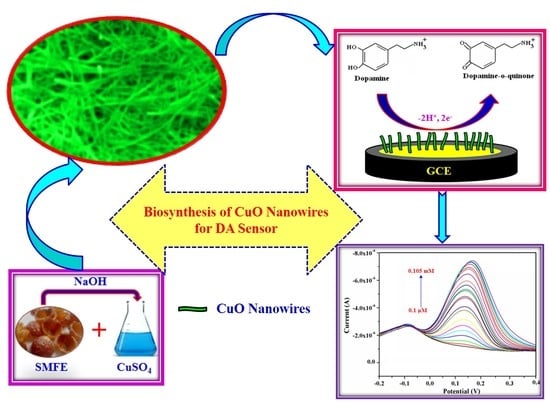
Biosynthesis of Copper Oxide (CuO) Nanowires and Their Use for the Electrochemical Sensing of Dopamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sapindus Mukorossi Fruit Extract (SMFE)
2.3. Preparation of CuO Nanowires Using SMFE
2.4. Characterizations of CuO Nanowires
3. Results and Discussion
3.1. XRD Patterns of the CuO Nanowires
3.2. XPS Spectra of the CuO Nanowires
3.3. FTIR Spectra of the CuO Nanowires
3.4. FE-SEM Pictures of the CuO Nanowires Synthesized Using SMFE
3.5. HR-TEM Pictures of the CuO Nanowires Synthesized Using SMFE
3.6. Growth Mechanisms of CuO Nanowires
3.7. Electrochemical Investigations of the CuO Nanowires
3.8. Electrochemical Sensing Behavior of the CuO Nanowire/GCE for Dopamine
3.9. Redox Behavior of Dopamine
3.10. Influence of the Scan Rate on the Detection of Dopamine
3.11. Effect of Dopamine Concentration on the CuO Nanowires/GCE
3.12. Anti-Interference/Selectivity Study on the CuO Nanowires/GCE
3.13. Selectivity Study on the CuO Nanowires/GCEusing CV and DPV
3.14. Stability/Reproducibility of the CuO Nanowires/GCE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Z.P.; Buehler, M.J. Hierarchical nanostructures are crucial to mitigate ultrasmall thermal point loads. Nano Lett. 2009, 9, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Paladugu, M.; Zou, J.; Guo, Y.N.; Zhang, X.; Joyce, H.J.; Gao, Q.; Tan, H.H.; Jagadish, C.; Kim, Y. Formation of hierarchical InAs nanoring/GaAs nanowire heterostructures. Angew. Chem. Int. Ed. 2009, 48, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, C.; Liu, H.; Du, G.; He, X.; Xi, Y. Synthesis of CuO nanostructures and their application for non enzymatic glucose sensing. Sens. Actuator B Chem. 2010, 144, 220–225. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, I.S.; Kim, S.J.; Lee, C.Y.; Lee, J.H. CuO nanowire gas sensors for air quality control in automotive cabin. Sens. Actuator B Chem. 2008, 135, 298–303. [Google Scholar] [CrossRef]
- Zheng, S.F.; Hu, J.S.; Zhong, L.S.; Song, W.G.; Wan, L.J.; Guo, Y.G. Introducing dual functional CNT networks into CuO nanomicrospheres toward superior electrode materials for lithium-ion batteries. Chem. Mater. 2008, 20, 3617–3622. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, T.; Cheong, F.; Xu, X.; Lim, C.; Tan, V.; Thong, J.; Sow, C.H. Large-scale synthesis and field emission properties of vertically oriented CuO nanowire films. Nanotechnology 2005, 16, 88–92. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Park, K. CuO hollow nanostructures catalyze [3 + 2] cycloaddition of azides with terminal alkynes. Chem. Commun. 2010, 46, 439–441. [Google Scholar] [CrossRef]
- Xiang, J.; Tu, J.; Zhang, L.; Zhou, Y.; Wang, X.; Shi, S. Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries. J. Power Sources 2010, 195, 313–319. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Chen, J.M.; Lin, H.H.; Shih, H.C. Synthesis of well-ordered CuO nanofibers by a self-catalytic growth mechanism. Appl. Phys. Lett. 2003, 82, 3316–3318. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, G.; Wu, Y.; Bai, X.; Guo, D. Cupric oxide nanoflowers synthesized with a simple solution route and their field emission. J. Cryst. Growth 2008, 310, 3125–3130. [Google Scholar] [CrossRef]
- Zhu, J.; Bi, H.; Wang, Y.; Wang, X.; Yang, X.; Lu, L. Synthesis of flower-like CuO nanostructures via a simple hydrolysis route. Mater. Lett. 2007, 61, 5236–5238. [Google Scholar] [CrossRef]
- Teng, F.; Yao, W.; Zheng, Y.; Ma, Y.; Teng, Y.; Xu, T.; Liang, S.; Zhu, Y. Synthesis of flower-like CuO nanostructures as a sensitive sensor for catalysis. Sens. Actuator B Chem. 2008, 134, 761–768. [Google Scholar] [CrossRef]
- Tang, X.L.; Ling, R.; Sun, L.N.; Tian, W.G.; Cao, M.H.; Hu, C.W. A solvothermal route to Cu2O nanocubes and Cu nanoparticles. Chem. Res. Chin. Univ. 2006, 22, 547–551. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.Z.; Zhu, J.J.; Chen, H.Y. Preparation of CuO nanoparticles by microwave irradiation. J. Cryst. Growth 2002, 244, 88–94. [Google Scholar] [CrossRef]
- Das, S.K.; Khan, M.M.R.; Guhab, A.K.; Naskar, N. Bioinspired fabrication of silver nanoparticles on nanostructured silica: Characterization and application as a highly efficient hydrogenation catalyst. Green Chem. 2013, 15, 2548–2557. [Google Scholar] [CrossRef]
- Merims, D.; Giladi, N. Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson’s disease. Parkinsonism Relat. Disord. 2008, 4, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Snowden, M.E.; Unwin, P.R.; Macpherson, J.V. Single walled carbon nanotube channel flow electrode: Hydrodynamic voltammetry at the nanomolar level. Electrochem. Commun. 2011, 13, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, T.; Mirzagholipur, S. A Nafion-free non-enzymatic amperometric glucose sensor based on copper oxide nanoparticles–graphene nanocomposite. Sens. Actuator B Chem. 2014, 198, 438–447. [Google Scholar] [CrossRef]
- Yue, H.Y.; Huang, S.; Chang, J.; Heo, C.; Yao, F.; Adhikari, S.; Gunes, F.; Liu, L.C.; Lee, T.H.; Oh, E.S.; et al. ZnO nanowire arrays on 3D hierachical graphene foam: Biomarker detection of Parkinson’s disease. ACS Nano 2014, 8, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, A.R.; Kannan, R.; Krishnan, S.; Ramakrishnan, S.; Raj, S.M.; Kumaresan, D.; Kothurkar, N.; Rangarajan, M. Electrochemical Sensing of Dopamine, Uric Acid and Ascorbic Acid Using tRGO-TiO2 Nanocomposites. J. Nanosci. Nanotechnol. 2015, 15, 5042–5047. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, W. Electroanalysis of Dopamine at RuO2 Modified Vertically Aligned Carbon Nanotube Electrode. Electroanalysis 2009, 21, 1811–1815. [Google Scholar] [CrossRef]
- Salamon, J.; Sathishkumar, Y.; Ramachandran, K.; Lee, Y.S.; Yoo, D.J.; Kim, A.R.; Kumar, G.G. One-pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens. Bioelectron. 2015, 64, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, Q.; Lai, C.; Zhang, Y.; Deng, J.; Li, H.; Yao, S. A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@Au nanoparticles with graphene sheet. Biosens. Bioelectron. 2013, 48, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yuan, J.; Ye, H.; Song, P.; Hu, S. Facile ultrasonic synthesis of graphene/SnO2 nanocomposite and its application to the simultaneous electrochemical determination of dopamine, ascorbic acid, and uric acid. J. Electroanal. Chem. 2015, 749, 26–30. [Google Scholar] [CrossRef]
- Anithaa, A.C.; Lavanya, N.; Asokan, K.; Sekar, C. Highly sensitive and selective serotonin sensor based on gamma ray irradiated tungsten trioxide nanoparticles. Electrochim. Acta 2015, 167, 294–302. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, L.A.; Patil, D.R.; Jain, G.H.; Wagh, M.S. CuO-doped BSST thick film resistors for ppb level H2S gas sensing at room temperature. Sens. Actuator B Chem. 2007, 123, 246–253. [Google Scholar] [CrossRef]
- Li, C.; Su, Y.; Zhang, S.; Lv, X.; Xia, H.; Wang, Y. An improved sensitivity non-enzymatic glucose biosensor based on a CuxO modified electrode. Biosens. Bioelectron. 2010, 26, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, R.; Biji, P. Preparation of copper (I) oxide nanohexagon decorated reduced graphene oxide nanocomposite and its application in electrochemical sensing of dopamine. Mater. Sci. Eng. B 2016, 210, 10–18. [Google Scholar] [CrossRef]
- Steinhauer, S.; Brunet, E.; Maier, T.; Mutinati, G.C.; Kock, A.; Freudenberg, O.; Gspan, C.; Grogger, W.; Neuhold, A. Gas sensing properties of novel CuO nanowire devices. Sens. Actuator B Chem. 2013, 187, 50–57. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Li, J. Direct electrochemistry of hemoglobin immobilized in CuO nanowire bundles. Talanta 2010, 83, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Piraman, S.; Sundar, S.; Mariappan, R.; Kim, Y.Y.; Min, K. Nanospheres and nanoleaves of γ-Fe2O3 architecturing for magnetic and biomolecule sensing applications. Sens. Actuator B Chem. 2016, 234, 386–394. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Yuan, Z.; Sun, Y.; Chang, F.; Deng, H.; Xie, L.; Li, H. A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method. RSC Adv. 2016, 6, 79343–79349. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.; Mohammad, M. Tamarix gallica leaf extract mediated novel route for the green synthesis of CuO nanoparticles and their application for Narylation of nitrogen-containing heterocycles under ligand-free conditions. RSC Adv. 2015, 5, 40628–40635. [Google Scholar] [CrossRef]
- Vijay Kumar, P.P.N.; Shameem, U.; Pratap, K.; Kalyani, R.L.; Pammi, S.V.N. Green synthesis of copper oxide nanoparticles using Aloe vera leaf extract and its antibacterial activity against fish bacterial pathogens. BioNanoScience 2015, 5, 135–139. [Google Scholar] [CrossRef]
- Yin, M.; Wu, C.K.; Lou, Y.; Burda, C.; Koberstein, J.T.; Zhu, Y.; O’Brien, S. Copper Oxide Nanocrystals. J. Am. Chem. Soc. 2005, 127, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhang, J.; Zhu, J.; Qi, J.; Zhang, Z.; Sui, W.; Shi, H.; Xue, D. Vacancy-Mediated Magnetism in Pure Copper Oxide Nanoparticles. Nanoscale Res. Lett. 2010, 5, 769–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyquist, R.A.; Kagel, R.O. Infrared Spectra of Inorganic Compounds; Academic Press: New York, NY, USA; London, UK, 1997; p. 220. [Google Scholar]
- Ethiraj, A.S.; Kang, D.J. Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett. 2012, 7, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, M.; Islam, M.D.M.; Ansari, Z.; Ahammed, S.; Sen, K.; Islam, S.K.M. Biogenic Nano-CuO-Catalyzed Facile C−N Cross-Coupling Reactions: Scope and Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 648–657. [Google Scholar] [CrossRef]
- Sadia, S.; Arifa, T.; Tayyaba, A.; Yongsheng, C. Plant Mediated Green Synthesis of CuO Nanoparticles: Comparison of Toxicity of Engineered and Plant Mediated CuO Nanoparticles towards Daphnia magna. Nanomaterials 2016, 6, 205. [Google Scholar] [CrossRef]
- Umar, A.; Lee, J.H.; Kumar, R.; Al-Dossary, O.; Ibrahim, A.A.; Baskoutas, S. Development of highly sensitive and selective ethanol sensor based onlance-shaped CuO nanostructures. Mater. Des. 2016, 105, 16–24. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liang, J.; Hu, Z.; Li, S.; Peng, S.; Qian, Y. Synthesis of Copper Nanowires via a Complex-Surfactant-Assisted Hydrothermal Reduction Process. J. Phys. Chem. B 2003, 107, 12658–12661. [Google Scholar] [CrossRef]
- Liu, J.; Jin, J.; Deng, Z.; Huang, S.Z.; Hu, Z.Y.; Wang, L.; Wang, C.; Chen, L.H.; Li, Y.; VanTendeloo, G.; et al. Tailoring CuO nanostructures for enhanced photocatalytic property. J. Colloid Interface Sci. 2012, 384, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xiao, F.; Wang, J.; Su, X. 3D flower- and 2D sheet-like CuO nanostructures: Microwave-assisted synthesis and application in gas sensors. Sens. Actuator B Chem. 2015, 207, 177–185. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, F.; Chen, Y.; Zhou, Y.; Li, J.; Zhu, A.; Luo, Y.; Tian, Y.; Yang, J. Hierarchically Porous CuO Hollow Spheres Fabricated via a One-Pot Template-Free Method for High-Performance Gas Sensors. J. Phys. Chem. C 2012, 116, 11994–12000. [Google Scholar] [CrossRef]
- Devaraj, M.; Saravanan, R.; Deivasigamani, R.K.; Gupta, V.K.; Gracia, F.; Jayadevan, S. Fabrication of novel shape Cu and Cu/Cu2O nanoparticles modified electrode for the determination of dopamine and paracetamol. J. Mol. Liq. 2016, 221, 930–941. [Google Scholar] [CrossRef]
- Pandikumar, A.; How, G.T.S.; See, T.P.; Omar, F.S.; Jayabal, S.; Kamali, S.Z.; Yusoff, N.; Jamil, A.; Ramaraj, R.; John, S.A.; et al. Graphene and its nanocomposite material based electrochemical sensor platform for dopamine. RSC Adv. 2014, 4, 63296–63323. [Google Scholar] [CrossRef] [Green Version]
- Felix, S.; Kollu, P.; Raghupathy, B.P.C.; Jeong, S.K.; Grace, A.N. Electrocatalytic oxidation of carbohydrates and dopamine in alkaline and neutral medium using CuO nanoplatelets. J. Electroanal. Chem. 2015, 739, 1–9. [Google Scholar] [CrossRef]
- Reitz, E.; Jia, W.; Gentile, M.; Wang, Y.; Lei, Y. CuO Nanospheres Based Nonenzymatic Glucose Sensor. Electroanalysis 2008, 20, 2482–2486. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Yang, Y.; Li, S.; Yu, Q.; Xu, X.; Hu, X. Graphene–Au nanoparticles nanocomposite film for selective electrochemical determination of dopamine. Anal. Methods 2012, 4, 1725–1728. [Google Scholar] [CrossRef]
- Kim, Y.R.; Bong, S.; Kang, Y.J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, M.; Cai, B.; Wang, W.; Ye, Z.; Huang, J. 3D graphene network@WO3 nanowire composites: A multifunctional colorimetric and electrochemical biosensing platform. Chem. Commun. 2014, 50, 11135–11138. [Google Scholar] [CrossRef] [PubMed]
- Aparna, T.K.; Sivasubramanian, R.; Dar, A.H. One-pot synthesis of Au Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Gao, F.; Cai, X.; Wang, X.; Gao, C.; Liu, S.; Gao, F.; Wang, Q. Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode. Sens. Actuator B Chem. 2013, 186, 380–387. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.E.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuator B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, X.; Ding, Y.; Luo, L.; Xu, D.; Ning, Y. Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan. Talanta 2016, 146, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Ku, S.; Chen, S.M. Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim. Acta 2013, 180, 1037–1042. [Google Scholar] [CrossRef]
- Reddy, S.; Swamya, B.E.K.; Jayadevappa, H. CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Kaur, B.; Pandiyan, T.; Satpati, B.; Srivastava, R. Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid, and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloids Surf. B 2013, 111, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, G.; Yin, Y.; Yang, R.; Li, J.; Qu, L. Nano-sized copper oxide/multi-wall carbon nanotube/Nafion modified electrode for sensitive detection of dopamine. J. Electroanal. Chem. 2013, 703, 45–51. [Google Scholar] [CrossRef]
- Aravind, S.S.J.; Ramaprabhu, S. Dopamine biosensor with metal oxide nanoparticles decorated multi-walled carbon nanotubes. Nanosci. Methods 2012, 1, 102–114. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Sudh, V.; Kumar, S.M.S.; Thangamuthu, R. Simultaneous determination of dopamine and uric acid using copper oxide nano-rice modified electrode. J. Alloys Compd. 2018, 748, 338–347. [Google Scholar] [CrossRef]
- Suna, W.; Wang, X.; Wang, Y.; Ju, X.; Xu, L.; Li, G.; Suna, Z. Application of graphene–SnO2 nanocomposite modified electrode for the sensitive electrochemical detection of dopamine. Electrochim. Acta 2013, 87, 317–322. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Zheng, X.Q.; Xu, J.Y.; Bao, W.J.; Wang, F.B.; Xia, X.H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]














| Electrode Material | Technique | LOD | Linear Range | Electrolyte | Ref. |
|---|---|---|---|---|---|
| Au-graphene | DPV | 1.86 mM | 5–1000 mM | pH 6.0 | [50] |
| Graphene | DPV | 2.64 mM | 4–100 mM | pH 7.0 | [51] |
| 3D-GN@WO3 nanowire | CA | 238 µM | 10–150 mM | pH 6.0 | [52] |
| Au-Cu2O/rGO | DPV | 3.9 µM | 10–90 µM | pH 7.0 | [53] |
| GO | DPV | 0.27 mM | 1–15 mM | pH 5.0 | [54] |
| Au/RGO/GCE | DPV | 1.40 μM | 6.8–41 μM | pH 7.0 | [55] |
| NiO-CuO/GR/GCE | SWV | 0.167 μM | 0.5–20 μM | pH 8.0 | [56] |
| RGO-Pd-NPs | LSV | 0.23 μM | 1–150 μM | pH 7.0 | [57] |
| Rod shaped CuO nanoparticles/MCPE | DPV | 0.18 μM | 0.3–1.4 μM | pH 6.0 | [58] |
| Ag/RGO | LSV | 5.4 µM | 10–800 µM | pH 6.0 | [59] |
| CuO/MWNTs/Nafion/GCE | DPV | 0.4 µM | 1.0–80 µM | pH 6.0 | [60] |
| ZnO/MWNTs/GCE | CV | 3 µM | 3—200 µM | pH 7.0 | [61] |
| CuO nano-rice/GCE | DPV | 0.42 µM | 1–150 µM | pH 7.0 | [62] |
| GR-SnO2/CILE | DPV | 0.5 µM | 5–500 µM | pH 6.0 | [63] |
| N-doped graphene | DPV | 0.25 µM | 0.5–170 µM | pH 6.0 | [64] |
| GR-CS/GCE | DPV | 5 µM | 15–175 µM | pH 7.0 | [65] |
| CuO nanowire/GCE | DPV | 0.1 µM | 0.1–105 µM | pH 7.4 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundar, S.; Venkatachalam, G.; Kwon, S.J. Biosynthesis of Copper Oxide (CuO) Nanowires and Their Use for the Electrochemical Sensing of Dopamine. Nanomaterials 2018, 8, 823. https://doi.org/10.3390/nano8100823
Sundar S, Venkatachalam G, Kwon SJ. Biosynthesis of Copper Oxide (CuO) Nanowires and Their Use for the Electrochemical Sensing of Dopamine. Nanomaterials. 2018; 8(10):823. https://doi.org/10.3390/nano8100823
Chicago/Turabian StyleSundar, Sasikala, Ganesh Venkatachalam, and Seong Jung Kwon. 2018. "Biosynthesis of Copper Oxide (CuO) Nanowires and Their Use for the Electrochemical Sensing of Dopamine" Nanomaterials 8, no. 10: 823. https://doi.org/10.3390/nano8100823
APA StyleSundar, S., Venkatachalam, G., & Kwon, S. J. (2018). Biosynthesis of Copper Oxide (CuO) Nanowires and Their Use for the Electrochemical Sensing of Dopamine. Nanomaterials, 8(10), 823. https://doi.org/10.3390/nano8100823