Preparation of GST Inhibitor Nanoparticle Drug Delivery System and Its Reversal Effect on the Multidrug Resistance in Oral Carcinoma
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis and Characterization of MPEG–PLA–SS–ECA Polymer

2.2. Preparation and Related Physicochemical Properties of MPEG–PLA–SS–ECA Nanoparticles

2.3. Identification of the Induced Drug-Resistant SCC15/CBP and SCC15/PYM Cells

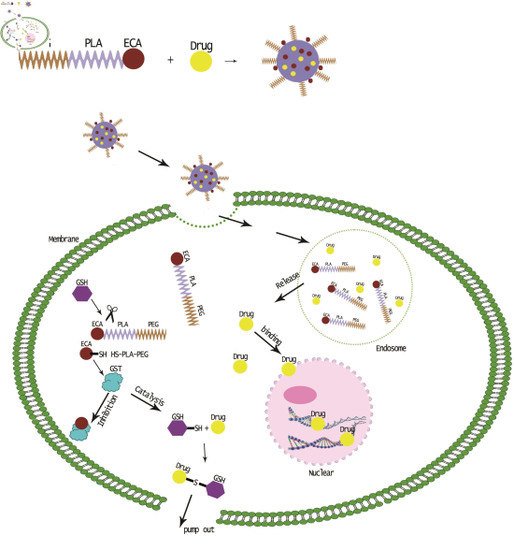
2.4. Endocytosis of MPEG–PLA–SS–ECA Nanoparticles in Drug-Resistant Oral Squamous Cell Carcinoma Cells

2.5. Effects of MPEG–PLA–SS–ECA on MDR of the Two Drug-Resistant Cells

2.6. Inhibition of MPEG–PLA–SS–ECA on GST-π

3. Experimental Section
3.1. Reagents
3.2. Synthesis of MPEG–PLA–SS–ECA Polymer
3.3. Preparation of Self-Assembled MPEG–PLA–SS–ECA Nanoparticles
3.4. Detection of Physicochemical Properties of MPEG–PLA–SS–ECA Nanoparticles and Study on ECA Release
3.5. Study on Drug Delivery Properties of the MPEG–PLA–SS–ECA Nanoparticles
3.6. Study on the Induction and Identification of Drug-Resistant Cells
3.7. Study on Nanoparticle Endocytosis of the Cells
3.8. Effects of MPEG–PLA–SS–ECA Nanoparticles on MDR of the Drug-Resistant Cells
3.9. Detection of the Expression of GST-π Protein in Drug-Resistant Oral Squamous Cell Carcinoma Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Ferreira, J.A.; Peixoto, A.; Lima, L.; Barroso, S.; Sarmento, B.; Santos, L.L. New trends in guided nanotherapies for digestive cancers: A systematic review. J. Control. Release 2015, 209, 288–307. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Cameron, D.; Argyle, D. Species differences in tumour responses to cancer chemotherapy. Philos. T. R. Soc. B. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M. Prognostic value of clinicopathological parameters and outcome in 484 patients with oral squamous cell carcinoma: Microvascular invasion (V plus ) is an independent prognostic factor for OSCC. Clin. Transl. Oncol. 2012, 14, 870–880. [Google Scholar] [CrossRef]
- Hanabata, Y.; Nakajima, Y.; Morita, K.-I.; Kayamori, K.; Omura, K. Coexpression of SGLT1 and EGFR is associated with tumor differentiation in oral squamous cell carcinoma. Odontology 2012, 100, 156–163. [Google Scholar] [CrossRef]
- Peng, B.; Yi, S.; Gu, Y.; Zheng, G.; He, Z. Purification and biochemical characterization of a novel protein-tongue cancer chemotherapy resistance-associated protein1 (TCRP1). Protein Expr. Purif. 2012, 82, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sayans, M.; Suarez-Penaranda, J.M.; Pilar, G.-D.; Barros-Angueira, F.; Gandara-Rey, J.M.; Garcia-Garcia, A. Hypoxia-inducible factors in OSCC. Cancer Lett. 2011, 313, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, M.J. Expression of multidrug resistance-related genes in oral squamous cell carcinomas. Oral Oncol. 2001, 37, 652–659. [Google Scholar] [CrossRef]
- Liu, S.; Liao, C.; Wang, D. The clinical application and evaluation of combined chemotherapy in comprehensive treatment for oral squamous cell carcinoma. West China J. Stomatol. 2003, 21, 109–111. (in Chinese). [Google Scholar]
- Saiyin, W.; Wang, D.; Li, L.; Zhu, L.; Liu, B.; Sheng, L.; Li, Y.; Zhu, B.; Mao, L.; Li, G.; et al. Sequential Release of Autophagy Inhibitor and Chemotherapeutic Drug with Polymeric Delivery System for Oral Squamous Cell Carcinoma Therapy. Mol. Pharm. 2014, 11, 1662–1675. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Wang, D.-Z.; Chen, H.-Q.; He, J.; Leng, W. Induction of drug resistance in Tca8113 cell line by exposing to chemotherapy drug. West China J. Stomatol. 2007, 25, 184–187. (in Chinese). [Google Scholar]
- Qiu, Z.-Q.; Qiu, Z.-R. Sensitivity of gastric cancer cells to chemotherapy drugs in elderly patients and its correlation with cyclooxygenase-2 expression. Asian Pac. J. Cancer Prev. 2015, 16, 3447–3450. [Google Scholar] [PubMed]
- Rocha, G.D.G.; Oliveira, R.R.; Coelho Kaplan, M.A.; Gattass, C.R. 3β-Acetyl tormentic acid reverts MRP1/ABCC1 mediated cancer resistance through modulation of intracellular levels of GSH and inhibition of GST activity. Eur. J. Pharmacol. 2014, 741, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, S.; Chaturvedi, P.; Agarwal, G.; Lal, P.; Agrawal, S.; Mittal, R.D.; Mittal, B. Pharmacogenetic Influence of GST Polymorphisms on Anthracycline-Based Chemotherapy Responses and Toxicity in Breast Cancer Patients: A Multi-Analytical Approach. Mol. Diagn. Ther. 2013, 17, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Cheng, X.; Du, Y.; Yang, L.; Huang, L. Significance of MDR-related proteins in the postoperative individualized chemotherapy of gastric cancer. J. Cancer Res. Ther. 2015, 11, 46–50. [Google Scholar] [PubMed]
- Xu, Y.; Xia, F.; Ma, L.; Shan, J.; Shen, J.; Yang, Z.; Liu, J.; Cui, Y.; Bian, X.; Bie, P.; et al. MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett. 2011, 310, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Eady, S.J. Glutathione: Its implications for animal health, meat quality, and health benefits of consumers. Aust. J. Agric. Res. 2005, 56, 775–780. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu. Rev. Biomed. Eng. 2006, 8, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Bailon, P.; Won, C.-Y. PEG-modified biopharmaceuticals. Expert Opin. Drug Deliv. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: A strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 2011, 63, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. PEG-lipid micelles as drug carriers: Physiochemical attributes, formulation principles and biological implication. J. Drug Target. 2015, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Vachutinsky, Y.; Kataoka, K. PEG-based Polyplex Design for Gene and Nucleotide Delivery. Isr. J. Chem. 2010, 50, 175–184. [Google Scholar] [CrossRef]
- Gaowa, A.; Horibe, T.; Kohno, M.; Tabata, Y.; Harada, H.; Hiraoka, M.; Kawakami, K. Enhancement of anti-tumor activity of hybrid peptide in conjugation with carboxymethyl dextran via disulfide linkers. Eur. J. Pharm. Biopharm. 2015, 92, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Takeo, T.; Horikoshi, Y.; Nakao, S.; Sakoh, K.; Ishizuka, Y.; Tsutsumi, A.; Fukumoto, K.; Kondo, T.; Haruguchi, Y.; Takeshita, Y.; et al. Cysteine analogs with a free thiol group promote fertilization by reducing disulfide bonds in the zona pellucida of mice. Biol. Reprod. 2015, 92. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.D.; Zhang, R.; Han, X.; Kang, K.A.; Piao, M.J.; Maeng, Y.H.; Chang, W.Y.; Hyun, J.W. Involvement of glutathione and glutathione metabolizing enzymes in human colorectal cancer cell lines and tissues. Mol. Med. Rep. 2015, 12, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Schoeneberger, H.; Belz, K.; Schenk, B.; Fulda, S. Impairment of antioxidant defense via glutathione depletion sensitizes acute lymphoblastic leukemia cells for Smac mimetic-induced cell death. Oncogene 2015, 34, 4032–4043. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, P.; Liu, Z.; Chen, F.; Liu, Y.; Wang, D.; Dai, Y. A Facile One-Pot Synthesis of Layered Protonated Titanate Nanosheets Loaded with Silver Nanoparticles with Enhanced Visible-Light Photocatalytic Performance. Chem. Asian J. 2013, 8, 204–211. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Properties and energetics for design and characterization of chitosan nanoparticles used for drug encapsulation. RSC Adv. 2014, 4, 12653–12661. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.-T.; Liu, H.-Y.; Hong, H.; Lv, W.; Shang, Z.-H. Preparation of pingyangmycin PLGA microspheres and related in vitro/in vivo studies. Int. J. Pharm. 2010, 398, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, M.; Li, D.; Jiao, M.; Wang, L.; Zhang, H.; Liu, H.; Wang, D.; Han, B. Preparation, Characterization and Related In Vivo Release, Safety and Toxicity Studies of Long Acting Lanreotide Microspheres. Biol. Pharm. Bull. 2012, 35, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, Y.; Lv, W.; Jiao, C.; Duan, M.; Liu, H.; Han, B. Preparation of Bleomycin A(2)-PLGA Microspheres and Related In Vitro and In Vivo Studies. J. Pharm. Sci. 2011, 100, 2790–2800. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Eggers, P.K.; Raston, C.L.; Lim, L.Y. Development and validation of a LC/TOF MS method for the determination of carboplatin and paclitaxel in nanovesicles. Anal. Bioanal. Chem. 2014, 406, 2659–2667. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Wang, Y.; Wang, L.; Shang, Z.; Wang, S.; Pei, J. Preparation of GST Inhibitor Nanoparticle Drug Delivery System and Its Reversal Effect on the Multidrug Resistance in Oral Carcinoma. Nanomaterials 2015, 5, 1571-1587. https://doi.org/10.3390/nano5041571
Han B, Wang Y, Wang L, Shang Z, Wang S, Pei J. Preparation of GST Inhibitor Nanoparticle Drug Delivery System and Its Reversal Effect on the Multidrug Resistance in Oral Carcinoma. Nanomaterials. 2015; 5(4):1571-1587. https://doi.org/10.3390/nano5041571
Chicago/Turabian StyleHan, Bing, Yanli Wang, Lan Wang, Zuhui Shang, Shuang Wang, and Jin Pei. 2015. "Preparation of GST Inhibitor Nanoparticle Drug Delivery System and Its Reversal Effect on the Multidrug Resistance in Oral Carcinoma" Nanomaterials 5, no. 4: 1571-1587. https://doi.org/10.3390/nano5041571
APA StyleHan, B., Wang, Y., Wang, L., Shang, Z., Wang, S., & Pei, J. (2015). Preparation of GST Inhibitor Nanoparticle Drug Delivery System and Its Reversal Effect on the Multidrug Resistance in Oral Carcinoma. Nanomaterials, 5(4), 1571-1587. https://doi.org/10.3390/nano5041571