Short-Chain Sulfur Confined into Nitrogen-Doped Hollow Carbon Nanospheres for High-Capacity Potassium Storage
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Schematic Diagram of the SHC-450 Synthesis Process and Principle of Hollow Sphere Structure Formation
3.2. Morphology and Structural Characterization
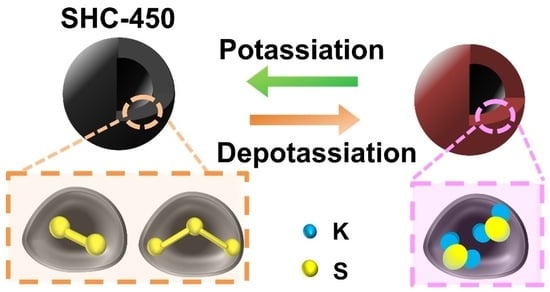
3.3. Electrochemical Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Min, X.; Xiao, J.; Fang, M.; Wang, W.; Zhao, Y.; Liu, Y.; Abdelkader, A.M.; Xi, K.; Kumar, R.V.; Huang, Z. Potassium-ion batteries: Outlook on present and future technologies. Energy Environ. Sci. 2021, 14, 2186–2243. [Google Scholar] [CrossRef]
- Wang, X.T.; Gu, Z.Y.; Ang, E.H.; Zhao, X.X.; Wu, X.L.; Liu, Y. Prospects for managing end-of-life lithium-ion batteries: Present and future. Interdiscip. Mater. 2022, 1, 417–433. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, X.; Meng, J.; Wang, H.; Zhao, Y.; Mai, L. Advances and perspectives on one-dimensional nanostructure electrode materials for potassium-ion batteries. Mater. Today 2022, 56, 114–134. [Google Scholar] [CrossRef]
- Zhao, L.F.; Hu, Z.; Lai, W.H.; Tao, Y.; Peng, J.; Miao, Z.C.; Wang, Y.X.; Chou, S.L.; Liu, H.K.; Dou, S.X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Dhir, S.; Wheeler, S.; Capone, I.; Pasta, M. Outlook on K-Ion Batteries. Chem 2020, 6, 2442–2460. [Google Scholar] [CrossRef]
- Hosaka, T.; Kubota, K.; Hameed, A.S.; Komaba, S. Research Development on K-Ion Batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dou, S.M.; Cui, X.Y.; Liu, W.D.; Zhang, Z.C.; Deng, Y.D.; Hu, W.B.; Chen, Y.N. Potassium-based electrochemical energy storage devices: Development status and future prospect. Energy Storage Mater. 2021, 34, 85–106. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Yu, R.; Wu, J. Unraveling the reaction mechanisms of electrode materials for sodium-ion and potassium-ion batteries by in situ transmission electron microscopy. Interdiscip. Mater. 2022, 1, 196–212. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; Liu, Y.; Han, Z.; Peng, L.; Li, S.; Yu, C.; Cheng, S.; Xie, J. Codoped porous carbon nanofibres as a potassium metal host for nonaqueous K-ion batteries. Nat. Commun. 2022, 13, 4911. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chong, S.; Yao, K.; Liu, H.K.; Dou, S.X.; Huang, W. Advanced anode materials for potassium batteries: Sorting out opportunities and challenges by potassium storage mechanisms. Matter 2023, 6, 3220–3273. [Google Scholar] [CrossRef]
- Salado, M.; Amores, M.; Pozo-Gonzalo, C.; Forsyth, M.; Lanceros-Méndez, S. Advanced and sustainable functional materials for potassium-ion batteries. Energy Mater. 2023, 3, 300037. [Google Scholar] [CrossRef]
- Liu, M.Q.; Wang, Y.H.; Wu, F.; Bai, Y.; Li, Y.; Gong, Y.T.; Feng, X.; Li, Y.; Wang, X.R.; Wu, C. Advances in Carbon Materials for Sodium and Potassium Storage. Adv. Funct. Mater. 2022, 32, 2203117. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Xing, Z.; Lam, C.W.K.; Pang, S.S.; Zhang, W.; Ju, Z. Advanced Carbon-Based Anodes for Potassium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1900343. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, Z.; Wang, W.; Alhajji, E.; Emwas, A.H.; Costa, P.; Cavallo, L.; Alshareef, H.N. A Site-Selective Doping Strategy of Carbon Anodes with Remarkable K-Ion Storage Capacity. Angew. Chem. Int. Ed. Engl. 2020, 59, 4448–4455. [Google Scholar] [CrossRef]
- Li, D.; Ren, X.; Ai, Q.; Sun, Q.; Zhu, L.; Liu, Y.; Liang, Z.; Peng, R.; Si, P.; Lou, J.; et al. Facile Fabrication of Nitrogen-Doped Porous Carbon as Superior Anode Material for Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1802386. [Google Scholar] [CrossRef]
- Liu, F.; Meng, J.; Xia, F.; Liu, Z.; Peng, H.; Sun, C.; Xu, L.; Van Tendeloo, G.; Mai, L.; Wu, J. Origin of the extra capacity in nitrogen-doped porous carbon nanofibers for high-performance potassium ion batteries. J. Mater. Chem. A 2020, 8, 18079–18086. [Google Scholar] [CrossRef]
- Li, J.; Qin, W.; Xie, J.; Lei, H.; Zhu, Y.; Huang, W.; Xu, X.; Zhao, Z.; Mai, W. Sulphur-doped reduced graphene oxide sponges as high-performance free-standing anodes for K-ion storage. Nano Energy 2018, 53, 415–424. [Google Scholar] [CrossRef]
- Mahmood, A.; Li, S.; Ali, Z.; Tabassum, H.; Zhu, B.; Liang, Z.; Meng, W.; Aftab, W.; Guo, W.; Zhang, H.; et al. Ultrafast Sodium/Potassium-Ion Intercalation into Hierarchically Porous Thin Carbon Shells. Adv. Mater. 2019, 31, e1805430. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Zhang, B.; Zhang, T.; Shen, T.; Zhao, Z.; Hou, Y. Sulfur-Doped Carbon for Potassium-Ion Battery Anode: Insight into the Doping and Potassium Storage Mechanism of Sulfur. ACS Nano 2022, 16, 21443–21451. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, H.; Wei, W.; Zheng, Y.; Tao, L.; Wang, Y.; Huang, M.; Shi, J.; Shi, Z.C.; Mitlin, D. Sulfur-Rich Graphene Nanoboxes with Ultra-High Potassiation Capacity at Fast Charge: Storage Mechanisms and Device Performance. ACS Nano 2021, 15, 1652–1665. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chen, Z.L.; Sun, Z.F.; Cai, M.T.; Liu, W.C.; Ye, W.B.; Gao, H.W.; Han, J.J.; Cheng, Y.; Zhang, Q.B.; et al. Unraveling the Origin of Enhanced K Storage of Carbonaceous Anodes Enabled by Nitrogen/Sulfur Co-Doping. Adv. Funct. Mater. 2023, 33, 11. [Google Scholar] [CrossRef]
- Chi, C.; Liu, Z.; Lu, X.; Meng, Y.; Huangfu, C.; Yan, Y.; Qiu, Z.; Qi, B.; Wang, G.; Pang, H.; et al. Balance of sulfur doping content and conductivity of hard carbon anode for high-performance K-ion storage. Energy Storage Mater. 2023, 54, 668–679. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Yi, Z.; Zhou, J.; Pan, Z.; Tian, J.; Wang, Y.; Sun, S.; Lin, N.; Qian, Y. Revealing the Double-Edged Behaviors of Heteroatom Sulfur in Carbonaceous Materials for Balancing K-Storage Capacity and Stability. Adv. Funct. Mater. 2020, 31, 2006875. [Google Scholar] [CrossRef]
- Goyenola, C.; Lai, C.-C.; Näslund, L.-Å.; Lu, J.; Högberg, H.; Hultman, L.; Rosen, J.; Gueorguiev, G.K. Theoretical Prediction and Synthesis of CSxFy Thin Films. J. Phys. Chem. C 2016, 120, 9527–9534. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, B.; Feng, J.; Wang, C.; Cai, X.; Qiao, K.; Qin, R. Electrochemical Insights, Developing Strategies, and Perspectives toward Advanced Potassium-Sulfur Batteries. Small 2020, 16, e2003386. [Google Scholar] [CrossRef] [PubMed]
- Haridas, A.K.; Huang, C. Advances and challenges in tuning the reversibility & cyclability of room temperature sodium–sulfur and potassium–sulfur batteries with catalytic materials. Mater. Today Energy 2023, 32, 101228. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, H.; Fan, W.; Zhong, C.; Hu, W.; Mitlin, D. Review of Emerging Potassium-Sulfur Batteries. Adv. Mater. 2020, 32, e1908007. [Google Scholar] [CrossRef]
- Lai, C.-C.; Goyenola, C.; Broitman, E.; Näslund, L.-Å.; Högberg, H.; Hultman, L.; Gueorguiev, G.K.; Rosen, J. Synthesis and properties of CSxFythin films deposited by reactive magnetron sputtering in an Ar/SF6discharge. J. Phys. Condens. Matter 2017, 29, 195701. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, H.; Zhou, H.; Feng, J.; Zheng, X.; Zhong, C.; Paek, E.; Hu, W.; Mitlin, D. Sulfur-Grafted Hollow Carbon Spheres for Potassium-Ion Battery Anodes. Adv. Mater. 2019, 31, e1900429. [Google Scholar] [CrossRef]
- Wang, C.Y.; Dong, W.D.; Zhou, M.R.; Wang, L.; Wu, L.; Hu, Z.Y.; Chen, L.; Li, Y.; Su, B.L. Gradient selenium-doping regulating interfacial charge transfer in zinc sulfide/carbon anode for stable lithium storage. J. Colloid Interface Sci. 2022, 619, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Xu, J.; Xu, C.; Zhang, J.; Liu, Y.; Wanyan, B.; Yu, H.; Yan, L.; Zhang, L.; Shu, J. Tailoring short-chain sulfur molecules to drive redox dynamics for sulfur-based aqueous battery. Proc. Natl. Acad. Sci. USA 2023, 120, e2307646120. [Google Scholar] [CrossRef]
- Han, C.H.; Liu, F.; Liu, J.S.; Li, Q.; Meng, J.S.; Shao, B.W.; He, Q.; Wang, X.P.; Liu, Z.; Mai, L.Q. Facile template-free synthesis of uniform carbon-confined VO hollow spheres for stable and fast lithium storage. J. Mater. Chem. A 2018, 6, 6220–6224. [Google Scholar] [CrossRef]
- Xu, F.; Zhai, Y.; Zhang, E.; Liu, Q.; Jiang, G.; Xu, X.; Qiu, Y.; Liu, X.; Wang, H.; Kaskel, S. Ultrastable Surface-Dominated Pseudocapacitive Potassium Storage Enabled by Edge-Enriched N-Doped Porous Carbon Nanosheets. Angew. Chem. Int. Ed. Engl. 2020, 59, 19460–19467. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Hong, Y.; Yang, Y.; Zhang, J.; Niu, X.; Wang, J.; Zeng, L.; Hao, W.; Guo, L.; Zhu, Y. Sulfurized Polyacrylonitrile as a High-Performance and Low-Volume Change Anode for Robust Potassium Storage. ACS Nano 2021, 15, 18419–18428. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Qu, J.; Sui, Y.; Ji, Q.-Y.; Zhang, T.-T.; Zhai, X.-Z.; Jing, Y.-Q.; Yu, Z.-Z. Constructing mesoporous hollow polysulfane spheres bonded with short-chain sulfurs (Sx, x ≤ 3) as high-performance sulfur cathodes in both ether and ester electrolytes. Energy Storage Mater. 2020, 27, 426–434. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, H.M.; Sun, Y.K. High performance potassium-sulfur batteries based on a sulfurized polyacrylonitrile cathode and polyacrylic acid binder. J. Mater. Chem. A 2018, 6, 14587–14593. [Google Scholar] [CrossRef]
- Xiong, P.; Han, X.; Zhao, X.; Bai, P.; Liu, Y.; Sun, J.; Xu, Y. Room-Temperature Potassium-Sulfur Batteries Enabled by Microporous Carbon Stabilized Small-Molecule Sulfur Cathodes. ACS Nano 2019, 13, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, W.; Liu, J.; Wu, Y.; Liu, Z. Sulfur/nitrogen/oxygen tri-doped carbon nanospheres as an anode for potassium ion storage. J. Energy Chem. 2023, 77, 338–347. [Google Scholar] [CrossRef]
- Wang, X.F.; Qian, Y.M.; Wang, L.N.; Yang, H.; Li, H.L.; Zhao, Y.; Liu, T.X. Sulfurized Polyacrylonitrile Cathodes with High Compatibility in Both Ether and Carbonate Electrolytes for Ultrastable Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1902929. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, Q.; Liu, H.; Xu, B.; Zhang, S.; Zhou, T.; Mao, J.; Pang, W.K.; Guo, Z.; Li, A.; et al. Graphitic Carbon Nanocage as a Stable and High Power Anode for Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801149. [Google Scholar] [CrossRef]
- Ma, R.F.; Fan, L.; Wang, J.; Lu, B.G. Confined and covalent sulfur for stable room temperature potassium-sulfur battery. Electrochim. Acta 2019, 293, 191–198. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L.; Hendrickson, K.E.; Tu, Z.; Archer, L.A. Metal-Sulfur Battery Cathodes Based on PAN-Sulfur Composites. J. Am. Chem. Soc. 2015, 137, 12143–12152. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Ye, W.; Xiao, B.; Sun, Z.; Cheng, Y.; Wang, M.S. Regulating the Wettability of Hard Carbon through Open Mesochannels for Enhanced K(+) Storage. Small 2023, 19, e2300605. [Google Scholar] [CrossRef]
- Hwang, T.H.; Jung, D.S.; Kim, J.S.; Kim, B.G.; Choi, J.W. One-dimensional carbon-sulfur composite fibers for Na-S rechargeable batteries operating at room temperature. Nano Lett. 2013, 13, 4532–4538. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Meng, J.S.; Jiang, G.P.; Li, J.T.; Wang, H.; Xiao, Z.T.; Yu, R.H.; Mai, L.Q.; Wu, J.S. Coordination engineering of metal single atom on carbon for enhanced and robust potassium storage. Matter 2021, 4, 4006–4021. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Luo, W.; Wang, C.; Feng, W.; Hong, X.; Mai, L. Dual sulfur-doped sites boost potassium storage in carbon nanosheets derived from low-cost sulfonate. Chem. Eng. J. 2022, 431, 134207. [Google Scholar] [CrossRef]
- Tao, L.; Yang, Y.; Wang, H.; Zheng, Y.; Hao, H.; Song, W.; Shi, J.; Huang, M.; Mitlin, D. Sulfur-nitrogen rich carbon as stable high capacity potassium ion battery anode: Performance and storage mechanisms. Energy Storage Mater. 2020, 27, 212–225. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Chen, Y.-F.; Feng, B.-Q.; Ye, C.-X.; Ye, X.-B.; Jin, H.; Zhou, E.; Zeng, X.; Zheng, Z.-L.; Chen, X.-L.; et al. Surface-dominated potassium storage enabled by single-atomic sulfur for high-performance K-ion battery anodes. Energy Environ. Sci. 2023, 16, 1540–1547. [Google Scholar] [CrossRef]
- Cheng, N.; Xu, P.; Lu, B.A.; Liu, Z.G. Covalent sulfur as stable anode for potassium ion battery. J. Energy Chem. 2021, 62, 645–652. [Google Scholar] [CrossRef]
- Guo, R.; Liu, X.; Wen, B.; Liu, F.; Meng, J.; Wu, P.; Wu, J.; Li, Q.; Mai, L. Engineering Mesoporous Structure in Amorphous Carbon Boosts Potassium Storage with High Initial Coulombic Efficiency. Nanomicro Lett 2020, 12, 148. [Google Scholar] [CrossRef]
- Ma, C.; Tang, X.; Jiang, J.; Ma, Z.; Li, H.; Ben, H.; Yuan, X.-Z. Constructing sulfur and nitrogen codoped porous carbon with optimized defect-sites and electronic structure promises high performance potassium-ion storage. Chem. Eng. J. 2023, 454, 140116. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Shi, T.; Liu, F.; Yang, C.; Qiao, F.; Han, K.; Han, C.; Meng, J.; Wang, X. Short-Chain Sulfur Confined into Nitrogen-Doped Hollow Carbon Nanospheres for High-Capacity Potassium Storage. Nanomaterials 2024, 14, 550. https://doi.org/10.3390/nano14060550
Liu W, Shi T, Liu F, Yang C, Qiao F, Han K, Han C, Meng J, Wang X. Short-Chain Sulfur Confined into Nitrogen-Doped Hollow Carbon Nanospheres for High-Capacity Potassium Storage. Nanomaterials. 2024; 14(6):550. https://doi.org/10.3390/nano14060550
Chicago/Turabian StyleLiu, Wenhan, Tengfei Shi, Fang Liu, Chen Yang, Fan Qiao, Kang Han, Chunhua Han, Jiashen Meng, and Xuanpeng Wang. 2024. "Short-Chain Sulfur Confined into Nitrogen-Doped Hollow Carbon Nanospheres for High-Capacity Potassium Storage" Nanomaterials 14, no. 6: 550. https://doi.org/10.3390/nano14060550
APA StyleLiu, W., Shi, T., Liu, F., Yang, C., Qiao, F., Han, K., Han, C., Meng, J., & Wang, X. (2024). Short-Chain Sulfur Confined into Nitrogen-Doped Hollow Carbon Nanospheres for High-Capacity Potassium Storage. Nanomaterials, 14(6), 550. https://doi.org/10.3390/nano14060550