Strongly Iridescent Hybrid Photonic Sensors Based on Self-Assembled Nanoparticles for Hazardous Solvent Detection
Abstract
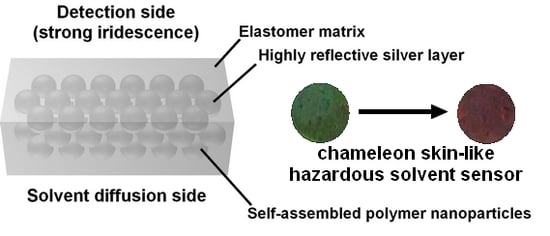
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Strongly Iridescent Hybrid Photonic Films
3.2. Hazardous Solvent Detection in Biomimetic Photonic Sensors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kristensen, P.; Irgens, L.M.; Kjersti Daltveit, A.; Andersen, A. Perinatal Outcome among Children of Men Exposed to Lead and Organic Solvents in the Printing Industry. Am. J. Epidemiol. 1993, 137, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.A. Effects of organic solvents on behavioral performance of workers in the paint industry. Neurobehav. Toxicol. Teratol. 1982, 4, 703–708. [Google Scholar]
- Agnesi, R.; Valentini, F.; Mastrangelo, G. Risk of spontaneous abortion and maternal exposure to organic solvents in the shoe industry. Int. Arch. Occup. Environ. Health 1997, 69, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Vohra, V.; Mróz, W.; Inaba, S.; Porzio, W.; Giovanella, U.; Galeotti, F. Low-Cost and Green Fabrication of Polymer Electronic Devices by Push-Coating of the Polymer Active Layers. ACS Appl. Mater. Interfaces 2017, 9, 25434–25444. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Eriksson, M.; Lenner, P.; Lundgren, E. Malignant lymphoma and exposure to chemicals, especially organic solvents, chlorophenols and phenoxy acids case-control study. Br. J. Cancer 1981, 43, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sallmén, M.; Lindbohm, M.-L.; Kyyrönen, P.; Nykyri, E.; Anttila, A.; Taskinen, H.; Hemminki, K. Reduced fertility among women exposed to organic solvents. Am. J. Ind. Med. 1995, 27, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.; Chin, B.; Huibers, P.D.; Garcia-Valls, R.; Hatton, T.A. Solvent replacement for green processing. Environ. Health Perspect. 1998, 106, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Sun, J.; Bhushan, B.; Tong, J. Structural coloration in nature. RSC Adv. 2013, 3, 14862–14889. [Google Scholar] [CrossRef]
- Teyssier, J.; Saenko, S.V.; van der Marel, D.; Milinkovitch, M.C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 2015, 6, 6368. [Google Scholar] [CrossRef] [PubMed]
- Mäthger, L.M.; Land, M.F.; Siebeck, U.E.; Marshall, N.J. Rapid colour changes in multilayer reflecting stripes in the paradise whiptail, Pentapodus paradiseus. J. Exp. Biol. 2003, 206, 3607–3613. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Matsuhana, B.; Tanaka, S.; Inouye, Y.; Oshima, N.; Kinoshita, S. Mechanism of variable structural colour in the neon tetra: Quantitative evaluation of the Venetian blind model. J. R. Soc. Interface 2011, 8, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Xiong, D.B.; Zhang, D. Biomimetic and Bioinspired Photonic Structures. Nano Adv. 2016, 1, 62–70. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, Q.; Shi, L.; Zhang, X.; Zhang, K.-Q. Bio-inspired Sensors Based on Photonic Structures of Morpho Butterfly Wings: A Review. J. Mater. Chem. C 2016, 4, 1752–1763. [Google Scholar] [CrossRef]
- Kuo, W.-K.; Weng, H.-P.; Hsu, J.-J.; Yu, H.H. A bioinspired color-changing polystyrene microarray as a rapid qualitative sensor for methanol and ethanol. Mater. Chem. Phys. 2016, 173, 285–290. [Google Scholar] [CrossRef]
- Burgess, B.; Loncar, M.; Aizenberg, J. Structural colour in colourimetric sensors and indicators. J. Mater. Chem. C 2013, 1, 6075–6086. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y. Nanofabrication and coloration study of artificial Morpho butterfly wings with aligned lamellae layers. Sci. Rep. 2015, 5, 16637. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Lin, C.-F.; Lin, H.-Y.; Lee, C.-L.; Chen, C.-D. Polymer-based photonic crystals fabricated with single-step electron-beam lithography. Adv. Mater. 2007, 19, 3052–3056. [Google Scholar] [CrossRef]
- Aryal, M.; Ko, D.-H.; Tumbleston, J.R.; Gadisa, A.; Samulski, E.T.; Lopez, R. Large area nanofabrication of butterfly wing’s three dimensional ultrastructures. J. Vac. Sci. Technol. B 2012, 30, 061802. [Google Scholar] [CrossRef]
- Siddique, R.H.; Hünig, R.; Faisal, A.; Lemmer, U.; Hölscher, H. Fabrication of hierarchical photonic nanostructures inspired by Morpho butterflies utilizing laser interference lithography. Opt. Mater. Express 2015, 5, 996–1005. [Google Scholar] [CrossRef]
- Vohra, V.; Galeotti, F.; Giovanella, U.; Anzai, T.; Kozma, E.; Botta, C. Investigating phase separation and structural coloration of self-assembled ternary polymer thin films. Appl. Phys. Lett. 2016, 109, 103702. [Google Scholar] [CrossRef]
- Zulian, L.; Emilitri, E.; Scavia, G.; Botta, C.; Colombo, M.; Destri, S. Structural Iridescent Tuned Colors from Self-Assembled Polymer Opal Surfaces. ACS Appl. Mater. Interfaces 2012, 4, 6071–6079. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ravaine, S. Bottom-Up Assembly and Applications of Photonic Materials. Crystals 2016, 6, 54. [Google Scholar] [CrossRef]
- Míguez, H.; López, C.; Meseguer, F.; Blanco, A.; Vázquez, L.; Mayoral, R.; Ocaña, M.; Fornés, V.; Mifsud, A. Photonic crystal properties of packed submicrometric SiO2 spheres. Appl. Phys. Lett. 1997, 71, 1148–1150. [Google Scholar] [CrossRef] [Green Version]
- Blanco, A.; Chomski, E.; Grabtchak, S.; Ibisate, M.; John, S.; Leonard, S.W.; Lopez, C.; Meseguer, F.; Miguez, H.; Mondia, J.P.; et al. Large-scale synthesis of a silicon photonic crystal with a complete three-dimensional bandgap near 1.5 micrometres. Nature 2000, 405, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; You, B.; Zhou, S.; Wu, L. Self-assembly of polymer colloids and their solvatochromic-responsive properties. J. Mater. Chem. 2011, 21, 687–692. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystal Based Sensor for Organic Solvents and for Solvent-Water Mixtures. Sensors 2012, 12, 16954–16963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shieh, J.-Y.; Kuo, J.-Y.; Weng, H.-P.; Yu, H.H. Preparation and Evaluation of the Bioinspired PS/PDMS Photochromic Films by the Self-Assembly Dip−Drawing Method. Langmuir 2013, 29, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Reculusa, S.; Ravaine, S. Synthesis of Colloidal Crystals of Controllable Thickness through the Langmuir–Blodgett Technique. Chem. Mater. 2003, 15, 598–605. [Google Scholar] [CrossRef]
- Kuo, W.-K.; Hsu, J.-J.; Nien, C.-K.; Yu, H.H. Moth-Eye-Inspired Biophotonic Surfaces with Antireflective and Hydrophobic Characteristics. ACS Appl. Mater. Interfaces 2016, 8, 32021–32030. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G.; Shin, D.H.; Lee, G.S.; Choi, U.S. Fabrication of colloidal crystals on hydrophilic/hydrophobic surface by spin-coating. Colloids Surf. A 2011, 385, 188–194. [Google Scholar] [CrossRef]
- Kuo, W.-K.; Weng, H.-P.; Hsu, J.-J.; Yu, H.H. Photonic Crystal-Based Sensors for Detecting Alcohol Concentration. Appl. Sci. 2016, 6, 67. [Google Scholar] [CrossRef]
- Stalder, A.F.; Kulik, G.; Sage, D.; Barbieri, L.; Hoffmann, P. A Snake-Based Approach to Accurate Determination of Both Contact Points and Contact Angles. Colloids Surf. A 2006, 286, 92–103. [Google Scholar] [CrossRef]
- Vohra, V.; Anzai, T.; Inaba, S.; Porzio, W.; Barba, L. Transfer-printing of active layers to achieve high quality interfaces in sequentially deposited multilayer inverted polymer solar cells fabricated in air. Sci. Technol. Adv. Mater. 2016, 17, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Zappia, S.; Scavia, G.; Ferretti, A.M.; Giovanella, U.; Vohra, V.; Destri, S. Water-Processable Amphiphilic Low Band Gap Block Copolymer: Fullerene Blend Nanoparticles as Alternative Sustainable Approach for Organic Solar Cells. Adv. Sustain. Syst. 2018, 2, 1700155. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Shishkin, I.; Rybin, M.V.; Samusev, K.B.; Golubev, V.G.; Limonov, M.F. Multiple Bragg diffraction in opal-based photonic crystals: Spectral and spatial dispersion. Phys. Rev. B 2014, 89, 035124. [Google Scholar] [CrossRef]
- Ng Lee, J.; Park, C.; Whitesides, G.M. Solvent Compatibility of Poly(dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]




| Solvent | THF | DME | CF | CB | DCM |
|---|---|---|---|---|---|
| Refractive index (nsolvent) | 1.40 | 1.38 | 1.45 | 1.52 | 1.42 |
| PDMS swelling ratio (mmol/g of PDMS) | 17.8 | 11.6 | 17.7 | 7.7 | 7.9 |
| Reflectance peak (nm) | 601 | 592 | 597 | 569 | 575 |
| Solvent Amount (μL) | 0 | 2 | 5 | 10 | 20 | 50 | 100 | |
| Reflectance Peak (nm) | THF | 550 | 552 | 567 | 574 | 599 | 602 | 601 |
| DME | 550 | 555 | 562 | 571 | 578 | 591 | 592 | |
| CF | 550 | 551 | 558 | 569 | 589 | 599 | 597 | |
| CB | 550 | 552 | 557 | 567 | 571 | 570 | 569 | |
| DCM | 550 | 554 | 561 | 569 | 574 | 574 | 575 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, A.; Ikeda, Y.; Yamaguchi, K.; Vohra, V. Strongly Iridescent Hybrid Photonic Sensors Based on Self-Assembled Nanoparticles for Hazardous Solvent Detection. Nanomaterials 2018, 8, 169. https://doi.org/10.3390/nano8030169
Sato A, Ikeda Y, Yamaguchi K, Vohra V. Strongly Iridescent Hybrid Photonic Sensors Based on Self-Assembled Nanoparticles for Hazardous Solvent Detection. Nanomaterials. 2018; 8(3):169. https://doi.org/10.3390/nano8030169
Chicago/Turabian StyleSato, Ayaka, Yuya Ikeda, Koichi Yamaguchi, and Varun Vohra. 2018. "Strongly Iridescent Hybrid Photonic Sensors Based on Self-Assembled Nanoparticles for Hazardous Solvent Detection" Nanomaterials 8, no. 3: 169. https://doi.org/10.3390/nano8030169
APA StyleSato, A., Ikeda, Y., Yamaguchi, K., & Vohra, V. (2018). Strongly Iridescent Hybrid Photonic Sensors Based on Self-Assembled Nanoparticles for Hazardous Solvent Detection. Nanomaterials, 8(3), 169. https://doi.org/10.3390/nano8030169