Thermally Robust Non-Wetting Ni-PTFE Electrodeposited Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
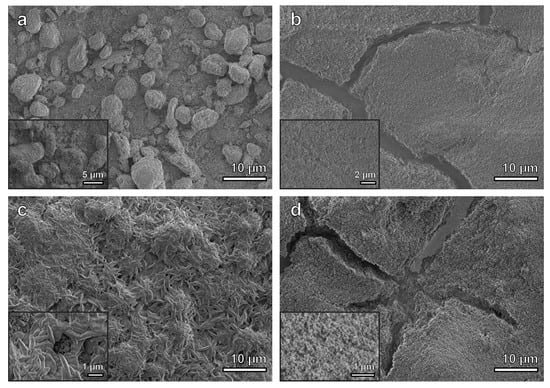
3.1. Characterization of As-Deposited Materials
3.2. High Temperature Exposure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Jung, Y.C. Micro- and nanoscale characterization of hydrophobic and hydrophilic leaf surfaces. Nanotechnology 2006, 17, 2758–2772. [Google Scholar] [CrossRef]
- Im, M.; Im, H.; Lee, J.-H.H.; Yoon, J.-B.B.; Choi, Y.-K.K. A robust superhydrophobic and superoleophobic surface with inverse-trapezoidal microstructures on a large transparent flexible substrate. Soft Matter 2010, 6, 1401. [Google Scholar] [CrossRef]
- Lee, C.H.; Drelich, J.; Yap, Y.K. Superhydrophobicity of boron nitride nanotubes grown on silicon substrates. Langmuir 2009, 25, 4853–4860. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Multifunctional surfaces produced by femtosecond laser pulses Multifunctional surfaces produced by femtosecond laser pulses. J. Appl. Phys. 2015, 117, 033103. [Google Scholar] [CrossRef]
- Baldacchini, T.; Carey, J.E.; Zhou, M.; Mazur, E. Superhydrophobic surfaces prepared by microstructuring of silicon using a femtosecond laser. Langmuir 2006, 22, 4917–4919. [Google Scholar] [CrossRef]
- Iacovetta, D.; Tam, J.; Erb, U. Synthesis, structure, and properties of superhydrophobic nickel–PTFE nanocomposite coatings made by electrodeposition. Surf. Coat. Technol. 2015, 279, 134–141. [Google Scholar] [CrossRef]
- Tam, J.; Jiao, Z.; Lau, J.J.C.F.J.; Erb, U. Wear stability of superhydrophobic nano Ni-PTFE electrodeposits. Wear 2017, 374–375, 1–4. [Google Scholar] [CrossRef]
- Su, C.; Xu, Y.; Gong, F.; Wang, F.; Li, C. The abrasion resistance of a superhydrophobic surface comprised of polyurethane elastomer. Soft Matter 2010, 6, 6068–6071. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ge, Q.; Yang, L.L.; Shi, X.J.; Li, J.J.; Yang, D.Q.; Sacher, E. Durable superhydrophobic PTFE films through the introduction of micro- and nanostructured pores. Appl. Surf. Sci. 2014, 339, 151–157. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Wu, L. Facile fabrication of self-repairing superhydrophobic coatings. Chem. Commun. 2014, 50, 11891–11894. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Kang, Z.; Li, W.; Zhang, J. A facile approach to fabricate a stable superhydrophobic film with switchable water adhesion on titanium surface. Surf. Coat. Technol. 2014, 239, 227–232. [Google Scholar] [CrossRef]
- Xue, F.; Jia, D.; Li, Y.; Jing, X. Facile preparation of a mechanically robust superhydrophobic acrylic polyurethane coating. J. Mater. Chem. A 2015, 3, 13856–13863. [Google Scholar] [CrossRef]
- Tam, J.; Palumbo, G.; Erb, U.; Azimi, G. Robust Hydrophobic Rare Earth Oxide Composite Electrodeposits. Adv. Mater. Interfaces 2017, 4, 1700850. [Google Scholar] [CrossRef]
- Xu, P.; Coyle, T.W.; Pershin, L.; Mostaghimi, J. Superhydrophobic ceramic coating: Fabrication by solution precursor plasma spray and investigation of wetting behavior. J. Colloid Interface Sci. 2018, 523, 35–44. [Google Scholar] [CrossRef]
- Zhang, X.; Mo, J.; Si, Y.; Guo, Z. How does substrate roughness affect the service life of a superhydrophobic coating? Appl. Surf. Sci. 2018, 441, 491–499. [Google Scholar] [CrossRef]
- Liang, Y.; Ju, J.; Deng, N.; Zhou, X.; Yan, J.; Kang, W.; Cheng, B. Super-hydrophobic self-cleaning bead-like SiO2@PTFE nanofiber membranes for waterproof-breathable applications. Appl. Surf. Sci. 2018, 442, 54–64. [Google Scholar] [CrossRef]
- Qiao, J.H.; Jin, X.; Qin, J.H.; Liu, H.T.; Luo, Y.; Zhang, D.K. A super-hard superhydrophobic Fe-based amorphous alloy coating. Surf. Coat. Technol. 2018, 334, 286–291. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, D.; Sun, L.; Zhao, Y.; Tiwari, M.K.; Carmalt, C.J.; Parkin, I.P.; Lu, Y. Super-durable, non-fluorinated superhydrophobic free-standing items. J. Mater. Chem. A 2017, 6, 357–362. [Google Scholar] [CrossRef]
- Nakayama, K.; Hiraga, T.; Zhu, C.; Tsuji, E.; Aoki, Y.; Habazaki, H. Facile preparation of self-healing superhydrophobic CeO 2 surface by electrochemical processes. Appl. Surf. Sci. 2017, 423, 968–976. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Huang, Y.; Yang, L.; Ye, Y.; Walsh, F.C.; Chen, J.; Wang, S. Robust Ni/WC superhydrophobic surfaces by electrodeposition. RSC Adv. 2017, 7, 44896–44903. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhang, Z.; Ren, G.; Yang, J.; Wang, K.; Xu, X.; Men, X.; Zhou, X. A novel superhydrophobic bulk material. J. Mater. Chem. 2012, 22, 20146. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Liu, T.; Xu, C.; Ge, Z. Controllable fabrication of superhydrophobic TiO2 coating with improved transparency and thermostability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 298–305. [Google Scholar] [CrossRef]
- Gong, G.; Gao, K.; Wu, J.; Sun, N.; Zhou, C.; Zhao, Y.; Jiang, L. A highly durable silica/polyimide superhydrophobic nanocomposite film with excellent thermal stability and abrasion-resistant performance. J. Mater. Chem. A 2015, 3, 713–718. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, M.; Guo, Z. Robust, heat-resistant and multifunctional superhydrophobic coating of carbon microflowers with molybdenum trioxide nanoparticles. J. Colloid Interface Sci. 2017, 506, 649–658. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, B.; Men, X.; Li, Y. Mechanically durable, superhydrophobic coatings prepared by dual-layer method for anti-corrosion and self-cleaning. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 182–188. [Google Scholar] [CrossRef]
- Velayi, E.; Norouzbeigi, R. Annealing temperature dependent reversible wettability switching of micro/nano structured ZnO superhydrophobic surfaces. Appl. Surf. Sci. 2018, 441, 156–164. [Google Scholar] [CrossRef]
- Lee, Y.; You, E.A.; Ha, Y.G. Facile one-step construction of covalently networked, self-healable, and transparent superhydrophobic composite films. Appl. Surf. Sci. 2018, 445, 368–375. [Google Scholar] [CrossRef]
- Cha, S.C.; Her, E.K.; Ko, T.J.; Kim, S.J.; Roh, H.; Lee, K.R.; Oh, K.H.; Moon, M.W. Thermal stability of superhydrophobic, nanostructured surfaces. J. Colloid Interface Sci. 2013, 391, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hong, X.; Lin, J. Transparent superhydrophobic hollow films (TSHFs) with superior thermal stability and moisture resistance. RSC Adv. 2018, 8, 491–498. [Google Scholar] [Green Version]
- Yang, H.; Cheng, Y.; Xiao, F. Thermal stable superhydrophobic polyphenylsilsesquioxane/nanosilica composite coatings. Appl. Surf. Sci. 2011, 258, 1572–1580. [Google Scholar] [CrossRef]
- Tam, J.; Palumbo, G.; Erb, U. Recent Advances in Superhydrophobic Electrodeposits. Materials 2016, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Walsh, F.C.; Ponce de Leon, C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Mahidashti, Z.; Aliofkhazraei, M.; Lotfi, N. Review of Nickel-Based Electrodeposited Tribo-Coatings. Trans. Indian Inst. Met. 2018, 71, 257–295. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; Mohamed, A.M.A. Electrodeposition of Nanostructured Nickel-Ceramic Composite Coatings: A review. Int. J. Electrochem. Sci. 2014, 1942–1963. [Google Scholar]
- Zimmerman, A.F.; Palumbo, G.; Aust, K.T.; Erb, U. Mechanical properties of nickel silicon carbide nanocomposites. Mater. Sci. Eng. A 2002, 328, 137–146. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Lond. Sect. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The Cleavage Strength of Polycrystals. J. Iron Steel Inst. 1953, 174, 22–28. [Google Scholar]
- El-Sherik, A.M.M.; Erb, U.; Palumbo, G.; Aust, K.T.T. Deviations from hall-petch behaviour in as-prepared nanocrystalline nickel. Scr. Metall. Mater. 1992, 27, 1185–1188. [Google Scholar] [CrossRef]
- Victor, J.J.; Erb, U. Influence of Weather Conditions on the Surface Morphology and Wetting Behaviour of Superhydrophobic Quaking Aspen Leaves. Am. J. Plant Sci. 2013, 4, 61–68. [Google Scholar] [CrossRef]
- Rust-Oleum. Rust-Oleum Multi Component Product Information Sheet 274232; Rust-Oleum: Vemon Hills, IL, USA, 2015. [Google Scholar]
- Conesa, A.; Font, R. Polytetrafluorethylene Decomposition in Air and Nitrogen. Polym. Eng. Sci. 1994, 41, 2137–2147. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering: An Integrated Approach, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Chen, Y.C.; Lin, H.C.; Lee, Y. Der The effects of phenyltrimethoxysilane coupling agents on the properties of PTFE/silica composites. J. Polym. Res. 2004, 11, 1–7. [Google Scholar] [CrossRef]
- Sim, L.C.; Ramanan, S.R.; Ismail, H.; Seetharamu, K.N.; Goh, T.J. Thermal characterization of Al2O3and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim. Acta 2005, 430, 155–165. [Google Scholar] [CrossRef]
- Rae, P.J.; Dattelbaum, D.M. The properties of poly(tetrafluoroethylene) (PTFE) in compression. Polymer 2004, 45, 7615–7625. [Google Scholar] [CrossRef]
- Bassett, D.C.; Davitt, R. On Crystallization Phenomena in Polytetrafluoroethylene. Polymer 1974, 15, 721–728. [Google Scholar] [CrossRef]
- ASTM. ASTM D3359-09e2 Standard Test Methods for Measuring Adhesion by Tape Test; ASTM: West Conshohocken, PA, USA, 2009. [Google Scholar]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, J.; Lau, J.C.F.; Erb, U. Thermally Robust Non-Wetting Ni-PTFE Electrodeposited Nanocomposite. Nanomaterials 2019, 9, 2. https://doi.org/10.3390/nano9010002
Tam J, Lau JCF, Erb U. Thermally Robust Non-Wetting Ni-PTFE Electrodeposited Nanocomposite. Nanomaterials. 2019; 9(1):2. https://doi.org/10.3390/nano9010002
Chicago/Turabian StyleTam, Jason, Jonathan Chun Fung Lau, and Uwe Erb. 2019. "Thermally Robust Non-Wetting Ni-PTFE Electrodeposited Nanocomposite" Nanomaterials 9, no. 1: 2. https://doi.org/10.3390/nano9010002
APA StyleTam, J., Lau, J. C. F., & Erb, U. (2019). Thermally Robust Non-Wetting Ni-PTFE Electrodeposited Nanocomposite. Nanomaterials, 9(1), 2. https://doi.org/10.3390/nano9010002