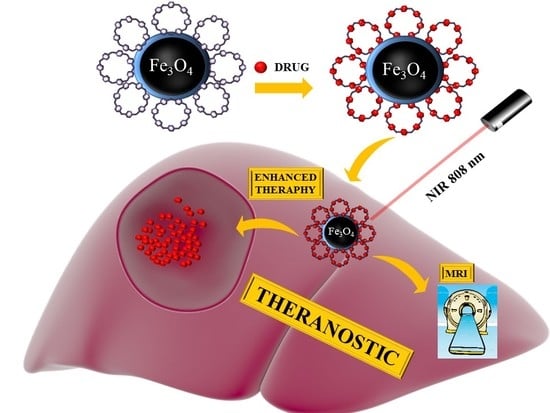
Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe3O4@PDA (Nanomaterial A) and Fe3O4@PDA@SH-βCD (Nanomaterial B)
2.2. Nuclear and Magnetic Resonance Imaging (NMR and MRI) Studies
2.2.1. Transverse Relaxation T2 for Fe3O4@PDA in Water Suspensions
2.2.2. Transverse Relaxation T2 in Agarose Gels from Multi-Echo Multi-Slice MEMS MRI
2.3. FT-IR, UV-VIS, XPS, TGA, Zeta Potential, DLS and Magnetic Measurements
2.4. TEM and Confocal Microscopy Imaging
2.5. Loading and Release of Doxorubicin from Fe3O4@PDA@SH-βCD (Nanomaterial B + DOXO)
2.6. NIR Laser Irradiation of Fe3O4@PDA@SH-βCD (Nanomaterial B)
2.7. Cytotoxicity Assays
2.8. Combined Chemo- and Photothermal Therapy
3. Results and Discussion
3.1. Characterization of Fe3O4@PDA and Fe3O4@PDA@SH-βCD
3.2. Transverse Relaxation T2 in Fe3O4@PDA in Water
3.3. Transverse Relaxation T2 of Water for Fe3O4@PDA and Fe3O4@PDA@SH-βCD in Agarose
3.4. NIR Laser Irradiation
3.5. Drug Loading and Release
3.6. Activity of Nanomaterials in Anticancer Therapy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Hu, Y.; Shen, M.; Shi, X.; Zhang, G. Folic acid-targeted iron oxide nanoparticles as contrast agents for magnetic resonance imaging of human ovarian cancer. J. Ovarian Res. 2016, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Shetake, N.G.; Barick, K.C.; Pandey, B.N.; Salunke, H.G.; Hassan, P.A. Folic acid conjugated Fe3O4 magnetic nanoparticles for targeted delivery of doxorubicin. Dalton Trans. 2016, 45, 17401–17408. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-C.; Chen, Y.-C.; Chiang, C.-F.; Mo, L.-R.; Wei, S.-Y.; Hsieh, W.-Y.; Lin, W.-L. Doxorubicin-modified magnetic nanoparticles as a drug delivery system for magnetic resonance imaging-monitoring magnet-enhancing tumor chemotherapy. Int. J. Nanomed. 2016, 11, 2021–2037. [Google Scholar]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic nanomedicine for cancer detection and treatment. J. Food Drug. Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Heo, G.S.; Lim, S.-M.; Sun, G.; Wooley, K.L. Polymeric nanostructures for imaging and therapy. Chem. Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chao, Y.; Liu, J.; Huang, J.; Pan, J.; Guo, W.; Wu, J.; Sheng, M.; Yang, K.; Wang, J.; et al. Polydopamine coated single-walled carbon nanotubes as a versatile platform with radionuclide labeling for multimodal tumor imaging and therapy. Theranostics 2016, 6, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, R.; Lu, J.; Zhao, C.; Deng, X.; Wu, Y. Mesoporous silica coated polydopamine functionalized reduced graphene oxide for synergistic targeted chemo-photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wei, Y. Upconversion nanoparticle as a theranostic agent for tumor imaging and therapy. J. Innov. Opt. Health. Sci. 2016, 9, 1630006. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Curry, T.; Kopelman, R.; Shilo, M.; Popovtzer, R. Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol. Imaging 2014, 9, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.-H.; Choi, Y.; Kim, S.; Cheon, J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rev. 2015, 44, 4501–4516. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic iron oxide nanoparticles as mri contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Szczeszak, A.; Ekner-Grzyb, A.; Runowski, M.; Szutkowski, K.; Mrówczyńska, L.; Kaźmierczak, Z.; Grzyb, T.; Dąbrowska, K.; Giersig, M.; Lis, S. Spectroscopic, structural and in vitro cytotoxicity evaluation of luminescent, lanthanide doped core@shell nanomaterials GdVO4:Eu3+5%@SiO2@NH2. J. Colloid Interface Sci. 2016, 481, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Zavareh, S.; Mahdi, M.; Erfanian, S.; Hashemi-Moghaddam, H. Synthesis of polydopamine as a new and biocompatible coating of magnetic nanoparticles for delivery of doxorubicin in mouse breast adenocarcinoma. Cancer Chemother. Pharmacol. 2016, 78, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and eumelanin: From structure–property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyński, R.; Markiewicz, R.; Liebscher, J. Chemistry of polydopamine analogues. Polym. Int. 2016, 65, 1288–1299. [Google Scholar] [CrossRef]
- Mrówczyński, R. Polydopamine-based multifunctional (nano)materials for cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 7541–7561. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Hegab, H.M.; Clarke, S.R.; Ginic-Markovic, M. Versatile surface modification using polydopamine and related polycatecholamines: Chemistry, structure, and applications. Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Jurga-Stopa, J.; Markiewicz, R.; Coy, L.; Emerson, J.S.; Woźniak, A. Assessment of polydopamine coated magnetic nanoparticles in doxorubicin delivery. RSC Adv. 2016, 6, 5936–5943. [Google Scholar] [CrossRef]
- Woźniak, A.; Walawender, M.; Tempka, D.; Coy, E.; Załęski, K.; Grześkowiak, B.F.; Mrówczyński, R. In vitro genotoxicity and cytotoxicity of polydopamine-coated magnetic nanostructures. Toxicol. In Vitro 2017, 44, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-C.; Chen, Y.; Li, Y.-H.; Yin, X.-B. Magnetic resonance imaging-guided multi-drug chemotherapy and photothermal synergistic therapy with PH and NIR-stimulation release. ACS Appl. Mater. Interfaces 2017, 9, 22278–22288. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, C.; Zhang, D.; Wang, Y.; Ren, X.; Ai, K.; Chen, X.; Lu, L. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017, 47, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, G.; Li, C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano 2010, 4, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Otero-Espinar, F.J.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C.; Blanco-Méndez, J. Cyclodextrins in drug delivery systems. J. Drug Deliv. Sci. Technol. 2010, 20, 289–301. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Bauer, N.A.; Chauhan, N.; Kumar, D.; Jaggi, M.; Chauhan, S.C. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int. J. Nanomedicine 2012, 7, 1761–1779. [Google Scholar] [PubMed]
- Huang, L.; Wang, H.; Li, B.; Li, E.; Zhou, Y.; Yang, Y.; Dong, C.; Shuang, S. β-cyclodextrin derivatives hybrid Fe3O4 magnetic nanoparticles as the drug delivery for ketoprofen. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 209–215. [Google Scholar] [CrossRef]
- Chen, P.; Song, H.; Yao, S.; Tu, X.; Su, M.; Zhou, L. Magnetic targeted nanoparticles based on β-cyclodextrin and chitosan for hydrophobic drug delivery and a study of their mechanism. RSC Adv. 2017, 7, 29025–29034. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Oroujeni, M.; Kaboudin, B.; Xia, W.; Jönsson, P.; Ossipov, D.A. Conjugation of cyclodextrin to magnetic Fe3O4 nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.; Hadade, N.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyński, R.; Magerusan, L.; Turcu, R.; Liebscher, J. Diazo transfer at polydopamine—A new way to functionalization. Polym. Chem. 2014, 5, 6593–6599. [Google Scholar]
- Bloch, F. Nuclear induction. Phys. Rev. 1946, 70, 460–474. [Google Scholar] [CrossRef]
- Hahn, E.L. Spin echoes. Phys. Rev. 1950, 80, 580–594. [Google Scholar] [CrossRef]
- Hills, B.P.; Wright, K.M.; Belton, P.S.; Proton, N.M.R. Studies of chemical and diffusive exchange in carbohydrate systems. Mol. Phys. 1989, 67, 1309–1326. [Google Scholar] [CrossRef]
- Carver, J.P.; Richards, R.E. A general two-site solution for the chemical exchange produced dependence of T2 upon the Carr-Purcell pulse separation. J. Magn. Reson. 1972, 6, 89–105. [Google Scholar] [CrossRef]
- Szutkowski, K.; Stilbs, P.; Jurga, S. Proton chemical exchange in aqueous solutions of dodecylammonium chloride: Effects of micellar aggregation. J. Phys. Chem. C 2007, 111, 15613–15619. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, S.; Tian, Y.; Jiang, X.; Fu, D.; Shen, S.; Yang, W. Polydopamine-coated magnetic composite particles with an enhanced photothermal effect. ACS Appl. Mater. Interfaces 2015, 7, 15876–15884. [Google Scholar] [CrossRef] [PubMed]
- Szutkowski, K.; Klinowski, J.; Jurga, S. NMR studies of restricted diffusion in lyotropic systems. Solid State Nucl. Magn. Reson. 2002, 22, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Li, X.; Lin, M.; Wang, D.; Li, S.; Liu, S.; Tang, Q.; Liu, Y.; Jiang, J.; Liu, L.; et al. Fe3O4@polydopamine composite theranostic superparticles employing preassembled Fe3O4 nanoparticles as the core. ACS Appl. Mater. Interfaces 2016, 8, 22942–22952. [Google Scholar] [CrossRef] [PubMed]








| Concentration [mM] | FWHM [Hz] | R2eff [s−1] |
|---|---|---|
| 0.15 | 240 | 754 |
| 0.20 | 252 | 792 |
| 0.30 | 353 | 1108 |
| 0.40 | 357 | 1121 |
| 0.50 | 370 | 1162 |
| 0.60 | 380 | 1193 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrówczyński, R.; Jędrzak, A.; Szutkowski, K.; Grześkowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 170. https://doi.org/10.3390/nano8030170
Mrówczyński R, Jędrzak A, Szutkowski K, Grześkowiak BF, Coy E, Markiewicz R, Jesionowski T, Jurga S. Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials. 2018; 8(3):170. https://doi.org/10.3390/nano8030170
Chicago/Turabian StyleMrówczyński, Radosław, Artur Jędrzak, Kosma Szutkowski, Bartosz F. Grześkowiak, Emerson Coy, Roksana Markiewicz, Teofil Jesionowski, and Stefan Jurga. 2018. "Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy" Nanomaterials 8, no. 3: 170. https://doi.org/10.3390/nano8030170
APA StyleMrówczyński, R., Jędrzak, A., Szutkowski, K., Grześkowiak, B. F., Coy, E., Markiewicz, R., Jesionowski, T., & Jurga, S. (2018). Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials, 8(3), 170. https://doi.org/10.3390/nano8030170