Iron Oxide and Gold Based Magneto-Plasmonic Nanostructures for Medical Applications: A Review
Abstract
:1. Introduction
2. Structure and Synthesis of Magneto-Plasmonic Nanostructures Based on a Sequential Growth in Solution
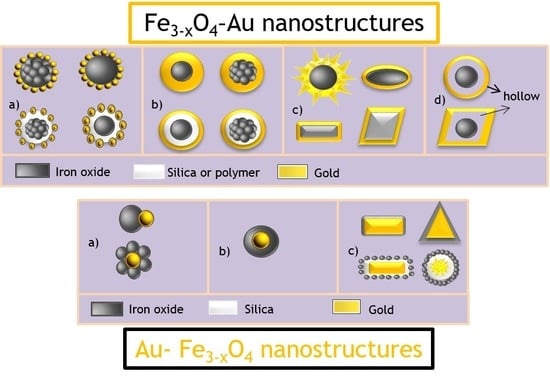
2.1. Fe3−xO4-Au Nanostructures (FANSs)
2.1.1. Different FANS Architectures
Fe3−xO4-Au Core-Satellite Structure
Spherical Fe3−xO4-Au Core-Shell Structure
Non-Spherical Fe3−xO4-Au Core-Shell Structure
Fe3−xO4-Au Hollow Structure
2.1.2. FANS Synthesis
2.2. Au-Fe3−xO4 Nanostructures (AFNSs)
2.2.1. Different Types of AFNSs
Au-Fe3−xO4 Dumbbell Structure
Au-Fe3−xO4 Core-Satellite Structure
Spherical Au-Fe3−xO4 Core-Shell Structure
Non-Spherical Au-Fe3−xO4 Core-Shell Structure
2.2.2. Synthesis of AFNSs
3. Magneto-Plasmonic Properties of FANSs and AFNSs
3.1. Optical Properties
3.2. Magnetic Properties
4. Application of Magneto-Plasmonic Hetero-Nanostructures
4.1. Multimodal Imaging
4.2. Multimodal Therapy
4.3. Biosensors and SERS Application
5. Conclusions
Acknowledgments
Conflicts of Interest
Glossary
| NPs | nanoparticles |
| CSNPS | core-shell nanoparticles |
| MRI | magnetic resonance imaging |
| NIR | near-infrared region |
| NSs | nanostructures |
| QDs | quantum dots |
| DOX | doxorubicin |
| FANSs | Fe3−xO4-Au nanostructures |
| AFNSs | Au-Fe3−xO4 nanostructures |
| TEM | transmission electron microscopy |
| PVP | polyvynilpyrolidone |
| PSA | serum albumin protein |
| DMF | N,N-dimethylformamide |
| APTMS | 3-aminopropyltrimethoxysilane |
| miRNA | micro ribonucleic acid |
| PEI | polyethylene imine |
| SPR | surface plasmon resonance |
| NDs | nanodumbbells |
| RF | Radio Frequency |
| DFM | dark field microscopy |
| OCT | optical coherence tomography |
| CT | computed tomography |
| PA | photoacoustic |
| SERS | surface enhanced Raman scattering |
| TREG | triethyleneglycol |
| UV-Vis | ultraviolet-visible |
| FC | field cooling |
| ZFC | zero field cooling |
References
- Yen, S.K.; Padmanabhan, P.; Selvan, S.T. Multifunctional iron oxide nanoparticles for diagnostics, therapy and macromolecule delivery. Theranostics 2013, 3, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Pradhan, L.; Devi, Y.P.; Meena, S.; Tewari, R.; Kumar, A.; Sharma, S.; Gajbhiye, N.; Vatsa, R.; Pandey, B.N.; et al. Induction heating studies of Fe3O4 magnetic nanoparticles capped with oleic acid and polyethylene glycol for hyperthermia. J. Mater. Chem. 2011, 21, 13388–13398. [Google Scholar] [CrossRef]
- Araújo-Neto, R.; Silva-Freitas, E.; Carvalho, J.; Pontes, T.; Silva, K.; Damasceno, I.; Egito, E.; Dantas, A.L.; Morales, M.A.; Carriço, A.S. Monodisperse sodium oleate coated magnetite high susceptibility nanoparticles for hyperthermia applications. J. Magn. Magn. Mater. 2014, 364, 72–79. [Google Scholar] [CrossRef]
- Múzquiz-Ramos, E.; Guerrero-Chávez, V.; Macías-Martínez, B.; López-Badillo, C.; García-Cerda, L. Synthesis and characterization of maghemite nanoparticles for hyperthermia applications. Ceram. Int. 2015, 41, 397–402. [Google Scholar] [CrossRef]
- Parak, W.J.; Osinski, M.; Yamamoto, K.I.; Stover, R.; Murthy, A.; Gourisankar, S.; Nie, G.; Martinez, M.; Truskett, T.; Sokolov, K.; et al. Plasmonic biodegradable gold nanoclusters with high NIR-absorbance for biomedical imaging. Int. Soc. Opt. Photonics 2014, 8955, 89550T. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, B.; Xing, D. Bio-modified Fe3O4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cells. J. Mater. Chem. 2012, 22, 470–477. [Google Scholar] [CrossRef]
- Becker, J.; Trügler, A.; Jakab, A.; Hohenester, U.; Sönnichsen, C. The optimal aspect ratio of gold nanorods for plasmonic bio-sensing. Plasmonics 2010, 5, 161–167. [Google Scholar] [CrossRef]
- Li, F.; Yu, Z.; Zhao, L.; Xue, T. Synthesis and application of homogeneous Fe3O4 core/Au shell nanoparticles with strong SERS effect. RSC Adv. 2016, 6, 10352–10357. [Google Scholar] [CrossRef]
- Mezni, A.; Balti, I.; Mlayah, A.; Jouini, N.; Smiri, L.S. Hybrid Au-Fe3O4 nanoparticles: Plasmonic, surface enhanced raman scattering, and phase transition properties. J. Phys. Chem. C 2013, 117, 16166–16174. [Google Scholar] [CrossRef]
- Niu, W.; Chua, Y.A.; Zhang, W.; Huang, H.; Lu, X. Highly symmetric gold nanostars: Crystallographic control and surface-enhanced raman scattering property. J. Am. Chem. Soc. 2015, 137, 10460–10463. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, P.; Osório, I.; Dória, G.; Carvalho, P.A.; Pereira, A.; Langer, J.; Araújo, J.P.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Franco, R.; et al. Star-shaped magnetite@gold nanoparticles for protein magnetic separation and SERS detection. RSC Adv. 2014, 4, 3690–3698. [Google Scholar] [CrossRef]
- Espinosa, A.; Bugnet, M.; Radtke, G.; Neveu, S.; Botton, G.A.; Wilhelm, C.; Abou-Hassan, A. Can magneto-plasmonic nanohybrids efficiently combine photothermia with magnetic hyperthermia? Nanoscale 2015, 7, 18872–18877. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Chen, J.; Zhu, S.; Liu, X.; Zhou, L.; Shi, P.; Niu, D.; Gu, J.; Shi, J. Multifunctional gold nanostar-based nanocomposite: Synthesis and application for noninvasive MR-SERS imaging-guided photothermal ablation. Biomaterials 2015, 60, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Jihye Choi, J.Y.; Jang, E.; Suh, J.; Huh, Y.; Lee, K.; Haam, S. Gold nanostructures as photothermal therapy agent for cancer. Anti-Cancer Agents Med. Chem. 2011, 11, 953–964. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Hyaluronic acid-modified Fe3O4/Au core/shell nanostars for multimodal imaging and photothermal therapy of tumours. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Shelton, M.; Singh, A.K.; Senapati, D.; Khan, S.A.; Ray, P.C. Multifunctional plasmonic shell-magnetic core nanoparticles for targeted diagnostics, isolation, and photothermal destruction of tumour cells. ACS Nano 2012, 6, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, M.; Oza, G.; Velumani, S.; Ramirez, J.T.; Garcia-Sierra, F.; Andrade, N.B.; Vera, A.; Leija, L.; Garza-Navarro, M.A. Plasmonic/magnetic multifunctional nanoplatform for cancer theranostics. Sci. Rep. 2016, 6, 34874. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, D.; Hao, Y.; Hu, Y.; Niu, M.; Zheng, C.; Yanyan, Y.; Li, D.; Zhang, P.; Chang, J.; et al. A gold nanostar based multi-functional tumour-targeting nanoplatform for tumour theranostic applications. J. Mater. Chem. B 2016, 4, 5895–5906. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Mi, L.; Gong, H.; Jiang, S.; Yu, Q. Multifunctional magnetic-plasmonic nanoparticles for fast concentration and sensitive detection of bacteria using SERS. Biosens. Bioelectron. 2012, 31, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Jimenez de Aberasturi, D.; Winckelmans, N.; Langer, J.; Bals, S.; Liz-Marzan, L.M. Synthesis of Janus plasmonic-magnetic, star-sphere nanoparticles, and their application in SERS detection. Faraday Discuss. 2016, 191, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Mukai, Y.; Yoshikawa, M.; Kamei, K.; Yoshikawa, T.; Morita, M.; Inubushi, T.; Yamamoto, T.A.; Yoshioka, Y.; Okada, N.; et al. Simple PEG conjugation of SPIO via an Au−S bond improves its tumour targeting potency as a novel MR tumour imaging agent. Bioconjug. Chem. 2010, 21, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, Y.; Li, Y.; Wu, H.; Yu, F.; Zhou, S.; Xie, L.; Luo, F.; Lin, C.; Hou, Z. Drug/dye-loaded, multifunctional PEG–chitosan–iron oxide nanocomposites for methotraxate synergistically self-targeted cancer therapy and dual model imaging. ACS Appl. Mater. Interfaces 2015, 7, 11908–11920. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Hamishehkar, H.; Arsalani, N.; Entezami, A.A. Preparation of thermo and pH-responsive polymer@Au/Fe3O4 core/shell nanoparticles as a carrier for delivery of anticancer agent. J. Nanopart. Res. 2015, 17, 305. [Google Scholar] [CrossRef]
- Jin, R.; Song, D.; Xiong, H.; Ai, L.; Ma, P.; Sun, Y. Magnetic core/shell Fe3O4/Au nanoparticles for studies of quinolones binding to protein by fluorescence spectroscopy. Luminescence 2016, 31, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Q.; Fang, S.; Sun, Y.A.; Chen, Z.; Xie, G.; Du, Y. Multifunctional nanocomposites constructed from Fe3O4-Au nanoparticle cores and a porous silica shell in the solution phase. Dalton Trans. 2011, 40, 10857–10864. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhao, A.; Guo, H.; Chen, X.; Gan, Z.; Tao, W.; Zhang, M.; Wu, R.; Li, Z. Controlled synthesis of Au-Fe3O4 hybrid hollow spheres with excellent SERS activity and catalytic properties. Dalton Trans. 2014, 43, 7998–8006. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Hu, F.; Lee, S.-T.; Liao, F.; Li, Y.; Shao, M. Controllable Fe3O4/Au substrate for surface-enhanced infrared absorption spectroscopy. Appl. Phys. Lett. 2015, 106, 23107. [Google Scholar] [CrossRef]
- Zhu, Y.; Lei, J.; Tian, Y. Uniform iron oxide hollow spheres for high-performance delivery of insoluble anticancer drugs. Dalton Trans. 2014, 43, 7275–7281. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Xia, Q.; Fu, S.; Ren, G.; Chai, F.; Jiang, J.; Qu, F. Fabrication of Fe3O4@Au hollow spheres with recyclable and efficient catalytic properties. New J. Chem. 2016, 40, 818–824. [Google Scholar] [CrossRef]
- Xia, Q.; Fu, S.; Ren, G.; Chai, F.; Jiang, J.; Qu, F. Fabrication of magnetic bimetallic Fe3O4@Au–Pd hybrid nanoparticles with recyclable and efficient catalytic properties. RSC Adv. 2016, 6, 55248–55256. [Google Scholar] [CrossRef]
- Feng, B.; Hong, R.; Wang, L.; Guo, L.; Li, H.; Ding, J.; Zheng, Y.; Wei, D. Synthesis of Fe3O4/APTES/PEG diacid functionalized magnetic nanoparticles for MR imaging. Colloids Surf. A Physicochem. Eng. Asp. 2008, 328, 52–59. [Google Scholar] [CrossRef]
- Lou, L.; Yu, K.; Zhang, Z.; Huang, R.; Zhu, J.; Wang, Y.; Zhu, Z. Dual-mode protein detection based on Fe3O4-Au hybrid nanoparticles. Nano Res. 2012, 5, 272–282. [Google Scholar] [CrossRef]
- Del Mar Ramos-Tejada, M.; Viota, J.L.; Rudzka, K.; Delgado, A.V. Preparation of multi-functionalized Fe3O4-Au nanoparticles for medical purposes. Colloids Surf. B Biointerfaces 2015, 128, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Xie, S.; Zhu, Y.; Liu, Y.; Wang, L.; Xu, F. Graphene oxide wrapped Fe3O4-Au nanohybrid as SERS substrate for aromatic dye detection. Sens. Actuators B Chem. 2015, 221, 1084–1093. [Google Scholar] [CrossRef]
- Ke, F.; Wang, L.; Zhu, J. Multifunctional Au-Fe3O4@MOF core-shell nanocomposite catalysts with controllable reactivity and magnetic recyclability. Nanoscale 2015, 7, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Uribe Madrid, S.I.; Pal, U.; Kang, Y.S.; Kim, J.; Kwon, H.; Kim, J. Fabrication of Fe3O4@mSiO2 core-shell composite nanoparticles for drug delivery applications. Nanoscale Res. Lett. 2015, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, P.; Wang, J.; Rong, Z.; Pang, Y.; Xu, J.; Dong, P.; Xiao, R.; Wang, S. Polyethylenimine-interlayered core-shell-satellite 3D magnetic microspheres as versatile SERS substrates. Nanoscale 2015, 7, 18694–18707. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, S.; Zhang, K.; Li, M.; Li, Q.; Xiao, R.; Wang, S. Streptomycin-modified Fe3O4-Au@Ag core–satellite magnetic nanoparticles as an effective antibacterial agent. J. Mater. Sci. 2016, 52, 1357–1368. [Google Scholar] [CrossRef]
- An, P.; Zuo, F.; Wu, Y.P.; Zhang, J.H.; Zheng, Z.H.; Ding, X.B.; Peng, Y.X. Fast synthesis of dopamine-coated Fe3O4 nanoparticles through ligand-exchange method. Chin. Chem. Lett. 2012, 23, 1099–1102. [Google Scholar] [CrossRef]
- Qiu, Y.; Deng, D.; Deng, Q.; Wu, P.; Zhang, H.; Cai, C. Synthesis of magnetic Fe3O4-Au hybrids for sensitive SERS detection of cancer cells at low abundance. J. Mater. Chem. B 2015, 3, 4487–4495. [Google Scholar] [CrossRef]
- Gan, Z.; Zhao, A.; Zhang, M.; Tao, W.; Guo, H.; Gao, Q.; Mao, R.; Liu, E. Controlled synthesis of Au-loaded Fe3O4@C composite microspheres with superior SERS detection and catalytic degradation abilities for organic dyes. Dalton Trans. 2013, 42, 8597–8605. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.H.; Zhang, C.J.; Wei, C.; Xu, M.M.; Yuan, Y.X.; Gu, R.A.; Yao, J.L. Controlling dynamic SERS hot spots on a monolayer film of Fe3O4@Au nanoparticles by a magnetic field. Spectrochim. Acta Part A 2016, 152, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, D.; Cushing, B.L.; Caruntu, G.; O’Connor, C.J. Attachment of gold nanograins onto colloidal magnetite nanocrystals. Chem. Mater. 2005, 17, 3398–3402. [Google Scholar] [CrossRef]
- Ye, M.; Wei, Z.; Hu, F.; Wang, J.; Ge, G.; Hu, Z.; Shao, M.; Lee, S.-T.; Liu, J. Fast assembling microarrays of superparamagnetic Fe3O4@Au nanoparticle clusters as reproducible substrates for surface-enhanced raman scattering. Nanoscale 2015, 7, 13427–13437. [Google Scholar] [CrossRef] [PubMed]
- Kwizera, E.A.; Chaffin, E.; Shen, X.; Chen, J.; Zou, Q.; Wu, Z.; Gai, Z.; Bhana, S.; O’Connor, R.; Wang, L. Size-and shape-controlled synthesis and properties of magnetic–plasmonic core–shell nanoparticles. J. Phys. Chem. C 2016, 120, 10530–10546. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Tsai, P.J.; Chen, Y.C. Multifunctional Fe3O4@Au nanoeggs as photothermal agents for selective killing of nosocomial and antibiotic-resistant bacteria. Small 2009, 5, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S.; Hofmann, C.; Ali, T.A.; Kelly, A.T.; Morosan, E.; Nordlander, P.; Whitmire, K.H.; Halas, N.J. Magnetic-plasmonic core-shell nanoparticles. ACS Nano 2009, 3, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhong, J.; Rao, H.; Lu, Z.; Ge, H.; Chen, B.; Zou, P.; Wang, X.; He, H.; Zeng, X.; et al. Electrochemical dipyridamole sensor based on molecularly imprinted polymer on electrode modified with Fe3O4@Au/amine-multi-walled carbon nanotubes. J. Solid State Electrochem. 2017, 21, 3071–3082. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.; Xue, Y.; Liang, J.; Cui, L.; Li, Q.; Zhou, S.; Huang, Y.; Li, G.; Zhao, Y. A Fe3O4@Au-basedpseudo-homogeneous electrochemical immunosensor for AFP measurement using AFP antibody-GNPs-HRP as detection probe. Anal. Biochem. 2017, 534, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.; Mukai, Y.; Kojima, H.; Yoshikawa, T.; Yoshikawa, M.; Kiyohara, G.; Yamamoto, T.A.; Yoshioka, Y.; Okada, N.; Seino, S.; et al. Direct cell entry of gold/iron-oxide magnetic nanoparticles in adenovirus mediated gene delivery. Biomaterials 2009, 30, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Pang, X.; He, Y.; Wang, Y.; Chen, G.; Wang, W.; Lin, Z. Precisely size-tunable magnetic/plasmonic core/shell nanoparticles with controlled optical properties. Angew. Chem. 2015, 54, 12091–12096. [Google Scholar] [CrossRef] [PubMed]
- Karamipour, S.; Sadjadi, M.S.; Farhadyar, N. Fabrication and spectroscopic studies of folic acid-conjugated Fe3O4@Au core-shell for targeted drug delivery application. Spectrochim. Acta Part A 2015, 148, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Raja, S.O.; Sardar, M.; Gayathri, N.; Ghosh, B.; Dasgupta, A. Iron oxide nanoparticles coated with gold: Enhanced magnetic moment due to interfacial effects. J. Appl. Phys. 2011, 109, 123902. [Google Scholar] [CrossRef]
- Baskakov, A.O.; Solov’eva, A.Y.; Ioni, Y.V.; Starchikov, S.S.; Lyubutin, I.S.; Khodos, I.I.; Avilov, A.S.; Gubin, S.P. Magnetic and interface properties of the core-shell Fe3O4/Au nanocomposites. Appl. Surf. Sci. 2017, 422, 638–644. [Google Scholar] [CrossRef]
- Lyon, J.L.; Fleming, D.A.; Stone, M.B.; Schiffer, P.; Williams, M.E. Synthesis of Fe oxide core/Au shell nanoparticles by iterative hydroxylamine seeding. Nano Lett. 2004, 4, 719–723. [Google Scholar] [CrossRef]
- Abdulla-Al-Mamun, M.; Kusumoto, Y.; Zannat, T.; Horie, Y.; Manaka, H. Au-ultrathin functionalized core–shell (Fe3O4@Au) monodispersed nanocubes for a combination of magnetic/plasmonic photothermal cancer cell killing. RSC Adv. 2013, 3, 7816–7827. [Google Scholar] [CrossRef]
- Wang, H.; Brandl, D.W.; Le, F.; Nordlander, P.; Halas, N.J. Nanorice: A hybrid plasmonic nanostructure. Nano Lett. 2006, 6, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, X.; Chao, J.; Song, C.; Liu, B.; Zhu, D.; Sun, Y.; Yang, B.; Zhang, Q.; Chen, Y.; et al. Synthesis of magnetic core-branched Au shell nanostructures and their application in cancer-related miRNA detection via SERS. Sci. China Mater. 2017, 60, 1129–1144. [Google Scholar] [CrossRef]
- Zhou, H.; Choi, S.I.; Zou, F.; Oh, S.; Kim, J.E.; Hwang, D.Y.; Lee, J. Cytotoxicity and gene expression in sarcoma 180 cells in response to spiky magnetoplasmonic supraparticles. ACS Appl. Mater. Interfaces 2014, 6, 19680–19689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Kim, J.-P.; Bahng, J.H.; Kotov, N.A.; Lee, J. Self-assembly mechanism of spiky magnetoplasmonic supraparticles. Adv. Funct. Mater. 2014, 24, 1439–1448. [Google Scholar] [CrossRef]
- Li, C.; Chen, T.; Ocsoy, I.; Zhu, G.; Yasun, E.; You, M.; Wu, C.; Zheng, J.; Song, E.; Huang, C.Z.; et al. Gold-coated Fe3O4 nanoroses with five unique functions for cancer cell targeting, imaging and therapy. Adv. Funct. Mater. 2014, 24, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-P.; Liao, P.-Y.; Su, C.-H.; Yeh, C.-S. Formation of oligonucleotide-gated silica shell-coated Fe3O4-Au core–shell nanotrisoctahedra for magnetically targeted and near-infrared light-responsive theranostic platform. J. Am. Chem. Soc. 2014, 136, 10062–10075. [Google Scholar] [CrossRef] [PubMed]
- Urries, I.; Muñoz, C.; Gomez, L.; Marquina, C.; Sebastian, V.; Arruebo, M.; Santamaria, J. Magneto-plasmonic nanoparticles as theranostic platforms for magnetic resonance imaging, drug delivery and NIR hyperthermia applications. Nanoscale 2014, 6, 9230–9240. [Google Scholar] [CrossRef] [PubMed]
- Kirui, D.K.; Rey, D.A.; Batt, C.A. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology 2010, 21, 105105. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, Y.M.; Mak, K.Y.; Leung, C.W.; Pong, P.W.T. Novel hybrid Au-Fe3O4 magnetic octahedron-like nanoparticles with tunable size. IEEE Trans. Magn. 2014, 50, 1–5. [Google Scholar]
- Sheng, Y.; Xue, J. Synthesis and properties of Au-Fe3O4 heterostructured nanoparticles. J. Colloid Interface Sci. 2012, 374, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Umut, E.; Pineider, F.; Arosio, P.; Sangregorio, C.; Corti, M.; Tabak, F.; Lascialfari, A.; Ghigna, P. Magnetic, optical and relaxometric properties of organically coated gold–magnetite (Au-Fe3O4) hybrid nanoparticles for potential use in biomedical applications. J. Magn. Magn. Mater. 2012, 324, 2373–2379. [Google Scholar] [CrossRef]
- Yu, S.; Hachtel, J.A.; Chisholm, M.F.; Pantelides, S.T.; Laromaine, A.; Roig, A. Magnetic gold nanotriangles by microwave-assisted polyol synthesis. Nanoscale 2015, 7, 14039–14046. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, Y.; Zhang, Y.; Yang, H.; Zhou, Z.; Yang, S. Functionalized Au-Fe3O4 nanocomposites as a magnetic and colorimetric bimodal sensor for melamine. Sens. Actuators B Chem. 2016, 226, 512–517. [Google Scholar] [CrossRef]
- Leon Felix, L.; Coaquira, J.A.; Martinez, M.A.; Goya, G.F.; Mantilla, J.; Sousa, M.H.; Valladares, L.L.; Barnes, C.H.; Morais, P.C. Structural and magnetic properties of core-shell Au/Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 41732. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, B.; Sun, S. Dumbbell-like Au-Fe3O4 nanoparticles for target-specific platin delivery. J. Am. Chem. Soc. 2009, 131, 4216–4217. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Huang, Y.; Zhang, S.; Zhu, H.; Wu, Z.; Sun, S. Controlled synthesis of Au-Fe heterodimer nanoparticles and their conversion into Au-Fe3O4 heterostructured nanoparticles. Nanoscale 2016, 8, 17947–17952. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, F.; Aronova, M.; Zhu, L.; Lin, X.; Quan, Q.; Liu, G.; Zhang, G.; Choi, K.-Y.; Kim, K.; et al. Manipulating the power of an additional phase: A flower-like Au–Fe3O4 ptical nanosensor for imaging protease expressions in vivo. ACS Nano 2011, 5, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Fantechi, E.; Roca, A.G.; Sepúlveda, B.; Torruella, P.; Estradé, S.; Peiró, F.; Coy, E.; Jurga, S.; Bastús, N.G.; Nogués, J.; et al. Seeded growth synthesis of Au-Fe3O4 heterostructured nanocrystals: Rational design and mechanistic insights. Chem. Mater. 2017, 29, 4022–4035. [Google Scholar] [CrossRef]
- Lin, F.-H.; Doong, R.-A. Catalytic nanoreactors of Au@Fe3O4 yolk–shell nanostructures with various au sizes for efficient nitroarene reduction. J. Phys. Chem. C 2017, 121, 7844–7853. [Google Scholar] [CrossRef]
- Cai, W.; Shin, D.-W.; Chen, K.; Gheysens, O.; Cao, Q.; Wang, S.X.; Gambhir, S.S.; Chen, X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006, 6, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal organic frameworks as versatile hosts of Au nanoparticles in heterogeneous catalysis. ACS Catal. 2017, 7, 2896–2919. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Y.; Wang, S.; Xu, J.; Xu, S.; Li, G. Au-Fe3O4@SiO2 core/shell nanoparticles: The silica coating regulations with a single core for different core sizes and shell thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Huang, L.; Ao, L.; Hu, D.; Wang, W.; Sheng, Z.; Su, W. Magneto-plasmonic nanocapsules for multimodal-imaging and magnetically guided combination cancer therapy. Chem. Mater. 2016, 28, 5896–5904. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, L.; Zhang, J.; Ma, Z.; Song, C. Bifunctional Au-nanorod@Fe3O4 nanocomposites: Synthesis, characterization, and their use as bioprobes. J. Nanopart. Res. 2012, 14, 998. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, X.; Jiang, J. Facile synthesis of magnetic–plasmonic nanocomposites as T1 MRI contrast enhancing and photothermal therapeutic agents. Nano Res. 2016, 9, 787–799. [Google Scholar] [CrossRef]
- Xue, X.; Sukhotskiy, V.; Furlani, E.P. Optimization of optical absorption of colloids of SiO2@Au and Fe3O4@Au nanoparticles with constraints. Sci. Rep. 2016, 6, 35911. [Google Scholar] [CrossRef] [PubMed]
- Canet-Ferrer, J.; Albella, P.; Ribera, A.; Usagre, J.; Maier, S. Hybrid magnetite–gold nanoparticles as bifunctional magnetic–plasmonic systems: Three representative cases. Nanoscale Horiz. 2017, 2, 205–216. [Google Scholar] [CrossRef]
- Lin, F.H.; Doong, R.A. Characterization of interfacially electronic structures of gold-magnetite heterostructures using x-ray absorption spectroscopy. J. Colloid Interface Sci. 2014, 417, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Pineider, F.; Fernández, C.D.; Videtta, V.; Carlino, E.; al Hourani, A.; Wilhelm, F.; Rogalev, A.; Cozzoli, P.D.; Ghigna, P.; Sangregorio, C. Spin-polarization transfer in colloidal colloidal magnetic-plasmonic au/iron oxide hetero-nanocrystals. ACS Nano 2013, 7, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Hugounenq, P.; Levy, M.; Alloyeau, D.; Lartigue, L.; Dubois, E.; Cabuil, V.R.; Ricolleau, C.; Roux, S.P.; Wilhelm, C.; Gazeau, F.; et al. Iron oxide monocrystalline nanoflowers for highly efficient magnetic hyperthermia. J. Phys. Chem. C 2012, 116, 15702–15712. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from nature to improve the heat generation of iron-oxide nanoparticles for magnetic hyperthermia applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.; Stapf, M.; Dutz, S.; Müller, R.; Teichgräber, U.; Hilger, I. Structural properties of magnetic nanoparticles determine their heating behaviour-an estimation of the in vivo heating potential. Nanoscale Res. Lett. 2014, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, F.; Liu, L.; Duan, T.; Zhang, H.; Wang, Z. Lectin-conjugated Fe3O4@Au core@shell nanoparticles as dual mode contrast agents for in vivo detection of tumor. Mol. Pharm. 2014, 11, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Wang, X.; Liu, R.; Zhang, H.; Liu, X.; Zhang, Y. Facile synthesis of multifunctional Fe3O4@SiO2@Au magneto-plasmonic nanoparticles for mr/ct dual imaging and photothermal therapy. RSC Adv. 2017, 7, 18844–18850. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, Y.; Li, Y.; Jiang, J.; Cheng, L.; Liu, Z.; Guo, L.; Pan, Y.; Gu, H. Synthesis of Au-Fe3O4 heterostructured nanoparticles for in vivo computed tomography and magnetic resonance dual model imaging. Nanoscale 2014, 6, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Facile one-pot synthesis of Fe3O4@Au composite nanoparticles for dual-mode MR/CT imaging applications. ACS Appl. Mater. Interfaces 2013, 5, 10357–10366. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gao, W.; Zhang, X.; Mei, X. Au nanocage functionalized with ultra-small Fe3O4 nanoparticles for targeting T1-T2 dual MRI and CT imaging of tumor. Sci. Rep. 2016, 6, 28258. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Feldman, M.D.; Tam, J.M.; Paranjape, A.S.; Cheruku, K.K.; Larson, T.A.; Tam, J.O.; Ingram, D.R.; Paramita, V.; Villard, J.W.; et al. Small multifunctional nanoclusters for targeted cellular imaging and therapy. ACS Nano 2009, 3, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, R.; Wang, S.; Ding, L.; Li, J.; Luo, Y.; Wang, X.; Shen, M.; Shi, X. Multifunctional Fe3O4@Au core/shell nanostars: A unique platform for multimode imaging and photothermal therapy of tumors. Sci. Rep. 2016, 6, 28325. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Jarrett, B.R.; Louie, A.Y.; Kauzlarich, S.M. Gold-coated iron nanoparticles: A novel magnetic resonance agent for T1 and T2 weighted imaging. Nanotechnology 2006, 17, 640. [Google Scholar] [CrossRef]
- Larson, T.A.; Bankson, J.; Aaron, J.; Sokolov, K. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology 2007, 18, 325101. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y.; Lee, H.; Lee, M.; An, J.Y.; Shin, S.; Kwon, S.; Lee, D.S.; Park, J.H. Gold-nanoclustered hyaluronan nano-assemblies for photothermally maneuvered photodynamic tumor ablation. ACS Nano 2016, 10, 10858–10868. [Google Scholar] [CrossRef] [PubMed]
- Van de Broek, B.; Devoogdt, N.; D’Hollander, A.; Gijs, H.-L.; Jans, K.; Lagae, L.; Muyldermans, S.; Maes, G.; Borghs, G. Specific cell targeting with nanobody conjugated branched gold nanoparticles for photothermal therapy. ACS Nano 2011, 5, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Beji, Z.; Hanini, A.; Smiri, L.; Gavard, J.; Kacem, K.; Villain, F.; Greneche, J.-M.; Chau, F.; Ammar, S. Magnetic properties of zn-substituted MnFe3O4 nanoparticles synthesized in polyol as potential heating agents for hyperthermia. Evaluation of their toxicity on endothelial cells. Chem. Mater. 2010, 22, 5420–5429. [Google Scholar] [CrossRef]
- Manera, M.; Colombelli, A.; Rella, R.; Caricato, A.; Cozzoli, P.; Martino, M.; Vasanelli, L. TiO2 brookite nanostructured thin layer on magneto-optical surface plasmon resonance transductor for gas sensing applications. J. Appl. Phys. 2012, 112, 53524. [Google Scholar] [CrossRef]
- Chou, K.-H.; Lin, E.-P.; Chen, T.-C.; Lai, C.-H.; Wang, L.-W.; Chang, K.-W.; Lee, G.-B.; Lee, M.-C.M. Application of strong transverse magneto-optical Kerr effect on high sensitive surface plasmon grating sensors. Opt. Express 2014, 22, 19794–19802. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; García-Martín, A.; Cuevas, J.C. Hybrid magnetoplasmonic crystals boost the performance of nanohole arrays as plasmonic sensors. ACS Photonics 2016, 3, 203–208. [Google Scholar] [CrossRef]
- Grunin, A.; Mukha, I.; Chetvertukhin, A.; Fedyanin, A. Refractive index sensor based on magnetoplasmonic crystals. J. Magn. Magn. Mater. 2016, 415, 72–76. [Google Scholar] [CrossRef]
- Maccaferri, N.; Gregorczyk, K.E.; De Oliveira, T.V.; Kataja, M.; Van Dijken, S.; Pirzadeh, Z.; Dmitriev, A.; Åkerman, J.; Knez, M.; Vavassori, P. Ultrasensitive and label-free molecular-level detection enabled by light phase control in magnetoplasmonic nanoantennas. Nat. Commun. 2015, 6, 6150. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.G.; Ferreiro-Vila, E.; Garcia-Martin, J.M.; Garcia-Martin, A.; Rella, R. Enhanced antibody recognition with a magneto-optic surface plasmon resonance (MO-SPR) sensor. Biosens. Bioelectron. 2014, 58, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dostalek, J.; Knoll, W. Magnetic nanoparticle-enhanced biosensor based on grating-coupled surface plasmon resonance. Anal. Chem. 2011, 83, 6202–6207. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.; Di Carlo, D.; Judy, J.W. Rapid and dynamic intracellular patterning of cell-internalized magnetic fluorescent nanoparticles. Nano Lett. 2009, 9, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Shen, M.; Wu, S.; Zhao, C.; Ma, X.; Wang, Z. Graphene oxide wrapped Fe3O4/Au nanostructures as substrates for aptamer-based detection of Vibrio parahaemolyticus by surface-enhanced Raman spectroscopy. Microchim. Acta 2017, 184, 2653–2660. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Wang, Y.; Kuang, Q.; Shi, Y.; Zhong, L.; Zhang, Q. Febrication of cluster/shell/ Fe3O4/Au nanoparticles and application in protein detection via a SERS method. J. Phys. Chem. C 2010, 114, 19607–19613. [Google Scholar] [CrossRef]
- Han, Y.; Lei, S.L.; Lu, J.H.; He, Y.; Chen, Z.W.; Ren, L.; Zhou, X. Potential use of SERS-assisted theranostic strategy based on Fe3O4/Au cluster/shell nanocomposites for bio-detection, MRI, and magnetic hyperthermia. Mater. Sci. Eng. C 2016, 64, 199–207. [Google Scholar] [CrossRef] [PubMed]






















| Core Material | Shell Material | Reducing Agent | Application | Ref. |
|---|---|---|---|---|
| Fe3O4-SiO2-NH2 175 nm | Au 185 nm | Formaldehyde | Detection of rhodamine | [10] |
| Fe3O4-BPEI 380 nm | Au 420 nm | Electrostatic interaction | Detection of low abundance of cancer cell | [44] |
| Fe3O4-PEI 40 ± 2 nm | Fe3O4@Au 140 ± 5 nm | NH2OH·HCl | Detection of free PSA | [116] |
| Fe3O4 33 ± 9 nm | Star-shaped Au 146 ± 48 nm | DMF with PVP | Detection of dye molecule of Astra blue and protein magnetic separation | [13] |
| Fe3O4 16.2 ± 2.8 nm | Star-shaped Au 80 nm | DMF with PVP | Detection of crystal violet | [22] |
| Fe3O4-PEI 15 nm | Star-shaped Au 248 ± 36 nm | NH2OH | Detection of bacteria | [21] |
| Fe3O4-PEI 200 nm | Au 220 nm | NH2OH·HCl | Detection of free-Prostate specific antigen, MRI, magnetic hyperthermia | [117] |
| Fe3O4-SiO2-NH2 126 ± 15 nm | Star-shaped Au 160 ± 10–200 ± 16 nm | Formaldehyde; AgNO3 | Detection of cancer-related miRNA | [61] |
| Au 90 nm | Fe3−xO4 97 nm | TREG | Analysis of the structure and the chemical composition of the surrounding Fe3−xO4 shell | [11] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Mammeri, F.; Ammar, S. Iron Oxide and Gold Based Magneto-Plasmonic Nanostructures for Medical Applications: A Review. Nanomaterials 2018, 8, 149. https://doi.org/10.3390/nano8030149
Nguyen TT, Mammeri F, Ammar S. Iron Oxide and Gold Based Magneto-Plasmonic Nanostructures for Medical Applications: A Review. Nanomaterials. 2018; 8(3):149. https://doi.org/10.3390/nano8030149
Chicago/Turabian StyleNguyen, Thi Thuy, Fayna Mammeri, and Souad Ammar. 2018. "Iron Oxide and Gold Based Magneto-Plasmonic Nanostructures for Medical Applications: A Review" Nanomaterials 8, no. 3: 149. https://doi.org/10.3390/nano8030149
APA StyleNguyen, T. T., Mammeri, F., & Ammar, S. (2018). Iron Oxide and Gold Based Magneto-Plasmonic Nanostructures for Medical Applications: A Review. Nanomaterials, 8(3), 149. https://doi.org/10.3390/nano8030149