Study on the Performance of Nano-Titanium Nitride-Coated Stainless Steel Electrodes in Electro-Fenton Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TiN Powders
2.2. Preparation of the TiN/SUS304 Electrode
2.3. Electrode Characteristics and Performance Analysis
2.3.1. Analysis of Electrode Surface Micromorphology and Characteristics
2.3.2. Electrochemical Analysis
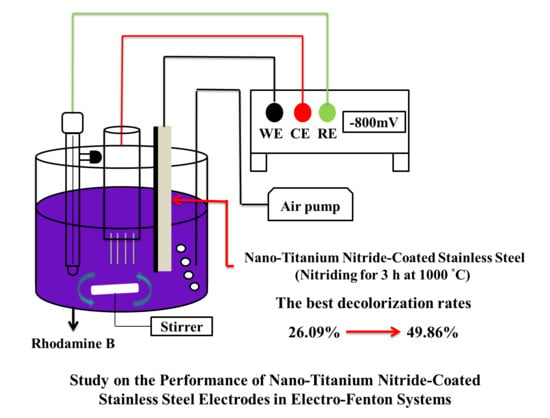
2.3.3. Tests on Rhodamine B (RhB) Decolorization Performance
3. Results and Discussion
3.1. Analysis of the TiN Morphologies and Compositions Corresponding to Different Nitriding Temperatures
3.1.1. Morphological and Compositional Analyses of TiN Powders Formed at Different Nitriding Temperatures
3.1.2. Analysis of TiN Powders Prepared at Different Nitriding Temperatures via X-ray Diffraction
3.2. Electrochemical Properties of TiN/SUS304
3.2.1. Tafel Corrosion Tests
3.2.2. Linear Sweep Voltammetry (LSV)
3.3.3. Cyclic Voltammetry Tests
3.3.4. Electro-Fenton RhB Decolorization Experiment
4. Conclusions
- The direct nitriding method was used to prepare TiN nanopowders. The powder formed at a nitriding temperature of 800 °C was a composite nanopowder containing TiO2 and TiN; TiO2 exhibited anatase crystal orientations in the (101) direction of 2θ = 26° and (004) direction of 2θ = 36°, whereas it exhibited rutile crystal peaks in all other directions.
- The nanopowders nitrided at different nitriding temperatures were used to coat electrodes, and it was observed that the TiN 1000 °C coating exhibited the best performance in electrochemical performance tests. In the Tafel test, it was shown that the corrosion potential and corrosion current of TiN 1000 °C were 116.94 mV and 205 nA/cm2, respectively. Based on the LSV tests, the optimal range of potentials for the production of H2O2 was −600 to −900 mV, and the potential of −800 mV produced the optimal response current (−8.5 mA). Cyclic voltammetry tests indicated that TiN 1000 °C has the highest electrochemically active surface area at 3.75 cm2; hence, the TiN 1000 °C coating can be used to increase the rate of EF reaction.
- The EF decolorization performance of the electrodes coated using nanopowders nitrided at different temperatures indicate that the decolorization performance is positively correlated with TiN crystallinity. The TiN 1000 °C/SUS304 electrode exhibited the best performance in EF decolorization. After 30 min of reaction time, the decolorization rate of TiN 1000 °C/SUS304 was 1.91 times that of the SUS304 substrate. In the two-hour-long decolorization experiment, it shown that the first-order kinetics of TiN 1000 °C/SUS304 exhibited the same slope in the short and long experiments, which demonstrates that the TiN 1000 °C coating possesses a high degree of electrochemical stability.
Author Contributions
Funding
Conflicts of Interest
References
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, Kinetics, and Thermodynamics of Methylene Blue Adsorption by Pine Tree Leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Qiang, Z.M. Removal of Selected Hazardous Organic Compounds by Electro-Fenton Oxidation Process. Ph.D. Thesis, Proquest Information and Learning Company, Ann Arbor, MI, USA, 2002; pp. 10–20. [Google Scholar]
- Garcia-Segura, S.; Centellas, F.; Arias, C.; Garrido, J.A.; Rodriguez, R.M.; Cabot, P.L.; Brillas, E. Comparative decolorization of monoazo, diazo and triazo dyes by electro-Fenton process. Desalination 2011, 58, 303–311. [Google Scholar] [CrossRef]
- Liu, W.; Ai, Z.; Zhang, L. Design of a neutral three-dimensional electro-Fenton system with foam nickel as particle electrodes for wastewater treatment. J. Hazard. Mater. 2012, 243, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Rivallin, M.; Cerneaux, S.; Lacour, S.; Petit, E.; Cretin, M. Coupling cathodic Electro-Fenton reaction to membrane filtration for AO7 dye degradation: A successful feasibility study. J. Membr. Sci. 2016, 510, 182–190. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Chou, S.; Huang, Y.H.; Lee, S.N.; Huang, G.H.; Huang, C. Treatment of high strength hexamine-containing wastewater by electro-Fenton method. Water Res. 1999, 33, 751–759. [Google Scholar] [CrossRef]
- Borràs, N.; Oliver, R.; Arias, C.; Brillas, E. Degradation of Atrazine by Electrochemical Advanced Oxidation Processes Using a Boron-Doped Diamond Anode. J. Phys. Chem. A 2010, 114, 6613–6621. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, A.D.; Merli, C.; Sirés, I.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E. Removal of the herbicide amitrole from water by anodic oxidation and electro-Fenton. Environ. Chem. Lett. 2005, 3, 7–11. [Google Scholar] [CrossRef]
- Ramírez, C.; Saldaña, A.; Hernández, B.; Acero, R.; Guerra, R.; Segura, S.G.; Brillas, E.; Hernández, J.M.P. Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology. J. Ind. Eng. Chem. 2013, 19, 571–579. [Google Scholar] [CrossRef]
- Rosales, E.; Pazos, M.; Longo, M.A.; Sanromán, M.A. Electro-Fenton decoloration of dyes in a continuous reactor: A promising technology in colored wastewater treatment. Chem. Eng. J. 2009, 155, 62–67. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F.; Jia, Q. Preparation of titanium nitride ultrafine powders by sol–gel and microwave carbothermal reduction nitridation methods. Ceram. Int. 2009, 35, 1071–1075. [Google Scholar] [CrossRef]
- Yousefi, E.; Ghorbani, M.; Dolati, A.; Yashiro, H. Facile synthesis of titanium nitride-graphene nanocomposite and its improved rate-dependent electroactivity with respect to lithium storage. Mater. Res. Bull. 2016, 84, 388–396. [Google Scholar] [CrossRef]
- Ru, J.; Hua, Y.; Xu, C.; Zhang, Q.; Wang, D.; Gong, K. Synthesis of TiN from FeTiO3 by microwave-assisted carbothermic reduction–nitridation. J. Alloys Compd. 2014, 583, 121–127. [Google Scholar] [CrossRef]
- Gou, H.P.; Zhang, G.H.; Chou, K.C. Phase evolution and reaction mechanism during reduction–nitridation process of titanium dioxide with ammonia. J. Mater. Sci. 2017, 52, 1255–1264. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Singh, V.B.; Singh, H.K. Structure and properties of electrodeposited functional Ni–Fe/TiN nanocomposite coatings. Surf. Coat. Technol. 2015, 278, 146–156. [Google Scholar] [CrossRef]
- Liu, G.; Pan, Z.; Zhang, B.; Xiao, J.; Xia, G.; Zhao, Q.; Shi, S.; Hu, G.; Xiao, C.; Wei, Z.; et al. A novel TiN coated CNTs nanocomposite CNTs@TiN supported Pt electrocatalyst with enhanced catalytic activity and durability for methanol oxidation reaction. Int. J. Hydrogen Energy 2017, 42, 12467–12476. [Google Scholar] [CrossRef]
- Mani, S.P.; Srinivasan, A.; Rajendran, N. Effect of nitrides on the corrosion behaviour of 316L SS bipolar plates for Proton Exchange Membrane Fuel Cell (PEMFC). Int. J. Hydrogen Energy 2015, 40, 3359–3369. [Google Scholar] [CrossRef]
- Su, J.; Lu, N.; Zhao, J.; Yu, H.; Huang, H.; Dong, X.; Quan, X. Nano-cubic structured titanium nitride particle films as cathodes for the effective electrocatalytic debromination of BDE-47. J. Hazard. Mater. 2012, 231, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, P.; Xiao, D. Enhanced Electrocatalytic Performance for Oxygen Reduction via Active Interfaces of Layer-By-Layered Titanium Nitride/Titanium Carbonitride Structures. Sci. Rep. 2014, 4, 6712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthik, T.; Rathinamoorthy, R.; Murugan, R. Enhancement of wrinkle recovery angle of cotton fabric using citric acid cross-linking agent with nano-TiO2 as a co-catalyst. Ind. Text. 2011, 42, 99–117. [Google Scholar] [CrossRef]
- Ma, J.; Wu, M.; Du, Y.; Chen, S.; Li, G.; Hu, J. Synthesis of nanocrystalline titanium nitride at low temperature and its thermal stability. J. Alloys Compd. 2009, 476, 603–605. [Google Scholar] [CrossRef]
- Mousset, E.; Wang, Z.; Hammaker, J.; Lefebvre, O. Physico-chemical properties of pristine graphene and its performance as electrode material for electro-Fenton treatment of wastewater. Electrochim. Acta 2016, 214, 217–230. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Fundamentals and applications. In Electrochemical Methods, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Grewal, Y.S.; Shiddiky, M.J.; Gray, S.A.; Weigel, K.M.; Cangelosi, G.A.; Trau, M. Label-free electrochemical detection of an Entamoeba histolytica antigen using cell-free yeast-scFv probes. Chem. Commun. 2013, 49, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Matthes, B.; Broszeit, E.; Aromaa, J.; Ronkainen, H.; Hannula, S.P.; Leyland, A.; Matthew, A. Corrosion performance of some titanium-based hard coatings. Surf. Coat. Technol. 1991, 49, 489–495. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetchakun, N.; Phanichphant, S. Effect of temperature on the degree of anatase–rutile transformation in titanium dioxide nanoparticles synthesized by the modified sol–gel method. Curr. Appl. Phys. 2008, 8, 343–346. [Google Scholar] [CrossRef]
- Dolat, D.; Moszyński, D.; Guskos, N.; Ohtani, B.; Morawski, A.W. Preparation of photoactive nitrogen-doped rutile. Appl. Surf. Sci. 2013, 266, 410–419. [Google Scholar] [CrossRef]
- Dolat, D.; Ohtani, B.; Mozia, S.; Moszyński, D.; Guskos, N.; Bieluń, Z.L.; Morawski, A.W. Preparation, characterization and charge transfer studies of nickel—Modified and nickel, nitrogen co-modified rutile titanium dioxide for photocatalytic application. Chem. Eng. J. 2014, 239, 149–157. [Google Scholar] [CrossRef]
- Bauer, D.A.; Herranen, M.; Ljungcrantz, H.; Carlsson, J.O.; Sundgren, J.E. Corrosion behaviour of monocrystalline titanium nitride. Surf. Coat. Technol. 1997, 91, 208–214. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Yuan, Z.; Shu, X.; Liu, E.; Han, Z. A comparative study of the corrosion performance of titanium (Ti), titanium nitride (TiN), titanium dioxide (TiO2) and nitrogen-doped titanium oxides (N–TiO2), as coatings for biomedical applications. Ceram. Int. 2015, 41, 11844–11851. [Google Scholar] [CrossRef]
- Valdez, B.; Kiyota, S.; Stoytcheva, M.; Zlatev, R.; Bastidas, J.M. Cerium-based conversion coatings to improve the corrosion resistance of aluminium alloy 6061-T6. Corros. Sci. 2014, 87, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.S.; Mul, G.; Moulijn, J.A. Electrochemical generation of hydrogen peroxide using surface area-enhanced Ti-mesh electrodes. Electrochim. Acta 2007, 52, 6304–6309. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, T.; Song, S.; Gui, X.; Liu, H.; Tsiakaras, P. Dimethyl phthalate degradation at novel and efficient electro-Fenton cathode. Appl. Catal. B Environ. 2014, 156–157, 1–7. [Google Scholar] [CrossRef]
- Sirés, I.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E.; Oturan, N.; Oturan, M.A. Catalytic behavior of the Fe3+/Fe2+ system in the electro-Fenton degradation of the antimicrobial chlorophene. Appl. Catal. B Environ. 2007, 72, 382–394. [Google Scholar] [CrossRef]








| Chemical Composition of SUS304 Stainless Steel | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Components | C | Si | Mn | P | S | Ni | Cr | N | Mo | Cu |
| Contents (%) | 0.042 | 0.47 | 0.97 | 0.029 | 0.004 | 8.09 | 18.06 | 0.058 | 0.00 | 0.02 |
| Element | O | N | Ti | |
|---|---|---|---|---|
| Powder | ||||
| 800 °C | 76.31 | - | Bal. | |
| 900 °C | - | 48.32 | Bal. | |
| 1000 °C | - | 49.42 | Bal. | |
| Properties | Ecorr (mV) | Icorr (nA/cm2) | Rp (kΩ/cm2) | P (10−4) | |
|---|---|---|---|---|---|
| Materials | |||||
| SUS304 | −233.607 | 325 | 28.093 | - | |
| TiO2 & TiN 800 °C/SUS304 | −96.522 | 85.8 | 114.65 | 0.1084 | |
| TiN 900 °C/SUS304 | 20.016 | 524 | 16.46 | 0.92 | |
| TiN 1000 °C/SUS304 | 116.94 | 205 | 41.367 | 10−3 | |
| Materials | Icorr (nA) | Reactive Surface Area (cm2) | Icorr (nA/cm2) |
|---|---|---|---|
| SUS304 | 14,365 | 1.72 | 8351.744 |
| TiO2 & TiN 800 °C/SUS304 | 3792.36 | 1.45 | 2615.421 |
| TiN 900 °C/SUS304 | 23,160.8 | 3.3 | 7018.424 |
| TiN 1000 °C/SUS304 | 9061 | 3.75 | 2416.267 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lin, Y. Study on the Performance of Nano-Titanium Nitride-Coated Stainless Steel Electrodes in Electro-Fenton Systems. Nanomaterials 2018, 8, 494. https://doi.org/10.3390/nano8070494
Wang Y, Lin Y. Study on the Performance of Nano-Titanium Nitride-Coated Stainless Steel Electrodes in Electro-Fenton Systems. Nanomaterials. 2018; 8(7):494. https://doi.org/10.3390/nano8070494
Chicago/Turabian StyleWang, Yita, and Youchen Lin. 2018. "Study on the Performance of Nano-Titanium Nitride-Coated Stainless Steel Electrodes in Electro-Fenton Systems" Nanomaterials 8, no. 7: 494. https://doi.org/10.3390/nano8070494
APA StyleWang, Y., & Lin, Y. (2018). Study on the Performance of Nano-Titanium Nitride-Coated Stainless Steel Electrodes in Electro-Fenton Systems. Nanomaterials, 8(7), 494. https://doi.org/10.3390/nano8070494