Influence of Nitrogen Doping on Device Operation for TiO2-Based Solid-State Dye-Sensitized Solar Cells: Photo-Physics from Materials to Devices
Abstract
:1. Introduction
2. Results
2.1. Properties of the Starting Powders
2.2. Photovoltaic Performance of ssDSSC Based on N-Doped TiO2 Electrodes
2.3. Charge Kinetics Probed by Transient Photo-Voltage at the Device Level
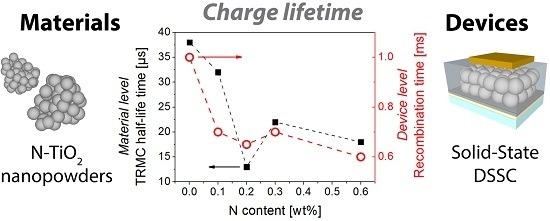
2.4. Charge Kinetics Probed by Transient Techniques at the Material Level
2.5. Photo-Conductivity Measurements on Porous Electrodes
3. Discussion
4. Materials and Methods
4.1. Synthesis of N-Doped TiO2 Nanocrystals
4.2. Device Fabrication
4.3. Characterization Techniques
4.4. Transient Photo-Voltage Measurements
4.5. Time-Resolved Microwave Conductivity Measurements (TRMC)
4.6. Photo-Conductivity Measurements
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bach, U.; Lupo, D.; Comte, P.; Moser, J.E.; Weissörtel, F.; Salbeck, J.; Spreitzer, H.; Grätzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar]
- Burschka, J.; Dualeh, A.; Kessler, F.; Baranoff, E.; Cevey-Ha, N.-L.; Yi, C.; Nazeeruddin, M.K.; Grätzel, M. Tris(2-(1H-pyrazol-1-yl)pyridine)cobalt(III) as p-Type Dopant for Organic Semiconductors and Its Application in Highly Efficient Solid-State Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2011, 133, 18042–18045. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gabrielsson, E.; Safdari, M.; Cheng, M.; Hua, Y.; Tian, H.; Gardner, J.M.; Kloo, L.; Sun, L. 1,1,2,2-Tetrachloroethane (TeCA) as a Solvent Additive for Organic Hole Transport Materials and Its Application in Highly Efficient Solid-State Dye-Sensitized Solar Cells. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Ahmad, S.; Guillén, E.; Kavan, L.; Grätzel, M.; Nazeeruddin, M.K. Metal free sensitizer and catalyst for dye sensitized solar cells. Energy Environ. Sci. 2013, 6, 3439–3466. [Google Scholar] [CrossRef]
- Li, L.L.; Diau, E.W.G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nano 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; de Marco, N.; Yang, Y.; Song, T.-B.; Chen, C.-C.; Zhao, H.; Hong, Z.; Zhou, H. Under the spotlight: The organic–inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Bui, T.T.; Goubard, F. Small organic molecule hole transporting materials for solid-state dye-sensitized solar cells. Mater. Tech. 2013, 101. [Google Scholar] [CrossRef]
- Aulakh, R.K.; Sandhu, S.; Tanvi; Kumar, S.; Mahajan, A.; Bedi, R.K. Designing and synthesis of imidazole based hole transporting material for solid state dye sensitized solar cells. Synth. Met. 2015, 205, 92–97. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, Y.C.; Lin, R.Y.Y.; Ho, K.C.; Lin, J.T. Solid-state dye-sensitized solar cells based on spirofluorene (spiro-OMeTAD) and arylamines as hole transporting materials. Phys. Chem. Chem. Phys. 2012, 14, 14099–14109. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.-Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P. Air-Stable Molecular Semiconducting Iodosalts for Solar Cell Applications: Cs2SnI6 as a Hole Conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Daniel, Q.; Pazoki, M.; Sveinbjornsson, K.; Zhang, J.; Sun, L.; Hagfeldt, A.; Boschloo, G. High-efficiency dye-sensitized solar cells with molecular copper phenanthroline as solid hole conductor. Energy Environ. Sci. 2015, 8, 2634–2637. [Google Scholar] [CrossRef]
- Bouclé, J.; Ackermann, J. Solid-state dye-sensitized and bulk heterojunction solar cells using TiO2 and ZnO nanostructures: Recent progress and new concepts at the borderline. Polym. Int. 2012, 61, 355–373. [Google Scholar] [CrossRef]
- Docampo, P.; Guldin, S.; Leijtens, T.; Noel, N.K.; Steiner, U.; Snaith, H.J. Lessons Learned: From Dye-Sensitized Solar Cells to All-Solid-State Hybrid Devices. Adv. Mater. 2014, 26, 4013–4030. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Xu, Y.F.; Kuang, D.B.; Su, C.Y. Recent advances in hierarchical macroporous composite structures for photoelectric conversion. Energy Environ. Sci. 2014, 7, 3887–3901. [Google Scholar] [CrossRef]
- Concina, I.; Vomiero, A. Metal oxide semiconductors for dye- and quantum-dot-sensitized solar cells. Small 2015, 11, 1744–1774. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, X.; Lu, B.; Chen, L.; Wang, Y.; Teng, F.; Wang, J.; Zhang, Z.; Pan, X.; Xie, E. A facile method to prepare SnO2 nanotubes for use in efficient SnO2–TiO2 core-shell dye-sensitized solar cells. Nanoscale 2012, 4, 3475–3481. [Google Scholar] [CrossRef] [PubMed]
- Antila, L.J.; Heikkilä, M.J.; Mäkinen, V.; Humalamäki, N.; Laitinen, M.; Linko, V.; Jalkanen, P.; Toppari, J.; Aumanen, V.; Kemell, M. ALD grown aluminum oxide submonolayers in dye-sensitized solar cells: The effect on interfacial electron transfer and performance. J. Phys. Chem. C 2011, 115, 16720–16729. [Google Scholar] [CrossRef]
- Liu, Y.; Zhai, H.; Guo, F.; Huang, N.; Sun, W.; Bu, C.; Peng, T.; Yuan, J.; Zhao, X. Synergistic effect of surface plasmon resonance and constructed hierarchical TiO2 spheres for dye-sensitized solar cells. Nanoscale 2012, 4, 6863–6869. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wang, L.; Jia, L.; Li, Q.; Song, Q.; Su, S.; Yang, H. A novel biomass coated Ag–TiO2 composite as a photoanode for enhanced photocurrent in dye-sensitized solar cells. RSC Adv. 2013, 3, 6369–6376. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Wang, P.; Zheng, J.; Zhang, X.; Cui, Y.; Feng, J.; Zhu, Y. Sulfur-doped TiO2 nanocrystalline photoanodes for dye-sensitized solar cells. J. Renew. Sustain. Energy 2012, 4. [Google Scholar] [CrossRef]
- Ma, T.; Akiyama, M.; Abe, E.; Imai, I. High-efficiency dye-sensitized solar cell based on a nitrogen-doped nanostructured titania electrode. Nano Lett. 2005, 5, 2543–2547. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, H.; Wang, J.; Wu, J.; Li, D.; Liu, M.; Su, P. Nitrogen-doped TiO2 nanoparticles better TiO2 nanotube array photo-anodes for dye sensitized solar cells. Electrochim. Acta 2014, 137, 744–750. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, J.; Li, H.; Wang, H.; Jia, X.; Su, P. The influence of yttrium dopant on the properties of anatase nanoparticles and the performance of dye-sensitized solar cells. Phys. Chem. Chem.Phys. 2015, 17, 14836–14842. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, T.; Mwabora, J.M.; Avandaño, E.; Jonsson, J.; Hoel, A.; Granqvist, C.G.; Lindquist, S.E. Photoelectrochemical and optical properties of nitrogen doped titanium dioxide films prepared by reactive DC magnetron sputtering. J. Phys. Chem. B 2003, 107, 5709–5716. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T. Nitrogen complex species and its chemical nature in TiO2 for visible-light sensitized photocatalysis. Chem. Phys. 2007, 339, 57–63. [Google Scholar] [CrossRef]
- Tian, H.; Hu, L.; Zhang, C.; Liu, W.; Huang, Y.; Mo, L.; Guo, L.; Sheng, J.; Dai, S. Retarded charge recombination in dye-sensitized nitrogen-doped TiO2 solar cells. J. Phys. Chem. C 2010, 114, 1627–1632. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Origin of the different photoactivity of N-doped anatase and rutile TiO2. Phys. Rev. B 2004, 70. [Google Scholar] [CrossRef]
- Melhem, H.; Simon, P.; Wang, J.; di Bin, C.; Ratier, B.; Leconte, Y.; Herlin-Boime, N.; Makowska-Janusik, M.; Kassiba, A.; Bouclé, J. Direct photocurrent generation from nitrogen doped TiO2 electrodes in solid-state dye-sensitized solar cells: Towards optically-active metal oxides for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2013, 117, 624–631. [Google Scholar] [CrossRef]
- Diker, H.; Varlikli, C.; Stathatos, E. N-doped titania powders prepared by different nitrogen sources and their application in quasi-solid state dye-sensitized solar cells. Int. J. Energy Res. 2014, 38, 908–917. [Google Scholar] [CrossRef]
- Pignon, B.; Maskrot, H.; Ferreol, V.G.; Leconte, Y.; Coste, S.; Gervais, M.; Pouget, T.; Reynaud, C.; Tranchant, J.F.; Herlin-Boime, N. Versatility of laser pyrolysis applied to the synthesis of TiO2 nanoparticles—Application to UV attenuation. Eur. J. Inorg. Chem. 2008, 2008, 883–889. [Google Scholar] [CrossRef]
- Melhem, H.; Simon, P.; Beouch, L.; Goubard, F.; Boucharef, M.; Di Bin, C.; Leconte, Y.; Ratier, B.; Herlin-Boime, N.; Bouclé, J. TiO2 Nanocrystals Synthesized by Laser Pyrolysis for the Up-Scaling of Efficient Solid-Stage Dye-Sensitized Solar Cells. Adv. Energy Mater. 2011, 1, 908–916. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Pinault, M.; Filoramo, A.; Fabert, M.; Ratier, B.; Bouclé, J.; Herlin-Boime, N. Single-Step Preparation of TiO2/MWCNT Nanohybrid Materials by Laser Pyrolysis and Application to Efficient Photovoltaic Energy Conversion. ACS Appl. Mater. Interfaces 2015, 7, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Spurr, R.A.; Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures; Wiley: New York, NY, USA, 1954. [Google Scholar]
- Kang, S.H.; Kim, H.S.; Kim, J.Y.; Sung, Y.E. Enhanced photocurrent of nitrogen-doped TiO2 film for dye-sensitized solar cells. Mater. Chem. Phys. 2010, 124, 422–426. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, G.; Dong, C.; Kim, K.H. Mechanical and optical properties of composite TiOxNy films prepared by pulsed bias arc ion plating. Curr. Appl. Phys. 2009, 9, S174–S178. [Google Scholar] [CrossRef]
- Amadelli, R.; Samiolo, L.; Borsa, M.; Bellardita, M.; Palmisano, L. N-TiO2 Photocatalysts highly active under visible irradiation for NOX abatement and 2-propanol oxidation. Catal. Today 2013, 206, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of Photocatalytic Activity of Nitrogen-Doped TiO2 Nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Shen, Y.; Wu, L.; Gao, Y.; Ma, T. Effect of N dopant amount on the performance of dye-sensitized solar cells based on N-Doped TiO2 electrodes. J. Phys. Chem. C 2011, 115, 21494–21499. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Bach, U.; Humphry-Baker, R.; Horiuchi, T.; Miura, H.; Ito, S.; Uchida, S.; Grätzel, M. Organic Dye for Highly Efficient Solid-State Dye-Sensitized Solar Cells. Adv. Mater. 2005, 17, 813–815. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, N.; Liu, Y.; Sun, W.; Mehnane, H.F.; You, S.; Wang, L.; Liu, W.; Guo, S.; Zhao, X.-Z. Photoelectrodes modification by N doping for dye-sensitized solar cells. Electrochim. Acta 2013, 93, 202–206. [Google Scholar] [CrossRef]
- Tian, H.; Hu, L.; Zhang, C.; Mo, L.; Li, W.; Sheng, J.; Dai, S. Superior energy band structure and retarded charge recombination for Anatase N, B codoped nano-crystalline TiO2 anodes in dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 9123–9130. [Google Scholar] [CrossRef]
- Guo, W.; Shen, Y.; Boschloo, G.; Hagfeldt, A.; Ma, T. Influence of nitrogen dopants on N-doped TiO2 electrodes and their applications in dye-sensitized solar cells. Electrochim. Acta 2011, 56, 4611–4617. [Google Scholar] [CrossRef]
- Guo, W.; Miao, Q.Q.; Xin, G.; Wu, L.Q.; Ma, T.L. Dye-sensitized solar cells based on nitrogen-doped titania electrodes. Key Eng. Mater. 2011, 451, 21–27. [Google Scholar] [CrossRef]
- Guo, W.; Wu, L.; Chen, Z.; Boschloo, G.; Hagfeldt, A.; Ma, T. Highly efficient dye-sensitized solar cells based on nitrogen-doped titania with excellent stability. J. Photochem. Photobiol. A 2011, 219, 180–187. [Google Scholar] [CrossRef]
- Guo, W.; Shen, Y.; Wu, L.; Gao, Y.; Ma, T. Performance of Dye-Sensitized Solar Cells Based on MWCNT/TiO2–xNx Nanocomposite Electrodes. Eur. J. Inorg. Chem. 2011, 2011, 1776–1783. [Google Scholar] [CrossRef]
- Kolen’ko, Y.V.; Churagulov, B.R.; Kunst, M.; Mazerolles, L.; Colbeau-Justin, C. Photocatalytic properties of titania powders prepared by hydrothermal method. Appl. Catal. B Environ. 2004, 54, 51–58. [Google Scholar] [CrossRef]
- Carneiro, J.T.; Savenije, T.J.; Moulijn, J.A.; Mul, G. How phase composition influences optoelectronic and photocatalytic properties of TiO2. J. Phys. Chem. C 2011, 115, 2211–2217. [Google Scholar] [CrossRef]
- Meichtry, J.M.; Colbeau-Justin, C.; Custo, G.; Litter, M.I. Preservation of the photocatalytic activity of TiO2 by EDTA in the reductive transformation of Cr(VI). Studies by Time Resolved Microwave Conductivity. Catal. Today 2014, 224, 236–243. [Google Scholar] [CrossRef]
- Segal-Peretz, T.; Leman, O.; Nardes, A.M.; Frey, G.L. On the origin of charge generation in hybrid TiOx/conjugated polymer photovoltaic devices. J. Phys. Chem. C 2012, 116, 2024–2032. [Google Scholar] [CrossRef]
- Abdi, F.F.; Savenije, T.J.; May, M.M.; Dam, B.; van de Krol, R. The origin of slow carrier transport in BiVO4 thin film photoanodes: A time-resolved microwave conductivity study. J. Phys. Chem. Lett. 2013, 4, 2752–2757. [Google Scholar] [CrossRef]
- Saeki, A.; Yasutani, Y.; Oga, H.; Seki, S. Frequency-modulated gigahertz complex conductivity of TiO2 nanoparticles: Interplay of free and shallowly trapped electrons. J. Phys. Chem. C 2014, 118, 22561–22572. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A.; Yamanaka, K.I.; Morikawa, T. Charge separation and trapping in N-doped TiO2 photocatalysts: A time-resolved microwave conductivity study. J. Phys. Chem. Lett. 2010, 1, 3261–3265. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjines, R.; Schmid, P.E.; Levy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and optical properties of anatase TiO2. Phys. Rev. B 2000, 61, 7459–7465. [Google Scholar] [CrossRef]
- Simon, P.; Pignon, B.; Miao, B.; Coste-Leconte, S.; Leconte, Y.; Marguet, S.; Jegou, P.; Bouchet-Fabre, B.; Reynaud, C.; Herlin-Boime, N. N-doped titanium monoxide nanoparticles with TiO rock-salt structure, low energy band gap, and visible light activity. Chem. Mater. 2010, 22, 3704–3711. [Google Scholar] [CrossRef]
- Lee, S.; Jeon, C.; Park, Y. Fabrication of TiO2 tubules by template synthesis and hydrolysis with water vapor. Chem. Mater. 2004, 16, 4292–4295. [Google Scholar] [CrossRef]









| Sample | N content (wt %) | Data extracted from XRD | BET analysis | ||
|---|---|---|---|---|---|
| Fraction of anatase (%) | Mean crystal diameter (nm) | Specific area (m²·g−1) | Mean grain diameter (nm) | ||
| TiO2 | <<0.1 | 94 | 15.6 | 77 | 20 |
| N-TiO2-0.1 | 0.1 | 80 | 12.0 | 86 | 18 |
| N-TiO2-0.2 | 0.2 | 90 | 11.4 | 86 | 18 |
| N-TiO2-0.3 | 0.3 | 94 | 12.4 | 90 | 17 |
| N-TiO2-0.6 | 0.6 | 94 | 15.0 | 96 | 16 |
| Sample | Relative contributions of XPS features | ||
|---|---|---|---|
| Substitutional N (peak at 396 eV) | Interstitial N (peak at 400 eV) | Surface N (peak at 402 eV) | |
| N-TiO2-0.1 | 11% | 89% | Not measurable |
| N-TiO2-0.2 | 16% | 74% | 9% |
| N-TiO2-0.3 | 19% | 58% | 23% |
| N-TiO2-0.6 | 15% | 46% | 39% |
| Nature of porous electrode | VOC (V) | JSC (mA·cm−2) | FF | η (%) |
|---|---|---|---|---|
| TiO2 | 0.77 | 8.31 | 0.62 | 4.0 |
| N-TiO2-0.1 | 0.79 | 8.31 | 0.62 | 4.1 |
| N-TiO2-0.2 | 0.82 | 7.86 | 0.60 | 3.9 |
| N-TiO2-0.3 | 0.77 | 7.00 | 0.61 | 3.3 |
| N-TiO2-0.6 | 0.78 | 6.55 | 0.60 | 3.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Tapio, K.; Habert, A.; Sorgues, S.; Colbeau-Justin, C.; Ratier, B.; Scarisoreanu, M.; Toppari, J.; Herlin-Boime, N.; Bouclé, J. Influence of Nitrogen Doping on Device Operation for TiO2-Based Solid-State Dye-Sensitized Solar Cells: Photo-Physics from Materials to Devices. Nanomaterials 2016, 6, 35. https://doi.org/10.3390/nano6030035
Wang J, Tapio K, Habert A, Sorgues S, Colbeau-Justin C, Ratier B, Scarisoreanu M, Toppari J, Herlin-Boime N, Bouclé J. Influence of Nitrogen Doping on Device Operation for TiO2-Based Solid-State Dye-Sensitized Solar Cells: Photo-Physics from Materials to Devices. Nanomaterials. 2016; 6(3):35. https://doi.org/10.3390/nano6030035
Chicago/Turabian StyleWang, Jin, Kosti Tapio, Aurélie Habert, Sebastien Sorgues, Christophe Colbeau-Justin, Bernard Ratier, Monica Scarisoreanu, Jussi Toppari, Nathalie Herlin-Boime, and Johann Bouclé. 2016. "Influence of Nitrogen Doping on Device Operation for TiO2-Based Solid-State Dye-Sensitized Solar Cells: Photo-Physics from Materials to Devices" Nanomaterials 6, no. 3: 35. https://doi.org/10.3390/nano6030035
APA StyleWang, J., Tapio, K., Habert, A., Sorgues, S., Colbeau-Justin, C., Ratier, B., Scarisoreanu, M., Toppari, J., Herlin-Boime, N., & Bouclé, J. (2016). Influence of Nitrogen Doping on Device Operation for TiO2-Based Solid-State Dye-Sensitized Solar Cells: Photo-Physics from Materials to Devices. Nanomaterials, 6(3), 35. https://doi.org/10.3390/nano6030035