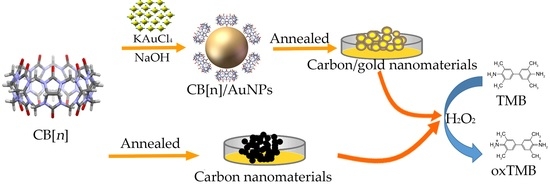
Cucurbit[n]uril (n = 6, 7) Based Carbon-Gold Hybrids with Peroxidase-Like Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cucurbit[n]uril Directed Synthesis of Gold Nanoparticles (AuNPs)
2.3. Preparation of Cucurbit[n]uril Based Carbon-Gold and Carbon Nanomaterials
2.4. Materials Characterization
2.5. Peroxidase-Like Activity
3. Results and Discussion
3.1. Characterization of Prepared Nanomaterials
3.2. Peroxidase-Like Activity
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ni, X.L.; Xiao, X.; Cong, H.; Liang, L.L.; Cheng, K.; Cheng, X.J.; Ji, N.N.; Zhu, Q.J.; Xue, S.F.; Tao, Z. Cucurbit[n]uril-based coordination chemistry: From simple coordination complexes to novel poly-dimensional coordination polymers. Chem. Soc. Rev. 2013, 42, 9480–9508. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Li, G.; Tao, C.; Li, Y.; Wu, Y.; Zhang, W. A general and efficient method to form self-assembled cucurbit[n]uril monolayers on gold surfaces. Chem. Commun. 2008, 0, 1989–1991. [Google Scholar] [CrossRef] [PubMed]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, S.; Idris, M.; Tuncel, D. Cucurbituril-based supramolecular engineered nanostructured materials. Org. Biomol. Chem. 2015, 13, 330–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Hong, C.; Ying, A.; Yang, X.; Ren, S. Ultrathin gold nanowire-functionalized carbon nanotubes for hybrid molecular sensing. ACS Nano 2013, 7, 7805–7811. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Fu, Q.; Xu, L.; Xu, Y.; Jiang, W.; Wang, D. Synthesis of pH-responsive nanocomposites of gold nanoparticles and graphene oxide and their applications in SERS and catalysis. RSC Adv. 2017, 7, 56519–56527. [Google Scholar] [CrossRef]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene-gold nanoparticles hybrid-synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Ondera, T.J.; Hamme, A.T. Gold nanopopcorn attached single-walled carbon nanotube hybrid for rapid detection and killing of bacteria. J. Mater. Chem. B 2014, 2, 7534–7543. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qu, L.; Zheng, R.; Liu, Z.; Ratinac, K.R.; Shen, L.; Yu, D.; Yang, L.J.; Barrow, C.; Ringer, S.P.; et al. Self-Assembly of gold nanowires along carbon nanotubes for ultrahigh-aspect-ratio hybrids. Chem. Mater. 2011, 23, 2760–2765. [Google Scholar] [CrossRef]
- Song, J.; Wang, F.; Yang, X.; Ning, B.; Harp, M.G.; Culp, S.H.; Hu, S.; Huang, P.; Nie, L.; Chen, J.; et al. Gold nanoparticle coated carbon nanotube ring with enhanced raman scattering and photothermal conversion property for theranostic applications. J. Am. Chem. Soc. 2016, 138, 7005–7015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, F. Application of graphene/graphene oxide in biomedicine and biotechnology. Curr. Med. Chem. 2014, 21, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Chen, R. Emerging assay platforms based on nanostructures. Curr. Nanosci. 2015, 11, 684. [Google Scholar] [CrossRef]
- Ma, T.; Liang, F.; Chen, R.; Liu, S.; Zhang, H. Synthesis of Au-Pd bimetallic nanoflowers for catalytic reduction of 4-nitrophenol. Nanomaterials 2017, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, W.; Liu, S.; Zhang, H.; Liang, F. A comparison reduction of 4-nitrophenol by gold nanospheres and gold nanostars. Catalysts 2017, 7, 38. [Google Scholar] [CrossRef]
- Shu, T.; Su, L.; Wang, J.; Lu, X.; Liang, F.; Li, C.; Zhang, X. Value of the debris of reduction sculpture: Thiol etching of Au nanoclusters for preparing water-soluble and aggregation-induced emission-active Au(I) complexes as phosphorescent copper ion sensor. Anal. Chem. 2016, 88, 6071–6077. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, T.; Shu, T.; Qu, X.; Zhang, X.; Liang, F.; Su, L. Combination of chemical etching of gold nanoclusters with aggregation-induced emission for preparation of new phosphors for the development of UV-driven phosphor-converted white light-emitting diodes. J. Mater. Chem. C 2016, 4, 11482–11487. [Google Scholar] [CrossRef]
- Han, Y.; Yang, X.; Liu, Y.; Ai, Q.; Liu, S.; Sun, C.; Liang, F. Supramolecular controlled cargo release via near infrared tunable cucurbit[7]uril-gold nanostars. Sci. Rep. 2016, 6, 22239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Y.; Chen, R.; Zhan, W.; Ni, H.; Zhang, P.; Liang, F. Antibacterial properties of multi-walled carbon nanotubes decorated with silver nanoparticles. Curr. Nanosci. 2016, 12, 411–415. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Chen, R.; Zhan, W.; Ni, H.; Liang, F. An antibacterial nonenzymatic glucose sensor composed of carbon nanotubes decorated with silver nanoparticles. Electroanalysis 2015, 27, 1138–1143. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Chen, R.; Zhang, H.; Liu, S.; Liang, F. In situimmobilization of copper nanoparticles on polydopamine coated graphene oxide for H2O2 determination. PLoS ONE 2016, 11, e0157926. [Google Scholar]
- Zhou, Y.; Liu, L.; Shen, Y.; Wu, L.; Yu, L.; Liang, F.; Xi, J. Carbon dots promoted vanadium flow batteries for all-climate energy storage. Chem. Commun. 2017, 53, 7565–7568. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Miao, F.; Zhao, Z.; Zhu, Y.; Liu, T.; Chen, R.; Liu, S.; Lv, Z.; Liang, F. Low-cost nanocarbon-based peroxidases from graphite and carbon fibers. Appl. Sci. 2017, 7, 924. [Google Scholar]
- Liu, Y.; Shen, Y.; Yu, L.; Liu, L.; Liang, F.; Qiu, X.; Xi, J. Holey-engineered electrodes for advanced vanadium flow batteries. Nano Energy 2018, 43, 55–62. [Google Scholar] [CrossRef]
- Lee, T.C.; Scherman, O.A. A facile synthesis of dynamic supramolecular aggregates of cucurbit[n]uril (n = 5–8) capped with gold nanoparticles in aqueous media. Chem. Eur. J. 2012, 18, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Scherman, O.A. Formation of dynamic aggregates in water by cucurbit[5]uril capped with gold nanoparticles. Chem. Commun. 2010, 46, 2438–2440. [Google Scholar] [CrossRef] [PubMed]
- Lanterna, A.; Pino, E.; Domenech-Carbo, A.; Gonzalez-Bejar, M.; Perez-Prieto, J. Enhanced catalytic electrochemical reduction of dissolved oxygen with ultraclean cucurbituril[7]-capped gold nanoparticles. Nanoscale 2014, 6, 9550–9553. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Esteve-Adell, I.; Atienzar, P.; Casas, J.A.; Hernández, P.; Quintana, C. Cucurbit[7]uril-stabilized gold nanoparticles as catalysts of the nitro compound reduction reaction. RSC Adv. 2016, 6, 86309–86315. [Google Scholar] [CrossRef]
- Jose, M.V.; Marx, S.; Murata, H.; Koepsel, R.R.; Russell, A.J. Direct electron transfer in a mediator-free glucose oxidase-based carbon nanotube-coated biosensor. Carbon 2012, 50, 4010–4020. [Google Scholar] [CrossRef]
- Cui, R.; Liu, C.; Shen, J.; Gao, D.; Zhu, J.-J.; Chen, H.-Y. Gold nanoparticle-colloidal carbon nanosphere hybrid material: Preparation, characterization, and application for an amplified electrochemical immunoassay. Adv. Funct. Mater. 2008, 18, 2197–2204. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Incorporating graphene oxide and gold nanoclusters: A synergistic catalyst with surprisingly high peroxidase-like activity over a broad pH range and its application for cancer cell detection. Adv. Mater. 2013, 25, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhang, M.; Ding, L.; Zheng, J.; Zeng, C.; Wen, Y.; Liu, G.; Aldalbahi, A.; Shi, J.; Song, S.; et al. Yolk-shell nanostructured Fe3O4@C magnetic nanoparticles with enhanced peroxidase-like activity for label-free colorimetric detection of H2O2 and glucose. Nanoscale 2017, 9, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Lv, X.; Ding, Y.; Zhang, Y.; Jing, J.; Xu, C. One-step synthesis of uniform nanoparticles of porphyrinfunctionalized ceria with promising peroxidase mimetics for H2O2and glucose colorimetric detection. Sens. Actuators. B Chem. 2017, 240, 726–734. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, W.; Liu, A.L.; Hong, L.; Deng, H.H.; Lin, X.H. Comparison of the peroxidase-like activity of unmodified, amino-modified, and citrate-capped gold nanoparticles. ChemPhysChem 2012, 13, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, Z.F.; Shi, M.J.; Xu, Y.; Wu, Y.L. Light emission of gold nanoparticles induced by the reaction of bis(2,4,6-trichlorophenyl) oxalate and hydrogen peroxide. Anal. Chem. 2005, 77, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Huang, Y.; Cole, A.J.; Yang, V.C. The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials 2009, 30, 4716–4722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, H.; Fan, Z.; Li, M.; Du, P.; Liu, C.; Li, Y.; Li, H.; Cao, H. A highly sensitive and selective hydrogen peroxide biosensor based on gold nanoparticles and three-dimensional porous carbonized chicken eggshell membrane. PLoS ONE 2015, 10, e0130156. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, H.; Dai, S.; Zhi, X.; Zhang, J.; Li, W. Glutathione-stabilized palladium nanozyme for colorimetric assay of silver(I) ions. Analyst 2015, 140, 6676–6683. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Zhang, J.; Fu, Y. Synthesis and sensing application of glutathione-capped platinum nanoparticles. Anal. Methods 2015, 7, 4464–4471. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, M.; Jiang, Y.; Chen, M.; Ding, Y.; Liu, Q. A facile preparation of montmorillonite-supported copper sulfide nanocomposites and their application in the detection of H2O2. Sens. Actuators B Chem. 2017, 239, 28–35. [Google Scholar] [CrossRef]







| Catalyst | Substrate | Km (mM) | Vmax (10−9 M/s) |
|---|---|---|---|
| CCB[6] | TMB | 2.4 ± 0.1 | 98.2 ± 3.8 |
| H2O2 | 2.2 ± 0.2 | 16.4 ± 1.8 | |
| CCB[7] | TMB | 2.5 ± 0.1 | 34.7 ± 2.9 |
| H2O2 | 1.4 ± 0.1 | 5.2 ± 0.8 | |
| CB[6]/AuNPs | TMB | 0.098 ± 0.008 | 10.1 ± 0.8 |
| H2O2 | 0.2 ± 0.07 | 3.0 ± 0.2 | |
| CB[7]/AuNPs | TMB | 0.038 ± 0.003 | 10.2 ± 0.2 |
| H2O2 | 0.097 ± 0.02 | 3.9 ± 0.1 | |
| CCB[6]/AuNPs | TMB | 1.9 ± 0.2 | 11.4 ± 1.2 |
| H2O2 | 1.3 ± 0.2 | 10.8 ± 1.5 | |
| CCB[7]/AuNPs | TMB | 0.63 ± 0.12 | 6.4 ± 1.0 |
| H2O2 | 0.74 ± 0.08 | 1.7 ± 1.1 | |
| HRP | TMB | 0.81 ± 0.11 | 47.6 ± 2.3 |
| H2O2 | 9.8 ± 0.04 | 87.6 ± 2.0 | |
| Fe3O4 magnetic nanoparticles [30] | TMB | 0.098 | 34.4 |
| H2O2 | 154 | 97.8 | |
| Glutathione-capped Pd nanoparticles [41] | TMB | 0.068 | 315 |
| H2O2 | 156 | 408 | |
| Glutathione-capped Pt nanoparticles [42] | TMB | 0.079 | 328 |
| H2O2 | 73.6 | 302 | |
| Montmorillonite-supported CuS nanoparticles [43] | TMB | 0.021 | 2.8 |
| H2O2 | 2.3 | 0.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zeng, Y.; Liu, S.; Liang, F. Cucurbit[n]uril (n = 6, 7) Based Carbon-Gold Hybrids with Peroxidase-Like Activity. Nanomaterials 2018, 8, 273. https://doi.org/10.3390/nano8050273
Zhang L, Zeng Y, Liu S, Liang F. Cucurbit[n]uril (n = 6, 7) Based Carbon-Gold Hybrids with Peroxidase-Like Activity. Nanomaterials. 2018; 8(5):273. https://doi.org/10.3390/nano8050273
Chicago/Turabian StyleZhang, Liangfeng, Yan Zeng, Simin Liu, and Feng Liang. 2018. "Cucurbit[n]uril (n = 6, 7) Based Carbon-Gold Hybrids with Peroxidase-Like Activity" Nanomaterials 8, no. 5: 273. https://doi.org/10.3390/nano8050273
APA StyleZhang, L., Zeng, Y., Liu, S., & Liang, F. (2018). Cucurbit[n]uril (n = 6, 7) Based Carbon-Gold Hybrids with Peroxidase-Like Activity. Nanomaterials, 8(5), 273. https://doi.org/10.3390/nano8050273