2.1. Characterization of Electrophoretically Prepared LDH-Agarose Composites
Figure 1 exhibited photographs and scanning electron microscopic (SEM) images of electrophoretically-prepared LDH-agarose (1 wt/v% agarose concentration) composite, namely C-1, along with those images of agarose (1 wt/v%) hydrogel (A-1). Photographs were taken with water containing the hydrogel state and SEM images were obtained with dehydrated hydrogel films. As shown in the photograph, C-1 contained a translucent white region below the white dotted line, while A-1 was homogeneously transparent. As we filled the anionic and cationic precursor solution to the white dotted line during electrophoresis, LDH nanoparticles might develop in that region. As the white color in the LDH developed region was fairly homogeneous, the formation of LDH nanoparticles was considered to occur uniformly throughout the hydrogel, as reported previously with hydrozincite [
17]. Thus, we could conclude that LDH nanoparticles existed in a colloid-like state being entrapped by the agarose moiety. According to this result, we could suggest the formation mechanism of LDH nanoparticles. Metal cations (Mg
2+ and Al
3+) in anodic compartment and anionic species (CO
32− and OH
−) in cathodic compartment moved to opposite directions, cathode and anode direction, respectively, upon potential application. It is thought that those ionic species (cations and anions) met inside the agarose hydrogel, resulting in development of metal hydroxycarbonate precipitates. As a result, LDH nanoparticles were formed inside the hydrogel.
Figure 1.
Photographs and scanning electron microscopic (SEM) images of agarose only hydrogel (1 wt/v% agarose concentration, A-1) and Layered double hydroxide (LDH)-agarose composite hydrogel (1 wt/v% agarose concentration, C-1). For SEM images, both A-1 and C-1 were dehydrated to film.
Figure 1.
Photographs and scanning electron microscopic (SEM) images of agarose only hydrogel (1 wt/v% agarose concentration, A-1) and Layered double hydroxide (LDH)-agarose composite hydrogel (1 wt/v% agarose concentration, C-1). For SEM images, both A-1 and C-1 were dehydrated to film.
The SEM image of C-1 (
Figure 1) showed small particles that were on the magnitude of ten nanometers in size and were homogeneously distributed through the smooth surface (agarose polymer matrix). On the other hand, the agarose only (A-1) showed a flat and clean surface, which was attributed to the strong intermolecular interaction of amorphous organic moiety [
19], and no significant particles were found. Compared with LDH nanoparticles in the powder form (
Figure S1), those in C-1 showed a blurred grain boundary and blunt edges, similar to that found in inorganic nanoparticles coated with organic moiety [
20]. Therefore, it could be suggested that every LDH nanoparticle was homogeneously coated with polysaccharide chains of agarose. This could be possible by two means. First, the LDH had a positive surface charge, which could strongly interact with the partial negative charges of the polysaccharides [
21]. Second, the crystal growth of the LDH nanoparticle occurred homogeneously among the agarose matrix and, thus, the particles readily interacted with adjacent polysaccharide.
Powder X-ray diffraction (XRD) analyses were employed in order to indirectly confirm that the small particles found in the composite were LDHs, utilizing reconstruction property of LDHs [
22]. LDHs (Mg
2Al(OH)
6(CO
32−)
0.5) are well known to transform into a mixed metal oxide (MMO) through dehydroxylation and decarbonation when they are calcined at ~600 °C. Then MMOs could recover their original LDH structure by treatment with water and carbonate ions, which is referred to as reconstruction [
23]. In this study, three kinds of LDH-agarose composites were electrophoretically-prepared starting with 0.5, 1 and 2 wt/v% agarose hydrogel and those composites were named C-0.5, C-1, and C-2, respectively, where the number represents concentration of agarose in the hydrogel. All of the XRD patterns of the calcined composites showed the periclase phase (JCPDS No. 71-1176) [
24], which is the typical form of calcined Mg
2Al(OH)
6(CO
32−)
0.5-LDH (
Figure 2A). The XRD patterns of the calcined composites after treatment with water and carbonate ions (
Figure 2B) exhibited well-crystallized hydrotalcite (Mg
2Al(OH)
6(CO
32−)
0.5-LDH) phase (JCPDS No. 14-0191) [
25]. These results strongly suggested that the particles found in each composite were LDH nanoparticles.
Figure 2.
X-ray diffraction (XRD) patterns of (A) calcined composites and (B) reconstructed ones for (a) LDH-agarose composite hydrogel (0.5 wt/v% agarose concentration, C-0.5), (b) LDH-agarose composite hydrogel (1 wt/v% agarose concentration, C-1) and (c) LDH-agarose composite hydrogel (2 wt/v% agarose concentration, C-2), respectively. XRD patterns of the calcined composite (A) showed a typical phase of periclase (JCPDS No. 71-1176), and those of reconstructed ones (B) exhibited the layered double hydroxide (LDH) phase (hydrotalcite, JCPDS No. 14-0191). The vertical lines under the XRD patterns are the corresponding JCPDS patterns.
Figure 2.
X-ray diffraction (XRD) patterns of (A) calcined composites and (B) reconstructed ones for (a) LDH-agarose composite hydrogel (0.5 wt/v% agarose concentration, C-0.5), (b) LDH-agarose composite hydrogel (1 wt/v% agarose concentration, C-1) and (c) LDH-agarose composite hydrogel (2 wt/v% agarose concentration, C-2), respectively. XRD patterns of the calcined composite (A) showed a typical phase of periclase (JCPDS No. 71-1176), and those of reconstructed ones (B) exhibited the layered double hydroxide (LDH) phase (hydrotalcite, JCPDS No. 14-0191). The vertical lines under the XRD patterns are the corresponding JCPDS patterns.
Given that the SEM measurement was carried out with a dehydrated composite film, LDH particles could gather together during dehydration, resulting in LDH-agarose agglomerates (
Figure 1). To verify that the inter-particle distance of the LDH was sufficient to form a colloidal state in the composite, we carried out atomic force microscopy (AFM) measurements on the composite (C-1) containing a water moiety (
Figure 3). For comparison, we also measured the surface topology of the agarose hydrogel (A-1) and LDH-agarose mixture (M-1). Here, the LDH-agarose mixture stands for the hydrogel monolith prepared by simply mixing a hot agarose solution and powdered LDH. The amount of agarose and LDH was set to be equivalent to that of the corresponding composite. The number at the end of the sample name, like in the case of the composites, represents the concentration of agarose in hydrogel. The AFM image of A-1 was fairly flat and smooth. The composite (C-1) showed several particles (arrows in
Figure 3b) sparsely distributed on a flat surface. The line profile of the single LDH nanoparticle showed a particle size approximately 20 nm (
Figure 3b), which corresponded to the particle size found in SEM (
Figure 1). In contrast, M-1 showed relatively large lumps of approximately micron-sized particles, suggesting that the powdered nanoparticles was not homogeneously distributed in the agarose hydrogel matrix through simple mixing. Thus, we proposed that the electrophoretically-prepared composite contained LDH nanoparticle moieties in a quasi-colloidal state. In the colloid, each particle was stably suspended in media through strong inter-particle repulsion [
26]. Similarly, the LDH nanoparticles in the composite stayed in the agarose hydrogel matrix maintaining an appropriate inter-particle distance.
Figure 3.
Three-dimensional atomic force microscopic (AFM) images and corresponding line profiles along the double-headed arrow for (a) agarose only hydrogel (1 wt/v% agarose concentration, A-1), (b) LDH-agarose composite hydrogel (1 wt/v% agarose concentration, C-1) and (c) LDH-agarose mixture hydrogel (1 wt/v% agarose concentration, M-1), respectively. Single-headed arrows in (b) stand for singe LDH nanoparticles distributed in agarose matrix.
Figure 3.
Three-dimensional atomic force microscopic (AFM) images and corresponding line profiles along the double-headed arrow for (a) agarose only hydrogel (1 wt/v% agarose concentration, A-1), (b) LDH-agarose composite hydrogel (1 wt/v% agarose concentration, C-1) and (c) LDH-agarose mixture hydrogel (1 wt/v% agarose concentration, M-1), respectively. Single-headed arrows in (b) stand for singe LDH nanoparticles distributed in agarose matrix.
2.3. Evaluation of Chromate Removal Efficacy of Composite and Effect of Quasi-Colloidal LDH
The anion scavenging ability of the composite, which was thought to contain quasi-colloidal LDH nanoparticles, was evaluated by time and concentration-dependent chromate (CrO42−) removal experiments. In order to determine the role of quasi-colloidal LDH nanoparticles in the composite on anion removal, the same experiments were also carried out on agarose only hydrogels and LDH-agarose mixture hydrogels.
As shown in
Figure 4, we verified that the adsorption reached equilibrium after 24 h at pH ~7.5.
Figure 4a–c showed that the chromate removal efficacy increased as the concentration of agarose hydrogel increased. Composites showed the highest removal efficacy (~41, ~68, and 101 mg CrO
42−/g dry weight) suggesting that homogeneous distribution of LDH nanoparticles in the composites facilitated chromate removal.
Figure 4.
Time-dependent chromate removal efficacy (mg chromate/g dry weight) of agarose only (A), LDH-agarose mixture (M) and LDH-agarose composite (C) samples for (a) 0.5 wt/v%; (b) 1 wt/v%; and (c) 2 wt/v% agarose-based materials. The experiments were carried out at initial chromate concentration of 400 ppm and at pH ~7.5.
Figure 4.
Time-dependent chromate removal efficacy (mg chromate/g dry weight) of agarose only (A), LDH-agarose mixture (M) and LDH-agarose composite (C) samples for (a) 0.5 wt/v%; (b) 1 wt/v%; and (c) 2 wt/v% agarose-based materials. The experiments were carried out at initial chromate concentration of 400 ppm and at pH ~7.5.
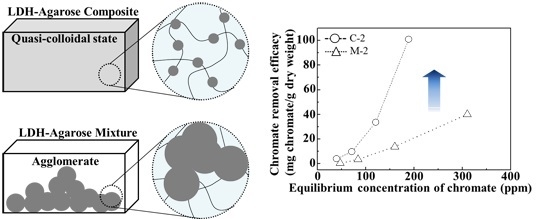
For adsorption isotherm examination, we utilized 24 h of incubation time and pH value of ~7. As shown in
Figure 5, all the tested samples showed concentration dependent chromate removal efficacy. The maximum removal efficacy at 310, 250 and 188 ppm equilibrium chromate solution was ~45, ~70, and 100 mg CrO
42−/g dry weight for C-0.5, C-1, and C-2, respectively. On the other hand, both A and M exhibited maximum removal of ~20, ~35, and 46 mg CrO
42−/g dry weight for 0.5, 1, and 2 wt/v% hydrogel based samples, respectively. Although the M sample contained an equivalent amount of LDHs (which have anion scavenging capacity), compared with C samples, they did not show any significant advantage of LDHs. In M samples, the LDH particles were agglomerated into a micron-sized particle (
Figure 3) and, thus, their surface could not fully play the role of an anion adsorption site. In contrast, the composites, where LDH nanoparticles were homogenously distributed in a quasi-colloidal state, could take maximum advantage of the LDH surface as the anion adsorption site.
Figure 5.
Isotherms of chromate removal efficacy (mg chromate/g dry weight) of agarose only (A), LDH-agarose mixture (M) and LDH-agarose composite (C) samples for (a) 0.5 wt/v%; (b) 1 wt/v%; and (c) 2 wt/v% agarose-based materials. The experiments were carried out at pH 7.2 ± 0.3 after 24 h.
Figure 5.
Isotherms of chromate removal efficacy (mg chromate/g dry weight) of agarose only (A), LDH-agarose mixture (M) and LDH-agarose composite (C) samples for (a) 0.5 wt/v%; (b) 1 wt/v%; and (c) 2 wt/v% agarose-based materials. The experiments were carried out at pH 7.2 ± 0.3 after 24 h.
In order to quantitatively evaluate the role of LDH in either C or M samples, we computed chromate removal by the LDH moiety itself by subtracting the chromate removal resulting from agarose (
Figure 6). As shown in
Figure 5, the agarose hydrogel itself seemed to have a chromate removal ability, possibly through concentration-gradient diffusion. Thus, the removal of chromates by agarose only (A-0.5, A-1, and A-2) was subtracted from that of the composites (C-0.5, C-1, and C-2) and the mixtures (M-0.5, M-1 and M-2), respectively. The baseline at 0 in
Figure 6 meant no adsorption effect by LDH itself. As expected, all composites exhibited positive values of chromate removal by the LDH moiety at all the tested chromate concentration. Furthermore, the anion scavenging functionality of LDH became higher with increasing agarose content in the composite. The LDH moiety in the mixture showed low or even negative effect, which again confirmed that a homogeneous quasi-colloidal dispersion of LDH nanoparticles in the composite promoted chromate removal. Interestingly, increasing the amount of agarose in the composite enhanced LDH’s chromate scavenging capacity. As the amount of LDH in the composites was similar regardless of agarose concentration, this result could be explained by modified environment around LDH nanoparticles inside the hydrogel. Polysaccharide chains having δ
− sites can be weakly bound to the positive surface of LDH and thus, LDH nanoparticles could be sufficiently separated at high amount of the agarose moiety. Thus, it could be expected that increasing the agarose concentration enhanced the colloidal stability of LDH nanoparticles inside of the hydrogel. When the strongly negative chromate ions approached LDH, polysaccharides were readily replaced by them.
Figure 6.
Estimated chromate removal efficacy (μmol/g) by LDH moiety in LDH-agarose composite (C) and LDH-agarose mixture (M) samples at initial chromate concentration of (a) 50 ppm, (b) 100 ppm, (c) 200 ppm, and (d) 400 ppm, respectively. For calculation, chromate removal by C or M sample was subtracted by the chromate removal by agarose only at the same chromate concentration.
Figure 6.
Estimated chromate removal efficacy (μmol/g) by LDH moiety in LDH-agarose composite (C) and LDH-agarose mixture (M) samples at initial chromate concentration of (a) 50 ppm, (b) 100 ppm, (c) 200 ppm, and (d) 400 ppm, respectively. For calculation, chromate removal by C or M sample was subtracted by the chromate removal by agarose only at the same chromate concentration.
LDHs are known to have a high anion capacity, utilizing their positive surface. We verified that the interlayer carbonate can be partially exchanged by chromate (
Figure S2). Thus, we could suggest that the chromate removal in current samples occurred by partial interlayer exchange as well as surface adsorption. Supposing that Mg
2Al(OH)
6(CO
3)
2−0.5-LDH with 20 nm size adsorbs anions on their surface only, an anion capacity of ~100 μmol(-)/g could be calculated. If that LDH particle uses interlayer space through an anion exchange reaction, the theoretical anion capacity could be as high as 4400 μmol(-)/g. The maximum chromate adsorption by the LDH itself (
Figure 6) was larger than 1000 μmol(-)/g for all three composites (considering that chromate had two sites of negative charges, the values in
Figure 6 was doubled.). Even the LDH moiety in C-2 showed a chromate adsorption of 2400 μmol(-)/g. This indicated that the LDH nanoparticles in the composite could adsorb chromate ions by multi-layer surface adsorption, partially utilizing their interlayer space. Thus, we fitted isotherm curves with
Figure 5 to the well-established multi-layer adsorption Freundlich model using the equation below:
where,
Qe: adsorbed amount at equilibrium in mg/g unit,
Ce: chromate concentration at equilibrium in mg/L unit K
F: the adsorption coefficient (
KF =
Kadsorption/
Kdesorption),
n: a correlation coefficient indicating adsorption behavior.
The fitting result, displayed in
Table 2, showed that the chromate removal of C samples was well-fitted to the Freundlich model, exhibiting a high coefficient of determination
R2F. The correlation coefficient
n is known to be related to the binding mode of adsorbate; adsorption is cooperative when
n < 1 and anti-cooperative with
n > 1. The coefficient
n of the C samples were lower than 1, suggesting that the composite displayed cooperative multi-layer chromate adsorption tendencies. It was interesting that the
n values of the composites decreased with increasing agarose content. As expected from
Figure 6, increasing the agarose content could enhance the colloidal stability of the LDH, resulting in facilitated adsorption of chromate ions. The adsorption coefficient K
F (= K
adsorption/K
desorption) revealed effective chromate removal of the C samples compared with corresponding M or A samples, exhibiting higher values than others.
Table 2.
Freundlich adsorption model fitting results for agarose only hydrogels (A), LDH-agarose composite hydrogels (C) and LDH-agarose mixture hydrogels (M) samples.
Table 2.
Freundlich adsorption model fitting results for agarose only hydrogels (A), LDH-agarose composite hydrogels (C) and LDH-agarose mixture hydrogels (M) samples.
| Samples | n | KF (dm3g−1) | R2F |
|---|
| A-0.5 | 1.10 | 0.633 | 0.856 |
| C-0.5 | 0.91 | 0.843 | 0.987 |
| M-0.5 | 1.03 | 0.725 | 0.937 |
| A-1 | 0.96 | 0.758 | 0.959 |
| C-1 | 0.85 | 0.797 | 0.981 |
| M-1 | 0.94 | 0.537 | 0.827 |
| A-2 | 0.90 | 0.785 | 0.976 |
| C-2 | 0.80 | 0.854 | 0.992 |
| M-2 | 0.93 | 0.559 | 0.854 |