Effect of MnxOy Nanoparticles Stabilized with Methionine on Germination of Barley Seeds (Hordeum vulgare L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MnxOy Nanoparticles
2.2. Samples Measurement
- Accelerating voltage 10 kV;
- Working distance (WD) 4.9 mm;
- In-beam secondary electron detector.
- Copper cathode;
- Emission wavelength 1.54 A;
- Current 35 mA;
- Voltage 40 kV;
- 2θ measurement range 10–90°;
- 2θ sampling frequency: 0.01°;
- Cylinder TL 1–8;
- Temperature 25 °C;
- The rotation speed of 0.1–200 rpm [66].
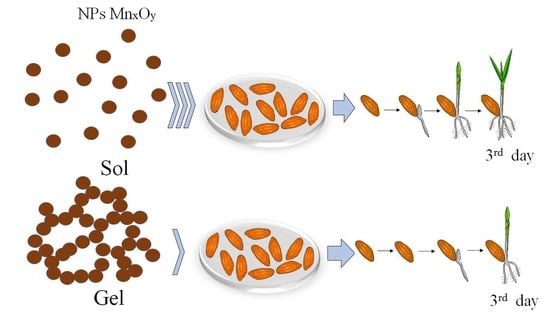
2.3. Treatment of the Hordeum vulgare L. Seeds
2.4. Statistical Data Processing
3. Results and Discussion
3.1. MnxOy Nanoparticles Characterization
3.2. Investigation of the Effect of MnxOy Nanoparticles Stabilized with Methionine on Germination of Barley Seeds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gevers, L.E.; Enakonda, L.R.; Shahid, A.; Ould-Chikh, S.; Silva, C.I.Q.; Paalanen, P.P.; Aguilar-Tapia, A.; Hazemann, J.-L.; Hedhili, M.N.; Wen, F.; et al. Unraveling the structure and role of Mn and Ce for NOx reduction in application-relevant catalysts. Nat. Commun. 2022, 13, 2960. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.; Miraz, S.M.; Khan, M.U.; Karekar, S.C.; Usman, M.; Khan, S.M.; Amin, U.; Rebezov, M.; Shariati, M.A.; Thiruvengadam, M. Heterologous expression and biophysical characterization of a mesophilic tannase following manganese nanoparticle immobilization. Colloids Surf. B Biointerfaces 2021, 207, 112011. [Google Scholar] [CrossRef] [PubMed]
- Koutani, M.; Hayashi, E.; Kamata, K.; Hara, M. Synthesis and Aerobic Oxidation Catalysis of Mesoporous Todorokite-Type Manganese Oxide Nanoparticles by Crystallization of Precursors. J. Am. Chem. Soc. 2022, 144, 14090–14100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liao, C.; Zhao, J.; Zeng, G.; Liu, W.; Gao, P.; Sun, D.; Du, J. Combined Process of Biogenic Manganese Oxide and Manganese-Oxidizing Microalgae for Improved Diclofenac Removal Performance: Two Different Kinds of Synergistic Effects. Toxics 2022, 10, 230. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Kakegawa, T. Mineralogical and Genomic Constraints on the Origin of Microbial Mn Oxide Formation in Complexed Microbial Community at the Terrestrial Hot Spring. Life 2022, 12, 816. [Google Scholar] [CrossRef]
- Srivastava, V.; Beg, M.; Sharma, S.; Choubey, A.K. Application of manganese oxide nanoparticles synthesized via green route for improved performance of water-based drilling fluids. Appl. Nanosci. 2021, 11, 2247–2260. [Google Scholar] [CrossRef]
- Kou, L.; Yang, Y.; Fan, Q.; Wang, J. Carboxyl group-assisted uniform growth of MnOx nanospheres on poly(acrylonitrile) fiber for enhanced removal of organic pollutants. Int. J. Environ. Sci. Technol. 2022, 7, 1–12. [Google Scholar] [CrossRef]
- Kerkar, R.D.; Salker, A.V. Highly active nano-composite of cobalt–copper–manganese oxides for room temperature CO oxidation. Appl. Nanosci. 2021, 11, 2861–2867. [Google Scholar] [CrossRef]
- Pardhiya, S.; Gaharwar, U.S.; Gautam, R.; Priyadarshini, E.; Nirala, J.P.; Rajamani, P. Cumulative effects of manganese nanoparticle and radiofrequency radiation in male Wistar rats. Drug Chem. Toxicol. 2022, 45, 1395–1407. [Google Scholar] [CrossRef]
- Khan, I.; Sadiq, M.; Khan, I.; Saeed, K. Manganese dioxide nanoparticles/activated carbon composite as efficient UV and visible-light photocatalyst. Environ. Sci. Pollut. Res. 2019, 26, 5140–5154. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, F.; Ji, X.; Yu, T.; Qiao, Y.; Yang, B.; Gao, M. An in-plane supercapacitor obtained by facile template method with high performance Mn–Co sulfide-based oxide electrode. Nanotechnology 2022, 33, 485401. [Google Scholar] [CrossRef]
- Chahal, P.; Madaswamy, S.L.; Lee, S.C.; Wabaidur, S.M.; Dhayalan, V.; Ponnusamy, V.K.; Dhanusuraman, R. Novel manganese oxide decorated polyaniline/graphitic carbon nitride nanohybrid material for efficient supercapacitor application. Fuel 2022, 330, 125531. [Google Scholar] [CrossRef]
- Godlaveeti, S.K.; Jangiti, S.; Somala, A.R.; Maseed, H.; Nagireddy, R.R. Different Phase and Morphology Effect of Manganese Oxide on Electrochemical Performance for Supercapacitor Application. J. Clust. Sci. 2021, 32, 703–710. [Google Scholar] [CrossRef]
- Ali, L.I.A.; Ismail, H.K.; Alesary, H.F.; Aboul-Enein, H.Y. A nanocomposite based on polyaniline, nickel and manganese oxides for dye removal from aqueous solutions. Int. J. Environ. Sci. Technol. 2021, 18, 2031–2050. [Google Scholar] [CrossRef]
- Islam, M.A.; Ali, I.; Karim, S.M.A.; Firoz, M.S.H.; Chowdhury, A.-N.; Morton, D.W.; Angove, M.J. Removal of dye from polluted water using novel nano manganese oxide-based materials. J. Water Process. Eng. 2019, 32, 100911. [Google Scholar] [CrossRef]
- Kim, E.-J.; Oh, D.; Lee, C.-S.; Gong, J.; Kim, J.; Chang, Y.-S. Manganese oxide nanorods as a robust Fenton-like catalyst at neutral pH: Crystal phase-dependent behavior. Catal. Today 2017, 282, 71–76. [Google Scholar] [CrossRef]
- Fayazi, M.; Afzali, D.; Ghanei-Motlagh, R.; Iraji, A. Synthesis of novel sepiolite–iron oxide–manganese dioxide nanocomposite and application for lead(II) removal from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 18893–18903. [Google Scholar] [CrossRef]
- Salam, M.A. Coating carbon nanotubes with crystalline manganese dioxide nanoparticles and their application for lead ions removal from model and real water. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 419, 69–79. [Google Scholar] [CrossRef]
- Chen, J.; Meng, H.; Tian, Y.; Yang, R.; Du, D.; Li, Z.; Qu, L.; Lin, Y. Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications. Nanoscale Horiz. 2019, 4, 321–338. [Google Scholar] [CrossRef]
- Wen, J.; Yang, K.; Sun, S. MnO2-Based nanosystems for cancer therapy. Chem. Commun. 2020, 56, 7065–7079. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, Y. Nanostructured manganese dioxide for anticancer applications: Preparation, diagnosis, and therapy. Nanoscale 2020, 12, 17982–18003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, W.; Chen, X.; Ren, M.; Zhang, H.; Xing, D.; Qin, H. Manganous-manganic oxide nanoparticle as an activatable microwave-induced thermoacoustic probe for deep-located tumor specific imaging in vivo. Photoacoustics 2022, 26, 100347. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, X.; Yi, T.; Zeng, Y.; Ma, J.; Li, L.; Pang, L.; Na Li, N.; Hu, H.; Zhan, Y. Tumor microenvironment responsive Mn3O4 nanoplatform for in vivo real-time monitoring of drug resistance and photothermal/chemodynamic synergistic therapy of gastric cancer. J. Nanobiotechnol. 2022, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yu, Y.; Shi, L.; Liao, Y.; Wang, Z.; Liu, X.; Lu, X.; Wang, J. Defect Engineering Triggers Exceptional Sonodynamic Activity of Manganese Oxide Nanoparticles for Cancer Therapy. ACS Appl. Bio Mater. 2022, 5, 4232–4243. [Google Scholar] [CrossRef]
- Xu, W.; Qing, X.; Liu, S.; Yang, D.; Dong, X.; Zhang, Y. Hollow Mesoporous Manganese Oxides: Application in Cancer Diagnosis and Therapy. Small 2022, 18, 2106511. [Google Scholar] [CrossRef]
- Su, Q.; Liu, C.; Zhu, J.; Ding, M.; Zhang, Z.; Li, J.; Zhang, Q. Albumin-Stabilized Manganese Oxide/Semiconducting Polymer Nanocomposites for Photothermal-Chemodynamic Therapy of Hepatic Carcinoma. Front. Bioeng. Biotechnol. 2022, 10, 919235. [Google Scholar] [CrossRef]
- Sun, W.; Xu, Y.; Yao, Y.; Yue, J.; Wu, Z.; Li, H.; Shen, G.; Liao, Y.; Wang, H.; Zhou, W. Self-oxygenation mesoporous MnO2 nanoparticles with ultra-high drug loading capacity for targeted arteriosclerosis therapy. J. Nanobiotechnol. 2022, 20, 88. [Google Scholar] [CrossRef]
- Zhou, F.; Zheng, T.; Abdel-Halim, E.S.; Jiang, L.; Zhu, J.-J. A multifunctional core–shell nanoplatform for enhanced cancer cell apoptosis and targeted chemotherapy. J. Mater. Chem. B 2016, 4, 2887–2894. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Adisa, I.O.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Effects of Manganese Nanoparticle Exposure on Nutrient Acquisition in Wheat (Triticum aestivum L.). Agronomy 2018, 8, 158. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Zhang, F.; Abdallah, Y.; Zhang, M.; Wang, Y.; Sun, G.; Qiu, W.; Li, B. Biosynthesis and characterization of magnesium oxide and manganese dioxide nanoparticles using Matricaria chamomilla L. extract and its inhibitory effect on Acidovorax oryzae strain RS-2. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2230–2239. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 97, 375–418. [Google Scholar] [CrossRef]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural Nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Agri, U.; Chaudhary, P.; Sharma, A. In vitro compatibility evaluation of agriusable nanochitosan on beneficial plant growth-promoting rhizobacteria and maize plant. Natl. Acad. Sci. Lett. 2021, 44, 555–559. [Google Scholar] [CrossRef]
- Nagdalian, A.A.; Blinov, A.V.; Siddiqui, S.A.; Gvozdenko, A.A.; Golik, A.B.; Maglakelidze, D.G.; Rzhepakovsky, I.V.; Kukharuk, M.Y.; Piskov, S.I.; Rebezov, M.B.; et al. Effect of selenium nanoparticles on biological and morphofunctional parameters of barley seeds (Hordéum vulgáre L.). Sci. Rep. 2023, 13, 6453. [Google Scholar] [CrossRef]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 17, 92. [Google Scholar] [CrossRef]
- Yan, X.; Chen, S.; Pan, Z.; Zhao, W.; Rui, Y.; Zhao, L. AgNPs-Triggered Seed Metabolic and Transcriptional Reprogramming Enhanced Rice Salt Tolerance and Blast Resistance. ACS Nano 2023, 17, 492–504. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Ye, Y.; Medina-Velo, I.A.; Cota-Ruiz, K.; Moreno-Olivas, F.; Gardea-Torresdey, J.L. Can abiotic stresses in plants be alleviated by manganese nanoparticles or compounds? Ecotoxicol. Environ. Saf. 2019, 184, 109671. [Google Scholar] [CrossRef]
- Azarin, K.; Usatov, A.; Minkina, T.; Plotnikov, A.; Kasyanova, A.; Fedorenko, A.; Duplii, N.; Vechkanov, E.; Rajput, V.D.; Mandzhieva, S.; et al. Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere 2022, 287, 132167. [Google Scholar] [CrossRef]
- Saod, W.M.; Hamid, L.L.; Alaallah, N.J.; Ramizy, A. Biosynthesis and antibacterial activity of manganese oxide nanoparticles prepared by green tea extract. Biotechnol. Rep. 2022, 34, e00729. [Google Scholar] [CrossRef]
- Saudy, H.S.; El–Samad, G.A.A.; El–Temsah, M.E.; El–Gabry, Y.A.E. Effect of Iron, Zinc, and Manganese Nano-Form Mixture on the Micronutrient Recovery Efficiency and Seed Yield Response Index of Sesame Genotypes. J. Soil Sci. Plant Nutr. 2021, 22, 732–742. [Google Scholar] [CrossRef]
- Rani, N.U.; Pavani, P.; Prasad Rao, P.T. Green synthesis of manganese oxide nanoparticles using Kigelia africana (lam.) Benth. Aqueous root extract and influence on chickpea (Cicer arietinum linn.) Seed germination and plant growth. Indian Drugs 2022, 59, 34–39. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanoparticle Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Galbraith, D.W. Silica breaks through in plants. Nat. Nanotechnol. 2007, 2, 272–273. [Google Scholar] [CrossRef]
- Juhel, G.; Batisse, E.; Hugues, Q.; Daly, D.; van Pelt, F.N.; O’halloran, J.; Jansen, M. Alumina nanoparticles enhance growth of Lemna minor. Aquat. Toxicol. 2011, 105, 328–336. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, J.; Li, M.; Li, Y.; Zhou, P.; Wang, Q.; Sun, Y.; Zhu, G.; Wang, Q.; Zhang, P.; et al. Effect of Silica-Based Nanomaterials on Seed Germination and Seedling Growth of Rice (Oryza sativa L.). Nanomaterials 2022, 12, 4160. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Zain, M.; Yousef, N.; Yinghai, Z.; Azeem, K.; Zhou, P.; White, J.C.; et al. Microplastic and Nanoplastic Interactions with Plant Species: Trends, Meta-Analysis, and Perspectives. Environ. Sci. Technol. Lett. 2022, 9, 482–492. [Google Scholar] [CrossRef]
- Margenot, A.J.; Rippner, D.A.; Dumlao, M.R.; Nezami, S.; Green, P.G.; Parikh, S.J.; McElrone, A.J. Copper oxide nanoparticle effects on root growth and hydraulic conductivity of two vegetable crops. Plant Soil 2018, 431, 333–345. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Allender, C.J. The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations (2010). Exp. Agric. 2011, 47, 574. [Google Scholar]
- Maryam, G.G.; Elahe, G.; Mohsen, S.; Zeinab, H.K. The effect of accelerated aging on germination characteristics, seed reserve utilization and malondialdehyde content of two wheat cultivars. J. Stress Physiol. Biochem. 2014, 10, 15–23. [Google Scholar]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Younis, M.; Abdel-Aziz, H.; Heikal, Y. Nanopriming technology enhances vigor and mitotic index of aged Vicia faba seeds using chemically synthesized silver nanoparticles. S. Afr. J. Bot. 2019, 125, 393–401. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influences of Nano-anatase TiO2 on the Nitrogen Metabolism of Growing Spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Elmer, W.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimkpa, C.; Gardea-Torresdey, J.; White, J.C. Effect of Metalloid and Metal Oxide Nanoparticles on Fusarium Wilt of Watermelon. Plant Dis. 2018, 102, 1394–1401. [Google Scholar] [CrossRef]
- Landa, P.; Cyrusova, T.; Jerabkova, J.; Drabek, O.; Vanek, T.; Podlipna, R. Effect of Metal Oxides on Plant Germination: Phytotoxicity of Nanoparticles, Bulk Materials, and Metal Ions. Water Air Soil Pollut. 2016, 227, 448. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Lal, R. Effects of Stabilized Nanoparticles of Copper, Zinc, Manganese, and Iron Oxides in Low Concentrations on Lettuce (Lactuca sativa) Seed Germination: Nanotoxicants or Nanonutrients? Water Air Soil Pollut. 2016, 227, 42. [Google Scholar] [CrossRef]
- Pradhan, S.; Patra, P.; Das, S.; Chandra, S.; Mitra, S.; Dey, K.K.; Akbar, S.; Palit, P.; Goswami, A. Photochemical Modulation of Biosafe Manganese Nanoparticles on Vigna radiata: A Detailed Molecular, Biochemical, and Biophysical Study. Environ. Sci. Technol. 2013, 47, 13122–13131. [Google Scholar] [CrossRef]
- Elmer, W.H.; Ma, C.; White, J.C. Nanoparticles for plant disease management. Curr. Opin. Environ. Sci. Health 2018, 6, 66–70. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum annuum L. through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.J.; Jayaprakasha, G.K.; Patil, B.S. Manganese Oxide Nanoparticles as Safer Seed Priming Agent to Improve Chlorophyll and Antioxidant Profiles in Watermelon Seedlings. Nanomaterials 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, D. In vitro effect of chromium, lead and manganese on seed germination, growth and mineral composition of finger millet (Eleusine coracana). Ann. Plant SOIL Res. 2021, 23, 129–134. [Google Scholar] [CrossRef]
- Wali, S.; Syed, L.; Rahim, F.; Tariq, F.; Rahman, K.U. Comparative effects of manganese and iron stress on seed germination and various growth parameters of Phaseolus lunatus L. Pak. J. Bot. 2021, 53, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Blinov, A.V.; Kravtsov, A.A.; Krandievskii, S.O.; Timchenko, V.P.; Gvozdenko, A.A.; Blinova, A.A. Synthesis of MnO2 Nanoparticles Stabilized by Methionine. Russ. J. Gen. Chem. 2020, 90, 283–286. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.V.; Maglakelidze, D.G.; Blinova, A.A.; Kobina, A.V.; et al. Effect of Selenium Nanoparticles on Germination of Hordéum Vulgáre Barley Seeds. Coatings 2021, 11, 862. [Google Scholar] [CrossRef]
- Blinov, A.V.; Siddiqui, S.A.; Nagdalian, A.A.; Blinova, A.A.; Gvozdenko, A.A.; Raffa, V.V.; Oboturova, N.P.; Golik, A.B.; Maglakelidze, D.G.; Ibrahim, S.A. Investigation of the influence of Zinc-containing compounds on the components of the colloidal phase of milk. Arab. J. Chem. 2021, 14, 103229. [Google Scholar] [CrossRef]
- Kaishev, V.G.; Khramtsov, A.G.; Blinov, A.V.; Gvozdenko, A.A.; Maglakelidze, D.G. Study of the Possibility of Application of Acoustic Spectroscopy in Dairy Products. In Intelligent Biotechnologies of Natural and Synthetic Biologically Active Substances; Lecture Notes in Networks and Systems Series; Springer: Berlin/Heidelberg, Germany, 2022; pp. 151–158. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO nanoparticles stabilized with gelatin for potential use in food packaging applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef]
- Sivakumar, S.; Prabu, L.N. Synthesis and Characterization of α-MnO2 nanoparticles for Supercapacitor application. Mater. Today Proc. 2021, 47, 52–55. [Google Scholar] [CrossRef]
- Pandey, V.; Adiba; Munjal, S.; Ahmad, T. Optical properties and spectroscopic investigation of single phase tetragonal Mn3O4 nanoparticles. Mater. Today Proc. 2020, 26, 1181–1183. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Blinov, A.V.; Yasnaya, M.A.; Golik, A.B.; Raffa, V.V.; Kramarenko, V.N.; Maglakelidze, D.G.; Shevchenko, I.M. Computer quantum-chemical simulation of multicomponent SiO2-MexOy systems. Phys. Chem. Asp. Study Clust. Nanostruct. Nanomater. 2020, 12, 394–404. [Google Scholar] [CrossRef]
- Blinov, A.; Gvozdenko, A.; Kravtsov, A.; Krandievsky, S.; Blinova, A.; Maglakelidze, D.; Vakalov, D.; Remizov, D.; Golik, A. Synthesis of nanosized manganese methahydroxide stabilized by cystine. Mater. Chem. Phys. 2021, 265, 124510. [Google Scholar] [CrossRef]
- Gilbert, A. Introduction to IQmol; QChem: New York, NY, USA, 2015; Volume 7, pp. 1–34. [Google Scholar]
- McBride, M.B. Adsorption and Oxidation of Phenolic Compounds by Iron and Manganese Oxides. Soil Sci. Soc. Am. J. 1987, 51, 1466–1472. [Google Scholar] [CrossRef]
- Minsky, A.; Meyer, A.Y.; Rabinovitz, M. Paratropicity and antiaromaticity: Role of the homo-lumo energy gap. Tetrahedron 1985, 41, 785–791. [Google Scholar] [CrossRef]
- Whangbo, M.-H.; Gordon, E.E.; Xiang, H.; Koo, H.-J.; Lee, C. Prediction of Spin Orientations in Terms of HOMO–LUMO Interactions Using Spin–Orbit Coupling as Perturbation. Accounts Chem. Res. 2015, 48, 3080–3087. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Shi, Y.; Gutman, I. Note on the HOMO-LUMO Index of Graphs. Match 2013, 70, 85–96. [Google Scholar]
- Brédas, J.L. Organic Electronics: Does a Plot of the HOMO–LUMO Wave Functions Provide Useful Information? Chem. Mater. 2017, 29, 477–478. [Google Scholar] [CrossRef]
- Ai, Z.; Zhang, L.; Kong, F.; Liu, H.; Xing, W.; Qiu, J. Microwave-assisted green synthesis of MnO2 nanoplates with environmental catalytic activity. Mater. Chem. Phys. 2008, 111, 162–167. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.-H.; Tang, X.; Wang, J.; Ooi, K. Synthesis and characterization of β-MnO2 single crystals with novel tetragonous morphology. Mater. Res. Bull. 2007, 42, 1432–1439. [Google Scholar] [CrossRef]
- Alzahrani, S.A.; Al-Thabaiti, S.A.; Al-Arjan, W.S.; Malik, M.A.; Khan, Z. Preparation of ultra long α-MnO2 and Ag@MnO2 nanoparticles by seedless approach and their photocatalytic performance. J. Mol. Struct. 2017, 1137, 495–505. [Google Scholar] [CrossRef]
- Ghaemi, M.; Ataherian, F.; Zolfaghari, A.; Jafari, S. Charge storage mechanism of sonochemically prepared MnO2 as supercapacitor electrode: Effects of physisorbed water and proton conduction. Electrochim. Acta 2008, 53, 4607–4614. [Google Scholar] [CrossRef]
- Yousefi, T.; Golikand, A.N.; Mashhadizadeh, M.H.; Aghazadeh, M. Template-free synthesis of MnO2 nanowires with secondary flower like structure: Characterization and supercapacitor behavior studies. Curr. Appl. Phys. 2012, 12, 193–198. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, Z.; Zhang, D.; Wang, H. Synthesis of mesoporous amorphous MnO2 from SBA-15 via surface modification and ultrasonic waves. Microporous Mesoporous Mater. 2006, 95, 257–264. [Google Scholar] [CrossRef]
- Corbi, P.P.; Cagnin, F.; Sabeh, L.P.; Massabni, A.C.; Costa-Neto, C.M. Synthesis, spectroscopic characterization and biological analysis of a new palladium(II) complex with methionine sulfoxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 66, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.A.J. Synthesis of Polymers Containing Residues of Biogenic Amino Acid Methionine, Methionine Sulfoxide and Methionine Sulfone and Their Application as Inhibitors of Mild Steel Corrosion. Int. J. Electrochem. Sci. 2019, 14, 1040–1068. [Google Scholar] [CrossRef]
- Yu, C.; Li, G.; Wei, L.; Fan, Q.; Shu, Q.; Yu, J.C. Fabrication, characterization of β-MnO2 microrod catalysts and their performance in rapid degradation of dyes of high concentration. Catal. Today 2014, 224, 154–162. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Li, C.-K.; Yu, J.-G.; Chen, X.-Q. MnO2/reduced graphene oxide nanoribbons: Facile hydrothermal preparation and their application in amperometric detection of hydrogen peroxide. Sensors Actuators B Chem. 2017, 239, 544–552. [Google Scholar] [CrossRef]
- Su, L.; Mosquera, J.; Mabesoone, M.F.J.; Schoenmakers, S.M.C.; Muller, C.; Vleugels, M.E.J.; Dhiman, S.; Wijker, S.; Palmans, A.R.A.; Meijer, E.W. Dilution-induced gel-sol-gel-sol transitions by competitive supramolecular pathways in water. Science 2022, 377, 213–218. [Google Scholar] [CrossRef]
- Gila-Vilchez, C.; Rodriguez-Arco, L.; Mañas-Torres, M.C.; de Cienfuegos, L.; Lopez-Lopez, M.T. Self-assembly in magnetic supramolecular hydrogels. Curr. Opin. Colloid Interface Sci. 2022, 62, 101644. [Google Scholar] [CrossRef]
- Neves, H.R.; Bini, R.A.; Barbosa, J.H.O.; Salmon, C.E.G.; Varanda, L.C. Dextran-Coated Antiferromagnetic MnO Nanoparticles for a T1-MRI Contrast Agent with High Colloidal Stability. Part. Part. Syst. Charact. 2016, 33, 167–176. [Google Scholar] [CrossRef]
- Ragupathy, P.; Vasan, H.N.; Munichandraiah, N. Synthesis and Characterization of Nano-MnO2 for Electrochemical Supercapacitor Studies. J. Electrochem. Soc. 2008, 155, A34–A40. [Google Scholar] [CrossRef]
- Gürer, F.; Kargl, R.; Bračič, M.; Makuc, D.; Thonhofer, M.; Plavec, J.; Mohan, T.; Kleinschek, K.S. Water-based carbodiimide mediated synthesis of polysaccharide-amino acid conjugates: Deprotection, charge and structural analysis. Carbohydr. Polym. 2021, 267, 118226. [Google Scholar] [CrossRef]
- Radoviciu, E.M.; Tomulescu, I.M.; Merca, V.V. Effects Induced Following the Treatments With Copper, Manganese and Zinc on Corn Seeds Germination (Carrera, Turda 200 and Hd-160). An. Univ. Din Oradea Fasc. Biol. 2009, 16, 105–107. [Google Scholar]
- Esper Neto, M.; Britt, D.W.; Jackson, K.A.; Coneglian, C.F.; Cordioli, V.R.; Braccini, A.L.; Batista, M.A. Assessments in early growth of corn seedlings after hausmanite (Mn3O4) nanoscale seed priming. J. Plant Nutr. 2021, 44, 1611–1620. [Google Scholar] [CrossRef]



















| Index | E, kcal/mol | HOMO | LUMO | η |
|---|---|---|---|---|
| Methionine Sulfoxide | −875.664 | −0.186 | −0.008 | 0.089 |
| MnO2 | −4200.463 | −0.087 | −0.046 | 0.0205 |
| Mn3O4 | −3825.168 | −0.115 | −0.74 | 0.205 |
| Interaction of oxygen of the sulfogroup of methionine sulfoxide and the hydroxogroup at the extreme manganese atom of Mn3O4 | −5000.96 | −0.086 | −0.032 | 0.027 |
| Interaction of the amino group of methionine sulfoxide and the hydroxo group at the extreme manganese atom in Mn3O4 | −5075.694 | −0.109 | −0.057 | 0.026 |
| Interaction of the carboxyl group of methionine sulfoxide and the hydroxo group at the extreme manganese atom of Mn3O4 | −5000.273 | −0.103 | −0.056 | 0.0235 |
| Interaction of oxygen of the sulfogroup of methionine sulfoxide and the hydroxogroup of the middle manganese atom in Mn3O4 | −5000.835 | −0.06 | −0.035 | 0.0125 |
| Interaction of the amino group of methionine sulfoxide and the hydroxo group of the middle manganese atom in Mn3O4 | −5076.435 | −0.088 | −0.043 | 0.225 |
| Interaction of the carboxyl group of methionine sulfoxide and the hydroxo group at the middle atom in Mn3O4 | −5000.29 | −0.1 | −0.054 | 0.023 |
| Interaction of oxygen of the sulfogroup of methionine sulfoxide and the hydroxogroup at the extreme manganese atom of MnO2 | −4624.686 | −0.103 | −0.051 | 0.026 |
| Interaction of the amino group of methionine sulfoxide and the hydroxo group at the extreme manganese atom in MnO2 | −4700.332 | −0.116 | −0.072 | 0.022 |
| Interaction of the carboxyl group of methionine sulfoxide and the hydroxo group at the extreme manganese atom of MnO2 | −4624.352 | −0.127 | −0.057 | 0.035 |
| Interaction of oxygen of the sulfogroup of methionine sulfoxide and the hydroxogroup of the middle manganese atom in MnO2 | −4624.778 | −0.064 | −0.033 | 0.0155 |
| Interaction of the amino group of methionine sulfoxide and the hydroxo group of the middle manganese atom in MnO2 | −4700.374 | −0.152 | −0.099 | 0.0265 |
| Interaction of the carboxyl group of methionine sulfoxide and the hydroxo group of the middle manganese atom in MnO2 | 4624.399 | −0.136 | −0.096 | 0.02 |
| Dressing Agent | Concentration (C), mg/mL | Length of Seedling, mm | Length of Roots, mm | Germination Energy, % |
|---|---|---|---|---|
| MnxOy stabilized with methionine | Control | 19.1 ± 2.08 a | 13.48 ± 2.66 c | 44.00 ± 1.78 d |
| 0.0005 | 23.13 ± 3.89 ab | 22.69 ± 3.37 a | 51.20 ± 3.56 ab | |
| 0.005 | 26.25 ± 4.59 b | 25.75 ± 3.33 b | 55.00 ± 0.71 bc | |
| 0.05 | 32.13 ± 3.40 c | 34.89 ± 2.34 d | 66.00 ± 2.55 e | |
| 0.5 | 25.04 ± 6.16 ab | 26.15 ± 4.34 b | 57.00 ± 0.70 c | |
| 5 | 21.96 ± 6.17 a | 19.82 ± 2.81 a | 50.50 ± 0.84 a | |
| KMnO4 | Control | 19.1 ± 2.08 a | 13.48 ± 2.66 a | 44.00 ± 1.78 b |
| 0.0005 | 21.44 ± 2.61 ab | 22.61 ± 3.05 bc | 56.00 ± 1.58 a | |
| 0.005 | 25.00 ± 4.03 c | 27.66 ± 3.69 d | 58.00 ± 1.00 a | |
| 0.05 | 26.10 ± 3.99 c | 26.29 ± 3.22 cd | 58.00 ± 1.58 a | |
| 0.5 | 22.35 ± 1.67 b | 21.47 ± 3.24 b | 56.00 ± 0.71 a | |
| 5 | 19.61 ± 3.00 a | 13.78 ± 4.06 a | 48.00 ± 1.58 c | |
| L-methionine | Control | 19.1± 2.08 a | 13.48 ± 2.66 b | 44.00 ± 1.78 c |
| 0.0005 | 20.56 ± 6.56 ab | 24.26 ± 1.80 a | 57.2 ± 1.79 ab | |
| 0.005 | 23.43 ± 4.92 b | 22.83± 1.85 a | 60.00 ± 1.41 a | |
| 0.05 | 21.75 ± 8.60 ab | 21.43 ± 3.54 a | 60.00 ± 1.00 a | |
| 0.5 | 18.55 ± 4.67 ac | 16.38 ± 3.69 b | 56.00 ± 1.22 b | |
| 5 | 14.09 ± 2.60 c | 9.70 ± 2.98 c | 49.00 ± 0.71 d |
| Index | MnxOy (Group 1) | L-Methionine (Group 2) | KMnO4 (Group 3) | Control (Group 4) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Me | 25 | 75 | Me | 25 | 75 | Me | 25 | 75 | Me | 25 | 75 | |
| Average length of seeding | 31.69 *** ### ●● | 29.50 | 35.00 | 22.15 §§§ | 16.00 | 28.00 | 25.77 *** §§ | 23.00 | 28.00 | 18.68 §§§ ●●● | 18.00 | 22.00 |
| Average length of roots | 34.89 *** ### ●●● | 33.75 | 35.50 | 21.43 §§§ | 18.30 | 25.00 | 26.29 *** §§§ | 25.00 | 29.00 | 13.48 §§§ ●●● | 12.40 | 15.60 |
| Germination energy | 66.00 *** ### ●●● | 64.00 | 68.00 | 60.00 *** §§§ | 59.00 | 61.00 | 58.00 ** §§§ | 57.00 | 59.00 | 44.00 §§§ ### ●● | 42.00 | 46.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinov, A.; Gvozdenko, A.; Golik, A.; Siddiqui, S.A.; Göğüş, F.; Blinova, A.; Maglakelidze, D.; Shevchenko, I.; Rebezov, M.; Nagdalian, A. Effect of MnxOy Nanoparticles Stabilized with Methionine on Germination of Barley Seeds (Hordeum vulgare L.). Nanomaterials 2023, 13, 1577. https://doi.org/10.3390/nano13091577
Blinov A, Gvozdenko A, Golik A, Siddiqui SA, Göğüş F, Blinova A, Maglakelidze D, Shevchenko I, Rebezov M, Nagdalian A. Effect of MnxOy Nanoparticles Stabilized with Methionine on Germination of Barley Seeds (Hordeum vulgare L.). Nanomaterials. 2023; 13(9):1577. https://doi.org/10.3390/nano13091577
Chicago/Turabian StyleBlinov, Andrey, Alexey Gvozdenko, Alexey Golik, Shahida A. Siddiqui, Fahrettin Göğüş, Anastasiya Blinova, David Maglakelidze, Irina Shevchenko, Maksim Rebezov, and Andrey Nagdalian. 2023. "Effect of MnxOy Nanoparticles Stabilized with Methionine on Germination of Barley Seeds (Hordeum vulgare L.)" Nanomaterials 13, no. 9: 1577. https://doi.org/10.3390/nano13091577
APA StyleBlinov, A., Gvozdenko, A., Golik, A., Siddiqui, S. A., Göğüş, F., Blinova, A., Maglakelidze, D., Shevchenko, I., Rebezov, M., & Nagdalian, A. (2023). Effect of MnxOy Nanoparticles Stabilized with Methionine on Germination of Barley Seeds (Hordeum vulgare L.). Nanomaterials, 13(9), 1577. https://doi.org/10.3390/nano13091577