Boosting the Initial Coulomb Efficiency of Sisal Fiber-Derived Carbon Anode for Sodium Ion Batteries by Microstructure Controlling
Abstract
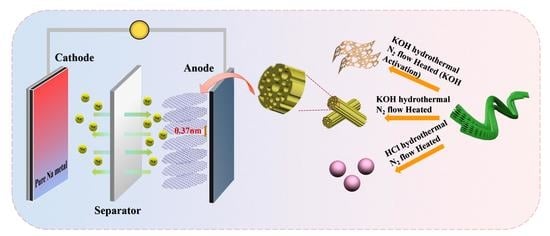
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tubular Sisal Fiber Carbon
2.3. Preparation of Sheet Sisal Fiber Carbon
2.4. Preparation of Sphere Sisal Fiber Carbon
2.5. Material Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
3.1. Morphology and Structure of Carbon Materials
3.2. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, W.; Lei, Y.; Ren, Q.; Fan, L.; Zhang, F.; Shi, Z. Facile hydrothermal treatment route of reed straw-derived hard carbon for high-performance sodium ion battery. Electrochim. Acta 2018, 219, 188–196. [Google Scholar]
- Yang, B.; Wang, J.; Zhu, Y.; Ji, K.; Xia, Y. Engineering hard carbon with high initial coulomb efficiency for practical sodium-ion batteries. J. Power Sources 2021, 492, 229656. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Z.; Chen, S.; Zhang, W.; Liu, X. Electrospinning synthesis of amorphous NiMoO4/graphene dendritic nanofibers as excellent anodes for sodium ion batteries. Nanotechnology 2020, 31, 505401. [Google Scholar] [CrossRef]
- Zhang, L.; He, W.; Ling, M.; Shen, K.; Liu, Y.; Guo, S. Self-standing MgMoO4/Reduced Graphene Oxide Nanosheet Arrays for Lithium and Sodium Ion Storage. ELectrochim. Acta 2017, 252, 322–330. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, Y.; Xiao, J.; Wang, W.; Liu, J. High capacity, reversible alloying reactions in SnSb/C nanocomposites for Na-ion battery applications. Chem. Commun. 2012, 48, 3321–3323. [Google Scholar] [CrossRef]
- Qian, J.; Wu, X.; Cao, Y.; Ai, X.; Yang, H. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angew. Chem. Int. Ed. 2013, 125, 4731–4734. [Google Scholar] [CrossRef]
- Xiao, J.; Mei, D.; Li, X.; Xu, W.; Wang, D.; Graff, G.; Bennett, W.; Nie, Z.; Saraf, L.; Aksay, I.; et al. Hierarchically porous graphene as a lithium-air battery electrode. Nano Lett. 2011, 11, 5071–5078. [Google Scholar] [CrossRef]
- Xiong, H.; Slater, M.D.; Balasubramanian, M.; Johnson, C.S.; Rajh, T. Amorphous TiO2 nanotube anode for rechargeable sodium ion batteries. J. Phys. Chem. Lett. 2011, 2, 2560–2565. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Yin, W.; Hirano, S.I. Carbon-coated graphene/antimony composite with a sandwich-like structure for enhanced sodium storage. J. Mater. Chem. A 2017, 5, 20623–20630. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Status on electrodeposited manganese dioxide and biowaste carbon for hybrid capacitors: The case of high-quality oxide composites, mechanisms, and prospects. J. Energy Storage 2022, 56, 106099. [Google Scholar] [CrossRef]
- Minakshi, M.; Visbal, H.; Mitchell, D.; Fichtner, M. Bio-waste chicken eggshells to store energy. Dalton Trans. 2018, 47, 16828–16834. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.; Dabare, R.; Gao, X.; Jiang, Z.; Wong, K. Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials 2022, 12, 1847. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, X.; Wang, Q.; Xu, X.; Zhou, X.; Bao, J. Kelp-derived hard carbons as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 5761–5769. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Li, Y.M.; Qiao, M.; Hu, Y.S.; Titirici, M.M.; Lieder, M. Hard carbon derived from rice husk as low cost negative electrodes in Na-ion batteries. J. Energy Chem. 2019, 29, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Pei, L.; Cao, H.; Yang, L.; Liu, P.; Zhao, M.; Xu, B.; Guo, J. Hard carbon derived from waste tea biomass as high-performance anode material for sodium-ion batteries. Ionics 2020, 26, 5535–5542. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Yu, C.; Zhang, X.; Lu, T.; Hu, B.; Li, H.; Pan, L. Nitrogen-doped CMs derived from oatmeal as high capacity and superior long life anode material for sodium ion battery. Electrochim. Acta 2016, 191, 385–391. [Google Scholar] [CrossRef]
- Duan, B.; Gao, X.; Yao, X.; Fang, Y.; Huang, L.; Zhou, J.; Zhang, L. Unique elastic N-doped carbon nanofibrous microspheres with hierarchical porosity derived from renewable chitin for high rate supercapacitors. Nano Energy 2016, 27, 482–491. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, K.; Wei, L.; Zhang, X.; Guo, X. Hierarchical porous microspheres of activated carbon with a high surface area from spores for electrochemical double-layer capacitors. J. Mater. Chem. A 2016, 4, 15968–15979. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.; Quan, H.; Huang, Z.; Chen, D.; Guo, L. Nitrogen-doped foam-like carbon plate consisting of carbon tubes as high performance electrode materials for supercapacitors. ChemElectroChem 2016, 3, 814–821. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, A.; Chen, S.; Liao, L.; Zhang, K.; Mo, Z. Hybrid Nanostructures of MoS2/Sisal fiber tubular carbon as anode material for lithium ion batteries. Int. J. Electrochem. Sci. 2018, 13, 2054–2068. [Google Scholar] [CrossRef]
- Wei, L.; Zhuang, S.; Qin, A.; Liao, L.; Chen, S.; Zhang, K. 2D Hybrid nanostructures of MoSe2⊥Sisal fiber activated carbon for enhanced Li storage performance. Mater. Express 2020, 10, 964–973. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, K.; Liao, L.; Chen, S.; Qin, A. Synthesis of nitrogen and sulfur Co-doped sisal fiber carbon and its electrochemical performance in lithium-ion battery. Int. J. Electrochem. Sci. 2019, 14, 102–113. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Chen, J.C.; Wu, M.Q.; Chen, C.; Song, Y.C.; Wang, Y.S. Effects of different atmosphere on electrochemical performance of hard carbon electrode in sodium ion battery. Electron. Mater. Lett. 2019, 15, 428–436. [Google Scholar] [CrossRef]
- Lu, H.; Chen, X.; Jia, Y.; Chen, H.; Wang, Y.; Ai, X.; Yang, H.; Cao, Y. Engineering Al2O3 atomic layer deposition: Enhanced hard carbon-electrolyte interface towards practical sodium ion batteries. Nano Energy 2019, 64, 103903. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and improving the initial coulombic efficiency of high-capacity anode materials for practical sodium ion batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.; Yang, Z.; Liu, J. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy-power rivals lithium ion capacitors. Energy Environ. Sci. 2015, 8, 941–955. [Google Scholar] [CrossRef]
- Simone, V.; Boulineau, A.; De Geyer, A.; Rouchon, D.; Simonin, L.; Martinet, S. Hard carbon derived from cellulose as anode for sodium ion batteries: Dependence of electrochemical properties on structure. J. Energy Chem. 2016, 25, 761–768. [Google Scholar] [CrossRef]
- Wang, P.T.; Wang, H.N.; Liang, C.; Yu, K.F. Two-dimensional porous flake biomass carbon with large layer spacing as an anode material for sodium ion batteries. Diam. Relat. Mater. 2023, 131, 109601. [Google Scholar] [CrossRef]
- Wei, C.H.; Dang, W.L.; Li, M.J.; Ma, X.; Li, M.Q.; Zhang, Y. Hard-soft carbon nanocomposite prepared by pyrolyzing biomass and coal waste as sodium-ion batteries anode material. Mater. Lett. 2023, 330, 133368. [Google Scholar] [CrossRef]
- Lee, G.H.; Moon, S.H.; Kim, M.C.; Kim, S.J.; Choi, S.; Kim, E.S.; Han, S.B.; Park, K.W. Molybdenum carbide embedded in carbon nanofiber as a 3D flexible anode with superior stability and high-rate performance for Li-ion batteries. Ceram. Int. 2018, 44, 7972–7977. [Google Scholar] [CrossRef]
- Chen, X.Y.; Tian, J.Y.; Li, P.; Fang, Y.L.; Fang, Y.J.; Liang, X.M.; Feng, J.W.; Dong, J.; Ai, X.P.; Yang, H.X. Overall understanding of sodium storage behaviors in hard carbons by an “Adsorption-Intercalation/Filling” hybrid mechanism. Adv. Energy Mater. 2022, 12, 2200886. [Google Scholar] [CrossRef]
- Lyu, T.; Wang, R.; Liang, L.; Chen, J.; Shen, P.K. Hierarchical porous oviform carbon capsules with double-layer shells derived from mushroom spores for efficient sodium ion storage. J. Electroanal. Chem. 2020, 8, 114310. [Google Scholar] [CrossRef]
- Wang, P.F.; Zhu, K.; Ye, K.; Gong, Z.; Liu, R.; Cheng, K.; Wang, G.L.; Yan, J.; Cao, D.X. Three-dimensional biomass derived hard carbon with reconstructed surface as a free-standing anode for sodium-ion batteries. J. Colloid Interface Sci. 2020, 561, 203–210. [Google Scholar] [CrossRef]
- Li, X.; Zeng, X.; Ren, T.; Zhao, J.; Zhu, Z.; Sun, S.; Zhang, Y. The transport properties of sodium-ion in the low potential platform region of oatmeal-derived hard carbon for sodium-ion batteries. J. Alloys Compd. 2019, 787, 229–238. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Liu, G.; Wei, M.; Ding, L.-X.; Wang, S.; Wang, H. Porous nitrogen doped carbon sphere as high performance anode of sodium-ion battery. Carbon 2015, 94, 888–894. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Y.; Sun, S.; Huang, Y.; Peng, J.; Luo, J.; Zhang, Q.; Qiu, Y.; Fang, C.; Han, J. Low-cost and high-performance hard carbon anode materials for sodium-ion batteries. ACS Omega 2017, 2, 1687–1695. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, X.C.; Yan, Z.H.; Huang, Z.Y.; Zhou, H.H.; Kuang, Y.F.; Li, H.X. Construction and preparation of nitrogen-doped porous carbon material based on waste biomass for lithium-ion batteries. Int. J. Hydrogen Energy 2021, 46, 17267–17281. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Xiong, J.; Gong, X.; Ciou, J.; Lee, P. Encapsulation of MnS Nanocrystals into N, S-Co-doped Carbon as Anode Material for Full Cell Sodium-Ion Capacitors. Nano-Micro Lett. 2020, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Lei, Y.Y.; Liang, X.M.; Qu, L.; Xu, K.; Hua, Y.N.; Feng, J.W. SnO2 nanoparticles composited with biomass N-doped carbon microspheres as low cost, environmentally friendly and high-performance anode material for sodium-ion and lithium-ion batteries. J. Power Sources 2022, 547, 232032. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.M.; Tong, L.P.; Cao, Y.; Jiao, H.; Long, Z.; Qiu, X.Q. Biomass hard carbon of high initial coulombic efficiency for sodium-ion batteries: Preparation and application. Electrochim. Acta 2022, 410, 140017. [Google Scholar] [CrossRef]
- Zhang, H.; Ming, H.; Zhang, W.; Cao, G.; Yang, Y. Coupled carbonization strategy toward advanced hard carbon for high-energy sodium-ion battery. ACS Appl. Mater. Interfaces 2017, 9, 23766–23774. [Google Scholar] [CrossRef]
- Campbell, B.; Ionescu, R.; Favors, Z.; Ozkan, C.S.; Ozkan, M. Bio-derived; binderless, hierarchically porous carbon anodes for Li-ion batteries. Sci. Rep. 2015, 5, 14575. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Qiu, J.S.; Xu, B. Understanding of sodium storage mechanism in hard carbons: Ongoing development under debate. Adv. Energy Mater. 2022, 12, 200715. [Google Scholar] [CrossRef]
- Yu, K.; Wang, X.; Yang, H.; Bai, Y.; Wu, C. Insight to defects regulation on sugarcane waste-derived hard carbon anode for sodium-ion batteries. J. Energy Chem. 2021, 55, 499–508. [Google Scholar] [CrossRef]
- Yu, K.; Zhao, H.; Wang, X.; Zhang, M.; Dong, R.; Li, Y.; Bai, Y.; Xu, H.; Wu, C. Hyperaccumulation route to Ca-rich hard carbon materials with cation self-incorporation and interlayer spacing optimization for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 10544–10553. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Di, S.; Zhai, B.; Li, L.; Wang, S. Advantageous tubular structure of biomass-derived carbon for high-performance sodium storage. ACS Appl. Energy Mater. 2021, 4, 4955–4965. [Google Scholar] [CrossRef]
- Cheng, H.; Tang, Z.; Luo, X.; Zheng, Z. Spartina alterniflora-derived porous carbon using as anode material for sodium-ion battery. Sci. Total Environ. 2021, 777, 146120. [Google Scholar] [CrossRef]
- Shaji, N.; Ho, C.; Nanthagopal, M.; Santhoshkumar, P.; Sim, G.; Lee, C. Biowaste-derived heteroatoms-doped carbon for sustainable sodium-ion storage. J. Alloys Compd. 2021, 872, 159670. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, X.; Lu, H.; Zhong, F.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. Hard carbon anode derived from camellia seed shell with superior cycling performance for sodium-ion batteries. J. Phys. D Appl. Phys. 2020, 53, 414002. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Xu, Y.; Li, X.; Zhang, K.; Pang, Q.; Qin, A. Boosting the Initial Coulomb Efficiency of Sisal Fiber-Derived Carbon Anode for Sodium Ion Batteries by Microstructure Controlling. Nanomaterials 2023, 13, 881. https://doi.org/10.3390/nano13050881
Luo Y, Xu Y, Li X, Zhang K, Pang Q, Qin A. Boosting the Initial Coulomb Efficiency of Sisal Fiber-Derived Carbon Anode for Sodium Ion Batteries by Microstructure Controlling. Nanomaterials. 2023; 13(5):881. https://doi.org/10.3390/nano13050881
Chicago/Turabian StyleLuo, Yuan, Yaya Xu, Xuenuan Li, Kaiyou Zhang, Qi Pang, and Aimiao Qin. 2023. "Boosting the Initial Coulomb Efficiency of Sisal Fiber-Derived Carbon Anode for Sodium Ion Batteries by Microstructure Controlling" Nanomaterials 13, no. 5: 881. https://doi.org/10.3390/nano13050881
APA StyleLuo, Y., Xu, Y., Li, X., Zhang, K., Pang, Q., & Qin, A. (2023). Boosting the Initial Coulomb Efficiency of Sisal Fiber-Derived Carbon Anode for Sodium Ion Batteries by Microstructure Controlling. Nanomaterials, 13(5), 881. https://doi.org/10.3390/nano13050881