Iron Oxide Nanoparticles Coated with a Phosphorothioate Oligonucleotide and a Cationic Peptide: Exploring Four Different Ways of Surface Functionalization
Abstract
:1. Introduction

2. Results and Discussion
2.1. Nanoparticle Synthesis and Li28 Surface Complexation
2.1.1. Li28 Structural Investigation at Acidic and Physiological pH



2.1.2. Iron Oxide Surface Functionalization with Li28 ODN

| Values | R | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 8.75 | 17.5 | 35 | 52.5 | 70 | 87.5 | 175 | |
| Dh (nm) | 602 | 135 | 89 | 59 | 55 | 53 | 55 | 56 |
| Z (mV) | - | −42 | −43 | −45 | −54 | −54 | −57 | −48 |

2.2. Li28 and Arg15 Peptide Complexation with Iron Oxide NP Surface
2.2.1. “Step by Step” Iron Oxide Surface Functionalization with Li28 ODN and Arg15 Peptide
2.2.2. “Layer by Layer” Iron Oxide Surface Functionalization with Poly-Arginine, Li28 ODN and Arg15 Peptide
| R | 0 | 17 | 17+Arg15 | 35 | 35+Arg15 | 69 | 69+Arg15 |
|---|---|---|---|---|---|---|---|
| Dh (nm) | 57 | 58 | 57 | 60 | 59 | 69 | 68 |
| Z (mV) | +63 | +59 | +59 | +52 | +51 | +50 | +49 |

2.2.3. “One Step Assembly” Iron Oxide Surface Functionalization with Li28 ODN and Arg15 Peptide


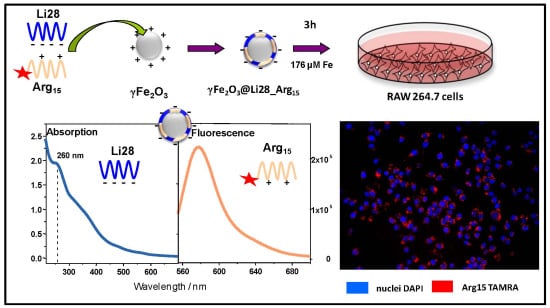
2.3. Uptake of Nanoparticle by Macrophages

3. Experimental Section
3.1. Physical Characterization
3.2. Penetration of NPs into RAW264.7 Cells in Culture
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligonucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.L.; Zamecnik, P.C. Inhibition of Rous sarcoma Viral RNA Translation by a Specific Oligonucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.W.; Campbell, B.J.; Cosstick, R. RNA interference: A chemist’s perspective. Chem. Soc. Rev. 2010, 39, 4169–4181. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P. An overview of sugar-modified oligonucleotides for antisense therapeutics. Chem. Biodivers. 2011, 8, 1616–1641. [Google Scholar] [CrossRef] [PubMed]
- Deleavey, G.F.; Damba, M.J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 2012, 19, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Thomas, S.M.; Kim, S.; Yeh, J.I.; Ferris, R.L.; Johnson, J.T.; Duvvuri, U.; Lee, J.; Sahu, N.; Joyce, S.; et al. First-in-Human trial of a STAT3 decoy oligonucleotide in head and neck tumors: Implications for cancer therapy. Cancer Discov. 2012, 2, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhao, X.; Lee, L.J.; Lee, R.J. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009, 11, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R.; Jaffer, F.A.; Weissleder, R. A macrophage-targeted theranostic nanoparticle for biomedical applications. Small 2006, 2, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Sumer, B.; Gao, J. Theranostic nanomedicine for cancer. Nanomedicine 2008, 3, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, S.; Son, S.; Kim, S.H.; Leary, J.F.; Choi, K.; Kwon, I.C. Theranostic nanoparticles for future personalized medicine. J. Control. Release 2014, 190, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Leary, J.F. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int. J. Nanomed. 2014, 9, 711–726. [Google Scholar]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine coating enhances the cellular uptrake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Epler, K.E.; Padilla, D.P.; Phillips, G.K.; Castillo, R.E.; Wilkinson, D.C.; Wilkinson, B.S.; Burgard, C.A.; Kalinich, R.M.; et al. Delivery of small interfering RNA by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers. ACS Nano 2012, 6, 2174–2188. [Google Scholar] [CrossRef] [PubMed]
- Buchman, Y.K.; Lellouche, E.; Zigdon, S.; Bechor, M.; Michaeli, S.; Lellouche, J.P. Silica nanoparticles and polyethyleneimine (PEI)-mediated functionalization: A new method of PEI covalent attachment for siRNA delivery applications. Bionconjug. Chem. 2013, 24, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.K.; Searson, P.C.; Leong, K.W. Multifonctional nanorods for gene delivery. Nat. Mater. 2003, 2, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Klibanov, A.M. Conjugation to gold nanoparticles enhances polyethylenimine’s transfer of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9138–9143. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, L.; He, Y.; Li, Y. Sandwich-type Au-PEI/DNA/PEI-Dexa nanocomplex for nucleus-targeted gene delivery in vitro and in vivo. ACS Appl. Mater. Interfaces 2014, 6, 14196–14206. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.J.; Tang, J.Q.; Zhu, S.G.; Nie, X.M.; Lu, H.B.; Shen, S.R.; Li, X.L.; Tang, K.; Zhou, M.; Li, G.Y. IONP-PLL: A novel non-viral vector for efficient gene delivery. J. Gene Med. 2003, 5, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, O.; Antequera, Y.S.; Vlaskou, D.; Plank, C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat. Protocol. 2007, 2, 2391–2411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Gordon, L.E.; Clark, G.J.; Nantz, M.H. Click assembly of magnetic nanovectors for gene delivery. Biomaterials 2011, 32, 2683–2688. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davis, T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef]
- Song, H.P.; Yang, J.Y.; Lo, S.L.; Wang, Y.; Fan, W.M.; Tang, X.S.; Xue, J.M.; Wang, S. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials 2010, 31, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Berti, L.; Woldeyesus, T.; Li, Y.; Lam, K.S. Maximization of loading and stability of ssDNA: Iron oxide nanoparticle complexes formed through electrostatic interaction. Langmuir 2010, 26, 18293–18299. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.C.; Moghimi, S.M. Cationic carriers of genetic material and cell death: A mitochondrial tale. Biochim. Biophys. Acta 2010, 1797, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, G.; Ingle, N.P.; Reineke, T.M. Interaction of poly(ethylenimine)-DNA polyplexes with mitochondria; implications for a mechanism of cytotoxicity. Mol. Pharm. 2011, 8, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Geinguenaud, F.; Souissi, I.; Fagard, R.; Motte, L.; Lalatonne, Y. Electrostatic assembly of a DNA superparamagnetic nano-tool for simultaneaous intracellular delivery and in situ monitoring. Nanomedicine 2012, 8, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Geinguenaud, F.; Souissi, I.; Fagard, R.; Lalatonne, Y.; Motte, L. Easily controlled grafting of oligonucleotides on γFe2O3 nanoparticles: Physicochemical characterization of DNA organization and biological activity studies. J. Phys. Chem. B 2014, 118, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Maubant, S.; Banissi, C.; Beck, S.; Chauvat, A.; Carpentier, A.F. Adjuvant properties of cytosine-phosphate-guanosine oligodeoxynucleotide in combination with various polycations in an ovalbumin-vaccine model. Nucleic Acid Ther. 2011, 21, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Lalatonne, Y.; Paris, C.; Serfaty, J.M.; Weinmann, P.; Lecouvey, M.; Motte, L. Bis-phosphonates-ultra small superparamagnetic iron oxide nanoparticles: A platform towards diagnosis and therapy. Chem. Commun. 2008, 22, 2553–2555. [Google Scholar] [CrossRef] [PubMed]
- Benyettou, F.; Lalatonne, Y.; Chebbi, I.; Di Benedetto, M.; Serfaty, J.-M.; Lecouvey, M.; Motte, L. A multimodal magnetic resonance imaging nanoplatform for cancer theranostics. Phys. Chem. Chem. Phys. 2011, 13, 10020–10027. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ohsumi, S.; Makino, K. Mechanistic studies on depurination and apurinic site breakage in oligodeoxyribonucleotides. Nucleic Acids Res. 1994, 22, 4997–5003. [Google Scholar] [CrossRef] [PubMed]
- Gilar, M.; Fountain, K.J.; Budman, Y.; Holyoke, J.L.; Davoudi, H.; Gebler, J.C. Characterization of therapeutic oligonucleotides using liquid chromatography with on-line mass spectrometry detection. Oligonucleotides 2003, 13, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.L.; Hirschbein, B.L.; Chiu, C.Y.; Quijano, M.R.; Zon, G. Phosphorothioate oligodeoxynucleotides: Large-scale synthesis and analysis, impurity characterization, and the effects of phosphorus stereochemistry. Ciba Found. Symp. 1997, 209, 19–31. [Google Scholar] [PubMed]
- Saenger, W. Principles of Nucleic Acid Structure, 2nd ed.; Springer-Verlag: New York, NY, USA, 1988. [Google Scholar]
- Mallajosyula, S.S.; Pati, S.K. Effect of protonation on the electronic properties of DNA base pairs: Applications for molecular electronics. J. Phys. Chem. B 2007, 111, 11614–11618. [Google Scholar] [CrossRef] [PubMed]
- Gilar, M.; Belenky, A.; Smisek, D.L.; Bourque, A.; Cohen, A.S. Kinetics of phosphorothioate oligonucleotide metabolism in biological fluids. Nucleic Acids Res. 1997, 25, 3615–3620. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, C.; Luck, G.; Venner, H.; Fric, J. Studies on the conformation of protonated DNA. Biopolymers 1968, 6, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, V.A.; Gladchenko, G.O.; Valeev, V.A. DNA protonation at low ionic strength of solution. Makromol. Chem. 1986, 187, 1053–1063. [Google Scholar] [CrossRef]
- Banyay, M.; Sarkar, M.; Gräslund, A. A library of IR bands of nucleic acids in solution. Biophys. Chem. 2003, 104, 477–488. [Google Scholar] [CrossRef]
- White, A.P.; Reeves, K.K.; Snyder, E.; Farrell, J.; Powell, J.W.; Mohan, V.; Griffey, R.H.; Sasmor, H. Hydration of single-stranded phosphodiester and phosphorothioate oligodeoxyribonucleotides. Nucleic Acids Res. 1996, 24, 3261–3266. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.; Bertrand, H.O.; Fermandjian, S. Sequence effects on energetic and structural properties of phosphorothioate DNA: A molecular modelling study. Nucleic Acids Res. 1999, 27, 3342–3347. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, Z.; Nsangou, M.; Hernandez, B.; Pflüger, F.; Liquier, J.; Ghomi, M. Geometrical and vibrational features of phosphate, phosphorothioate and phosphorodithioate linkages with hydrated cations: A DFT study. Spectrochim. Acta A 2009, 73, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Kyogoku, Y.; Shimanouchi, T. Infrared absorption spectra of protonated and deprotonated nucleosides. Biochim. Biophys. Acta 1962, 55, 1–12. [Google Scholar] [CrossRef]
- Paoletti, J.; le Pecq, J.B. Resonance energy transfer between ethidium bromide molecules bound to nucleic acids. Does intercalation wind or unwind the DNA helix? J. Mol. Biol. 1971, 59, 43–62. [Google Scholar] [CrossRef]
- Pigram, W.J.; Fuller, W.; Davies, M.E. Letter: Unwinding the DNA helix intercalation. J. Mol. Biol. 1973, 80, 361–365. [Google Scholar] [CrossRef]
- Cuniberti, C.; Guenza, M. Environment-induced changes in DNA conformation as probed by ethidium bromide fluorescence. Biophys. Chem. 1990, 38, 11–22. [Google Scholar] [CrossRef]
- Nelson, J.W.; Tinoco, I., Jr. Intercalation of ethidium ion into DNA and RNA oligonucleotides. Bioploymers 1984, 23, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Frantz, S. Determination of DNase activity by degradation of ethidium bromide-DNA complexes using a fluorescence plate reader. Anal. Biochem. 2015, 471, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Aloj, L.; Aurilio, M.; Morelli, G.; Tesauro, D. Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs. Int. J. Nanomed. 2014, 9, 1537–1557. [Google Scholar]
- Bolley, J.; Lalatonne, Y.; Haddad, O.; Letourneur, D.; Soussan, M.; Pérard-Viret, J.; Motte, L. Optimized multimodal nanoplatforms for targeting αvβ3 integrins. Nanoscale 2013, 5, 11478–11489. [Google Scholar] [CrossRef] [PubMed]
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B.; Danhier, F.; Préat, V. Comparison of active, passive and magnetic targeting to tumors of multifounctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release 2014, 194, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; He, Q.; Shi, J. MSN-Mediated sequential vascular-to-cell nuclear-targeted drug delivery for efficient tumor regression. Adv. Mater. 2014, 26, 6742–6748. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.H.; Weissleder, R. Arginine containing peptides as delivery vectors. Adv. Drug Deliv. Rev. 2003, 55, 281–294. [Google Scholar] [CrossRef]
- Traboulsi, H.; Larkin, H.; Bonin, M.A.; Volkov, L.; Lavoie, C.; Marsault, E. Macrocyclic cell penetrating peptides: A study of structure-penetration properties. Bioconjug. Chem. 2015, 26, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Motte, L.; Benyettou, F.; de Beaucorps, C.; Lecouvey, M.; Milesovic, I.; Lalatonne, Y. Multimodal Superparamagnetic Nanoplatform for Clinical Applications: Immunoassays, Imaging & Therapy. Faraday Discuss. 2011, 149, 211–225. [Google Scholar] [PubMed]
- Nikitin, P.I.; Vetoshko, P.M. Analysis of Biological and/or Chemical Mixtures Using Magnetic Particles. 7 March 2001. [Google Scholar]
- Nikitin, M.P.; Torno, M.; Chen, H.; Rosengart, A.; Nikitin, P.I. Quantitative Real-Time In Vivo Detection of Magnetic Nanoparticles by Their Nonlinear Magnetization. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Raynal, I.; Prigent, P.; Peyramaure, S.; Najid, A.; Rebuzzi, C.; Corot, C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: Mechanism and comparison of ferumoxides and ferumoxtran-10. Investig. Radiol. 2004, 39, 56–63. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geinguenaud, F.; Banissi, C.; Carpentier, A.F.; Motte, L. Iron Oxide Nanoparticles Coated with a Phosphorothioate Oligonucleotide and a Cationic Peptide: Exploring Four Different Ways of Surface Functionalization. Nanomaterials 2015, 5, 1588-1609. https://doi.org/10.3390/nano5041588
Geinguenaud F, Banissi C, Carpentier AF, Motte L. Iron Oxide Nanoparticles Coated with a Phosphorothioate Oligonucleotide and a Cationic Peptide: Exploring Four Different Ways of Surface Functionalization. Nanomaterials. 2015; 5(4):1588-1609. https://doi.org/10.3390/nano5041588
Chicago/Turabian StyleGeinguenaud, Frédéric, Claire Banissi, Antoine F. Carpentier, and Laurence Motte. 2015. "Iron Oxide Nanoparticles Coated with a Phosphorothioate Oligonucleotide and a Cationic Peptide: Exploring Four Different Ways of Surface Functionalization" Nanomaterials 5, no. 4: 1588-1609. https://doi.org/10.3390/nano5041588
APA StyleGeinguenaud, F., Banissi, C., Carpentier, A. F., & Motte, L. (2015). Iron Oxide Nanoparticles Coated with a Phosphorothioate Oligonucleotide and a Cationic Peptide: Exploring Four Different Ways of Surface Functionalization. Nanomaterials, 5(4), 1588-1609. https://doi.org/10.3390/nano5041588