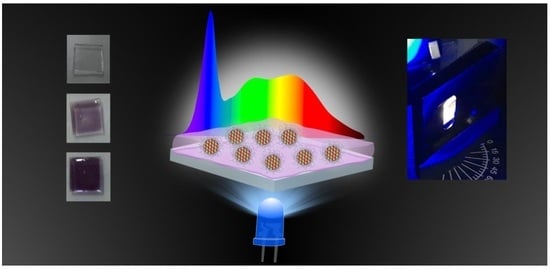
One-Pot Synthesis of Dual Color-Emitting CDs: Numerical and Experimental Optimization towards White LEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Fluorescent CDs
2.3. Transmission Electron Microscopy Investigation of Synthesized CDs
2.4. Spectroscopic Investigation
2.5. Preparation of CDs Powder Fluorophores
2.6. Preparation of CDs Nanocomposite Films
2.7. Scanning Electron Microscopy Investigation of Prepared Nanocomposites
2.8. Analysis of PL Spectra for Measurement of Colorimetric Properties
2.9. Numerical Estimation of Best Achievable White Colorimetric Properties
2.10. Experimental Measurement of Nanocomposite Colorimetric Properties
3. Results and Discussion
3.1. Characterization of CDs Fluorophores
3.2. Numerical Simulations for the Design of CDs Based Nanocomposite Films
3.3. Preparation and Characterization of Nanocomposite Films
3.3.1. Drop-Cast Films
3.3.2. Spin-Coating Deposited Films
3.4. Evaluation of Nanocomposite Film Color Converting Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. C 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Meng, T.; He, P.; Shi, Y.; Li, Y.; Li, X.; Fan, L.; Yang, S. Carbon Quantum Dots: An Emerging Material for Optoelectronic Applications. J. Mater. Chem. C 2019, 7, 6820–6835. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Pan, A.; Yang, B.; He, L.; Wu, Y. Rare Earth-Free Luminescent Materials for WLEDs: Recent Progress and Perspectives. Adv. Mater. Technol. 2021, 6, 2000648. [Google Scholar] [CrossRef]
- Fang, M.-H.; Bao, Z.; Huang, W.-T.; Liu, R.-S. Evolutionary Generation of Phosphor Materials and Their Progress in Future Applications for Light-Emitting Diodes. Chem. Rev. 2022, 122, 11474–11513. [Google Scholar] [CrossRef]
- Trapani, D.; Macaluso, R.; Crupi, I.; Mosca, M. Color Conversion Light-Emitting Diodes Based on Carbon Dots: A Review. Materials 2022, 15, 5450. [Google Scholar] [CrossRef]
- Kim, T.H.; Wang, W.; Li, Q. Advancement in Materials for Energy-Saving Lighting Devices. Front. Chem. Sci. Eng. 2012, 6, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Tang, B.; Wan, H.; Sun, H.; Zhou, S.; Dai, J.; Chen, C.; Liu, S.; Guo, L.J. Boosted Ultraviolet Electroluminescence of InGaN/AlGaN Quantum Structures Grown on High-Index Contrast Patterned Sapphire with Silica Array. Nano Energy 2020, 69, 104427. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, X.; Yan, H.; Chen, Z.; Liu, Y.; Liu, S. Highly Efficient GaN-Based High-Power Flip-Chip Light-Emitting Diodes. Opt. Express OE 2019, 27, A669–A692. [Google Scholar] [CrossRef]
- Zhou, S.; Wan, Z.; Lei, Y.; Tang, B.; Tao, G.; Du, P.; Zhao, X. InGaN Quantum Well with Gradually Varying Indium Content for High-Efficiency GaN-Based Green Light-Emitting Diodes. Opt. Lett. OL 2022, 47, 1291–1294. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, B.; Gong, L.; Bai, J.; Ping, J.; Zhou, S. Rational Construction of Staggered InGaN Quantum Wells for Efficient Yellow Light-Emitting Diodes. Appl. Phys. Lett. 2021, 118, 182102. [Google Scholar] [CrossRef]
- Schanda, J.; International Commission on Illumination (Eds.) Colorimetry: Understanding the CIE System; CIE/Commission Internationale de l’Eclairage: Vienna, Austria; Wiley-Interscience: Hoboken, NJ, USA, 2007; ISBN 978-0-470-04904-4. [Google Scholar]
- Ding, H.; Wei, J.-S.; Zhong, N.; Gao, Q.-Y.; Xiong, H.-M. Highly Efficient Red-Emitting Carbon Dots with Gram-Scale Yield for Bioimaging. Langmuir 2017, 33, 12635–12642. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% Efficient Red Emissive Carbon Quantum Dots for High Color Rendering and Stable Warm White-Light-Emitting Diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Panniello, A.; Madonia, A.; Carbonaro, C.M.; Mocci, F.; Sibillano, T.; Giannini, C.; Comparelli, R.; Ingrosso, C.; Depalo, N.; et al. Photostable Carbon Dots with Intense Green Emission in an Open Reactor Synthesis. Carbon 2022, 198, 230–243. [Google Scholar] [CrossRef]
- Meloni, M.; Stagi, L.; Sanna, D.; Garroni, S.; Calvillo, L.; Terracina, A.; Cannas, M.; Messina, F.; Carbonaro, C.M.; Innocenzi, P.; et al. Harnessing Molecular Fluorophores in the Carbon Dots Matrix: The Case of Safranin O. Nanomaterials 2022, 12, 2351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, S.; Xing, Z.; Wang, H.-B. Turning Waste into Treasure: Chicken Eggshell Membrane Derived Fluorescent Carbon Nanodots for the Rapid and Sensitive Detection of Hg2+ and Glutathione. Analyst 2021, 146, 7250–7256. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.; Xing, Z.; Gao, M.; Sun, M.; Wang, J.; Wang, H.-B. Green Synthesis of Carbon Nanodots for Direct and Rapid Determination of Theophylline through Fluorescence Turn on–off Strategy. Appl. Phys. A 2022, 128, 356. [Google Scholar] [CrossRef]
- Kausar, A. Polymer/Carbon-Based Quantum Dot Nanocomposite: Forthcoming Materials for Technical Application. J. Macromol. Sci. Part A 2019, 56, 341–356. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, S.; Peng, Z.; Leblanc, R. Polymers in Carbon Dots: A Review. Polymers 2017, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Dimos, K. Tuning Carbon Dots’ Optoelectronic Properties with Polymers. Polymers 2018, 10, 1312. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Bai, X.; Bai, J.; Pan, G.; Zhu, Y.; Zhai, Y.; Shao, H.; Chen, X.; Dong, B.; Zhang, H.; et al. Emitting Color Tunable Carbon Dots by Adjusting Solvent towards Light-Emitting Devices. Nanotechnology 2018, 29, 085705. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, X.; Li, D.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Zboril, R.; Rogach, A.L. Full-Color Inorganic Carbon Dot Phosphors for White-Light-Emitting Diodes. Adv. Opt. Mater. 2017, 5, 1700416. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Liu, C.; Li, Y.; Chen, M.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Preparation of Multicolour Solid Fluorescent Carbon Dots for Light-Emitting Diodes Using Phenylethylamine as a Co-Carbonization Agent. Int. J. Mol. Sci. 2022, 23, 11071. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, K.; Yang, Y.; Xie, G.; Ji, X.; He, Z. A Novel Method for the Synthesis of Carbon Dots Assisted by Free Radicals. Nano Res. 2022, 15, 9470–9478. [Google Scholar] [CrossRef]
- Da, X.; Han, Z.; Yang, Z.; Zhang, D.; Hong, R.; Tao, C.; Lin, H.; Huang, Y. Preparation of Multicolor Carbon Dots with High Fluorescence Quantum Yield and Application in White LED. Chem. Phys. Lett. 2022, 794, 139497. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, F.; Xu, J.; Zhang, H.; Chen, L. Solvent-Controlled Synthesis Strategy of Multicolor Emission Carbon Dots and Its Applications in Sensing and Light-Emitting Devices. Nano Res. 2022, 15, 414–422. [Google Scholar] [CrossRef]
- Perikala, M.; Bhardwaj, A. Waste to White Light: A Sustainable Method for Converting Biohazardous Waste to Broadband White LEDs. RSC Adv. 2022, 12, 11443–11453. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Mo, L.; Li, Y.; Pan, X.; Hu, G.; Lei, B.; Zhang, X.; Zheng, M.; Zhuang, J.; Liu, Y.; et al. Construction of Carbon Dots with Color-Tunable Aggregation-Induced Emission by Nitrogen-Induced Intramolecular Charge Transfer. Adv. Mater. 2021, 33, 2104872. [Google Scholar] [CrossRef]
- Wei, C.; Hu, S.; Liang, F.; Song, Z.; Liu, X. One-Pot Synthesis of Concentration and Excitation Dual-Dependency Truly Full-Color Photoluminescence Carbon Dots. Chin. Chem. Lett. 2022, 33, 4116–4120. [Google Scholar] [CrossRef]
- Madhu, M.; Chen, T.-H.; Tseng, W.-L. White-Light Emission of Single Carbon Dots Prepared by Hydrothermal Carbonization of Poly(Diallyldimethylammonium Chloride): Applications to Fabrication of White-Light-Emitting Films. J. Colloid Interface Sci. 2019, 556, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Panniello, A.; Fanizza, E.; Agostiano, A.; Curri, M.L.; Striccoli, M. Oil-Dispersible Green-Emitting Carbon Dots: New Insights on a Facile and Efficient Synthesis. Materials 2020, 13, 3716. [Google Scholar] [CrossRef] [PubMed]
- Panniello, A.; Di Mauro, A.E.; Fanizza, E.; Depalo, N.; Agostiano, A.; Curri, M.L.; Striccoli, M. Luminescent Oil-Soluble Carbon Dots toward White Light Emission: A Spectroscopic Study. J. Phys. Chem. C 2018, 122, 839–849. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, M.; Xiao, Y.; Dong, H.; Zhang, H.; Zhuang, J.; Hu, H.; Lei, B.; Liu, Y. A Self-Quenching-Resistant Carbon-Dot Powder with Tunable Solid-State Fluorescence and Construction of Dual-Fluorescence Morphologies for White Light-Emission. Adv. Mater. 2016, 28, 312–318. [Google Scholar] [CrossRef]
- Ganiga, M.; Cyriac, J. Direct Synthesis of Highly Stable Nitrogen Rich Carbon Dots toward White Light Emission. RSC Adv. 2015, 5, 101333–101337. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Zhang, F.; Chen, L.; Yang, Y.; Liu, X. Fluorescent Polyvinyl Alcohol Films Based on Nitrogen and Sulfur Co-Doped Carbon Dots towards White Light-Emitting Devices. New J. Chem. 2016, 40, 8710–8716. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhen, S.; Li, X.; Zhang, W.; Sun, X.; Xu, B.; Wang, X.; Gao, Z.; Meng, X. Gram-Scale Synthesis of 41% Efficient Single-Component White-Light-Emissive Carbonized Polymer Dots with Hybrid Fluorescence/Phosphorescence for White Light-Emitting Diodes. Adv. Sci. 2020, 7, 1902688. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, Y.; Miao, Y.; He, Y.; Yang, Y.; Liu, X. Optimal Nitrogen and Phosphorus Codoping Carbon Dots towards White Light-Emitting Device. Appl. Phys. Lett. 2016, 109, 083103. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuo, P.; Yin, H.; Fan, Y.; Zhang, J.; Liu, X.; Chen, Z. Solid-State Fluorescent Carbon Dots with Aggregation-Induced Yellow Emission for White Light-Emitting Diodes with High Luminous Efficiencies. ACS Appl. Mater. Interfaces 2019, 11, 24395–24403. [Google Scholar] [CrossRef]
- Ahmad, K.; Pal, A.; Pan, U.N.; Chattopadhyay, A.; Paul, A. Synthesis of Single-Particle Level White-Light-Emitting Carbon Dots via a One-Step Microwave Method. J. Mater. Chem. C 2018, 6, 6691–6697. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Zhuo, P.; Yin, H.; Fan, Y.; Liu, X.; Chen, Z. Carbon Dots Exhibiting Concentration-Dependent Full-Visible-Spectrum Emission for Light-Emitting Diode Applications. ACS Appl. Mater. Interfaces 2019, 11, 46054–46061. [Google Scholar] [CrossRef] [PubMed]
- Gavalas, S.; Kelarakis, A. Towards Red Emissive Systems Based on Carbon Dots. Nanomaterials 2021, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhai, Y.; Li, Z.; Zhu, P.; Mao, S.; Zhu, C.; Du, D.; Belfiore, L.A.; Tang, J.; Lin, Y. Red Carbon Dots: Optical Property Regulations and Applications. Mater. Today 2019, 30, 52–79. [Google Scholar] [CrossRef]
- Lee, J.M.; Yoo, J.G.; Kim, J.S.; Sohn, K.S. A Search for New Red and Green Phosphors Using a Computational Evolutionary Optimization Process. Mater. Sci. Forum 2005, 475–479, 1117–1120. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, L.; Zhang, H.; Zhou, T.; Zheng, P.; Liang, P. An Optimal Spectral Model for Phosphor-Converted White Light-Emitting Diodes Used in the Mesopic Vision. J. Am. Ceram. Soc. 2019, 102, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Sohn, K.-S.; Park, D.H.; Cho, S.H.; Kwak, J.S.; Kim, J.S. Computational Evolutionary Optimization of Red Phosphor for Use in Tricolor White LEDs. Chem. Mater. 2006, 18, 1768–1772. [Google Scholar] [CrossRef]
- Sohn, K.-S.; Kim, B.I.; Shin, N. Genetic Algorithm-Assisted Combinatorial Search for New Red Phosphors of High Efficiency at Soft Ultraviolet Excitation. J. Electrochem. Soc. 2004, 151, H243. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, K.; Zheng, L.; Shih, T.; Lu, Y.; Wu, T.; Lin, Y.; Zhang, Y.; Zheng, J.; Chen, J.; et al. Investigation on Three-Hump Phosphor-Coated White Light-Emitting Diodes for Healthy Lighting by Genetic Algorithm. IEEE Photonics J. 2019, 11, 8200110. [Google Scholar] [CrossRef]
- Cavinato, L.M.; Wölfl, S.; Pöthig, A.; Fresta, E.; Garino, C.; Fernandez-Cestau, J.; Barolo, C.; Costa, R.D. Multivariate Analysis Identifying [Cu(N^N)(P^P)]+ Design and Device Architecture Enables First-Class Blue and White Light-Emitting Electrochemical Cells. Adv. Mater. 2022, 34, 2109228. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, P.; He, G. Optimization of the Light-Emitting Diode Daylight Simulator Based on the CIE Metamerism Index Method. Color Res. Appl. 2022, 47, 65–73. [Google Scholar] [CrossRef]
- Ashdown, I. Solid-State Lighting: A Systems Engineering Approach. Opt. Amp Photonics News OPN 2007, 18, 24–30. [Google Scholar] [CrossRef]
- Xiao, H.; Li, Y.; Li, B.; Wang, G. An Investigation on CCT and Ra Optimization for Trichromatic White LEDs Using a Dual-Weight-Coefficient-Based Algorithm. Micromachines 2022, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Lien, J.-Y.; Chen, C.-J.; Chiang, R.-K.; Wang, S.-L. High Color-Rendering Warm-White Lamps Using Quantum-Dot Color Conversion Films. Opt. Express 2016, 24, A1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Liu, E.; Li, D.; Zhou, X.; Zhou, G.; Xiao, H.; Zhou, D.; Tian, P.; Guo, R.; Qu, S. Highly Emissive Carbon Dots in Solid State and Their Applications in Light-Emitting Devices and Visible Light Communication. ACS Sustain. Chem. Eng. 2019, 7, 9301–9308. [Google Scholar] [CrossRef]
- Qu, Y.; Bai, X.; Li, D.; Zhang, X.; Liang, C.; Zheng, W.; Qu, S. Solution-Processable Carbon Dots with Efficient Solid-State Red/near-Infrared Emission. J. Colloid Interface Sci. 2022, 613, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y.; et al. Near-Infrared Excitation/Emission and Multiphoton-Induced Fluorescence of Carbon Dots. Adv. Mater. 2018, 30, 1705913. [Google Scholar] [CrossRef]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward Efficient Orange Emissive Carbon Nanodots through Conjugated Sp2-Domain Controlling and Surface Charges Engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
- Madonia, A.; Martin-Sabi, M.; Sadaoui, A.; Ruhlmann, L.; Ammar, S.; Schaming, D. Dawson-Type Polyoxometalates Photosensitized with Carbon Dots for Photocatalytic Reduction of Silver Ions. Mater. Res. Bull. 2022, 149, 111721. [Google Scholar] [CrossRef]
- Jiang, K.; Feng, X.; Gao, X.; Wang, Y.; Cai, C.; Li, Z.; Lin, H. Preparation of Multicolor Photoluminescent Carbon Dots by Tuning Surface States. Nanomaterials 2019, 9, 529. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, X.; Xiang, G.; Jiang, S.; Li, L.; Wang, Y.; Li, Y.; Cao, Z.; Tang, X.; Ling, F.; et al. Full Color Fluorescent Carbon Quantum Dots Synthesized from Triammonium Citrate for Cell Imaging and White LEDs. Dye. Pigment. 2021, 193, 109478. [Google Scholar] [CrossRef]
- Aldrich, M. MATLAB Central File Exchange. Pspectro: Photometric and Colorimetric Calculations. Available online: https://www.mathworks.com/matlabcentral/fileexchange/28185-pspectro-photometric-and-colorimetric-calculations (accessed on 13 September 2022).
- Wang, J.; Yang, Y.; Liu, X. Solid-State Fluorescent Carbon Dots: Quenching Resistance Strategies, High Quantum Efficiency Control, Multicolor Tuning, and Applications. Mater. Adv. 2020, 1, 3122–3142. [Google Scholar] [CrossRef]
- Coblentz Soc. Collection. In Citric Acid in NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2022. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C77929&Mask=80#IR-Spec (accessed on 10 January 2023). [CrossRef]
- Sigma-Aldrich®. Sigma-Aldrich Library of FTIR Spectra; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-37679-8. [Google Scholar]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of Excitation Dependent Fluorescence in Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef]
- Gharat, P.M.; Chethodil, J.M.; Srivastava, A.P.; Praseetha, P.K.; Pal, H.; Choudhury, S.D. An Insight into the Molecular and Surface State Photoluminescence of Carbon Dots Revealed through Solvent-Induced Modulations in Their Excitation Wavelength Dependent Emission Properties. Photochem. Photobiol. Sci. 2019, 18, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Tan, K.; Chen, Q.; Xiong, J.; Gao, L. Origins of Efficient Multiemission Luminescence in Carbon Dots. Chem. Mater. 2019, 31, 4732–4742. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Srivastava, I.; Pan, D.; Gruebele, M. Unraveling the Fluorescence Mechanism of Carbon Dots with Sub -Single-Particle Resolution. ACS Nano 2020, 14, 6127–6137. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, A.; Marino, E.; van Dam, B.; Schall, P.; Cannas, M.; Messina, F. Solvatochromism Unravels the Emission Mechanism of Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3419–3423. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Corpino, R.; Salis, M.; Mocci, F.; Thakkar, S.V.; Olla, C.; Ricci, P.C. On the Emission Properties of Carbon Dots: Reviewing Data and Discussing Models. C 2019, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Nguyen, V.; Si, J.; Yan, L.; Hou, X. Electron–Hole Recombination Dynamics in Carbon Nanodots. Carbon 2015, 95, 659–663. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Świergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdał, D. Luminescence Phenomena of Carbon Dots Derived from Citric Acid and Urea—A Molecular Insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef]
- Yang, X.; Ai, L.; Yu, J.; Waterhouse, G.I.N.; Sui, L.; Ding, J.; Zhang, B.; Yong, X.; Lu, S. Photoluminescence Mechanisms of Red-Emissive Carbon Dots Derived from Non-Conjugated Molecules. Sci. Bull. 2022, 67, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chizhik, A.M.; Karedla, N.; Dekaliuk, M.O.; Gregor, I.; Schuhmann, H.; Seibt, M.; Bodensiek, K.; Schaap, I.A.T.; Schulz, O.; et al. Photoluminescence of Carbon Nanodots: Dipole Emission Centers and Electron–Phonon Coupling. Nano Lett. 2014, 14, 5656–5661. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A. Excitons in Carbonic Nanostructures. C 2019, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A.S.; Kasák, P.; Rogach, A.L. Molecular Fluorescence in Citric Acid-Based Carbon Dots. J. Phys. Chem. C 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Güner, T.; Köseoğlu, D.; Demir, M.M. Multilayer Design of Hybrid Phosphor Film for Application in LEDs. Opt. Mater. 2016, 60, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Guner, T.; Yuce, H.; Tascioglu, D.; Simsek, E.; Savaci, U.; Genc, A.; Turan, S.; Demir, M.M. Optimization and Performance of Nitrogen-Doped Carbon Dots as a Color Conversion Layer for White-LED Applications. Beilstein J. Nanotechnol. 2019, 10, 2004–2013. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-X.; Lin, C.-Y.; Ma, S.-H.; Sun, C.-C. Analysis of Position-Dependent Light Extraction of GaN-Based LEDs. Opt. Express 2005, 13, 4175. [Google Scholar] [CrossRef]
- Jarominski, J. Light Extraction Efficiency from LED Displays with Scattering Optical Cavities. Appl. Opt. 1982, 21, 3190. [Google Scholar] [CrossRef]
- Li, J.-S.; Tang, Y.; Li, Z.-T.; Li, Z.; Ding, X.-R.; Rao, L.-S. Investigation of the Emission Spectral Properties of Carbon Dots in Packaged LEDs Using TiO2 Nanoparticles. IEEE J. Select. Top. Quantum Electron. 2017, 23, 2000507. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Z.; He, L.; Jiao, B.; Hou, X. A Solvent/Non-Solvent System for Achieving Solution-Processed Multilayer Organic Light-Emitting Devices. Thin Solid Film. 2015, 589, 852–856. [Google Scholar] [CrossRef]
- Oleari, C. Standard Colorimetry: Definitions, Algorithms, and Software; John Wiley & Sons, Inc: Chichester, UK, 2016; ISBN 978-1-118-89445-3. [Google Scholar]
- Demir, H.V.; Nizamoglu, S.; Erdem, T.; Mutlugun, E.; Gaponik, N.; Eychmüller, A. Quantum Dot Integrated LEDs Using Photonic and Excitonic Color Conversion. Nano Today 2011, 6, 632–647. [Google Scholar] [CrossRef]
- Ding, W.; Wang, Y.; Chen, H.; Chou, S.Y. Plasmonic Nanocavity Organic Light-Emitting Diode with Significantly Enhanced Light Extraction, Contrast, Viewing Angle, Brightness, and Low-Glare. Adv. Funct. Mater. 2014, 24, 6329–6339. [Google Scholar] [CrossRef]
- Jarominski, J. Light Extraction Efficiency of LED Displays with Perpendicular Parallelepiped Transparent Optical Cavities. Appl. Opt. 1982, 21, 3184. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.; Chapman, M.; Euler, W.B. Modeling of Poly(Methylmethacrylate) Viscous Thin Films by Spin-Coating. Coatings 2021, 11, 198. [Google Scholar] [CrossRef]
- Baschenko, S.M.; Marchenko, L.S. On Raman spectra of water, its structure and dependence on temperature. Semicond. Phys. Quantum Electron. Optoelectron. 2011, 14, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Manifacier, J.C.; Gasiot, J.; Fillard, J.P. A Simple Method for the Determination of the Optical Constants n, h and the Thickness of a Weakly Absorbing Thin Film. J. Phys. E Sci. Instrum. 1976, 9, 1002–1004. [Google Scholar] [CrossRef]








| Sample | CD Powder Concentration (mg/mL) | PVA Concentration (g/mL) | Drop Volume (μL) |
|---|---|---|---|
| DC-A | 100 | 0.4 | 100 |
| DC-B | 50 | 0.4 | 100 |
| DC-C | 50 | 0.2 | 50 |
| DC-D | 2 | 0.2 | 50 |
| DC-E | 1 | 0.4 | 50 |
| Sample | CD Powder Concentration (mg/mL) | PVA Concentration (g/mL) | First Step Spin Speed (rpm) | dUV-Vis (μm) | dSEM (μm) |
|---|---|---|---|---|---|
| SC-A | 100 | 0.4 | 1000 | ~5 | 4.9 ± 0.1 |
| SC-B1 | 50 | 0.4 | 1000 | ~4 | 3.9 ± 0.1 |
| SC-B2 | 50 | 0.4 | 800 | ~4 | 3.9 ± 0.3 |
| Sample | (x, y) | CCT (K) | CRI |
|---|---|---|---|
| DC-A | (0.32, 0.40) | 5913 | 81 |
| DC-B | (0.31, 0.38) | 6341 | 80 |
| DC-C | (0.30, 0.36) | 6902 | 82 |
| DC-D | (0.24, 0.30) | 13,610 | 63 |
| DC-E | (0.22, 0.28) | 19,499 | 59 |
| SC-A | (0.30, 0.34) | 7100 | 77 |
| SC-B1 | (0.27, 0.31) | 9894 | 74 |
| SC-B2 | (0.27, 0.31) | 9894 | 73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minervini, G.; Madonia, A.; Panniello, A.; Fanizza, E.; Curri, M.L.; Striccoli, M. One-Pot Synthesis of Dual Color-Emitting CDs: Numerical and Experimental Optimization towards White LEDs. Nanomaterials 2023, 13, 374. https://doi.org/10.3390/nano13030374
Minervini G, Madonia A, Panniello A, Fanizza E, Curri ML, Striccoli M. One-Pot Synthesis of Dual Color-Emitting CDs: Numerical and Experimental Optimization towards White LEDs. Nanomaterials. 2023; 13(3):374. https://doi.org/10.3390/nano13030374
Chicago/Turabian StyleMinervini, Gianluca, Antonino Madonia, Annamaria Panniello, Elisabetta Fanizza, Maria Lucia Curri, and Marinella Striccoli. 2023. "One-Pot Synthesis of Dual Color-Emitting CDs: Numerical and Experimental Optimization towards White LEDs" Nanomaterials 13, no. 3: 374. https://doi.org/10.3390/nano13030374
APA StyleMinervini, G., Madonia, A., Panniello, A., Fanizza, E., Curri, M. L., & Striccoli, M. (2023). One-Pot Synthesis of Dual Color-Emitting CDs: Numerical and Experimental Optimization towards White LEDs. Nanomaterials, 13(3), 374. https://doi.org/10.3390/nano13030374