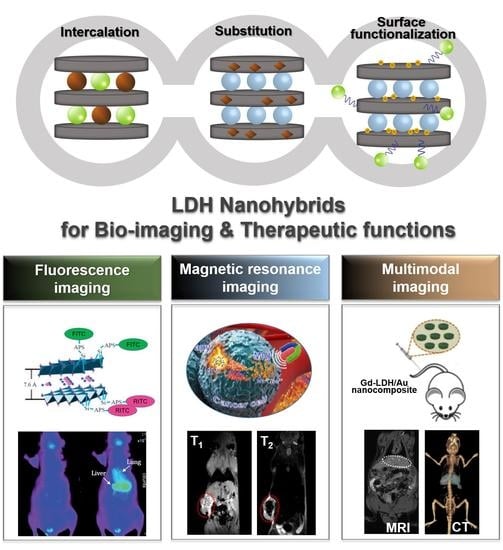
Functional Layered Double Hydroxide Nanohybrids for Biomedical Imaging
Abstract
:1. Introduction
2. Design of Functional LDH Nanohybrids for Biomedical Imaging
3. LDH Nanohybrids for Bio-Imaging Applications with Therapeutic Functions
3.1. Fluorescence Imaging
3.1.1. LDH Nanohybrids for Fluorescence Imaging
3.1.2. LDH Nanohybrids for Fluorescence Imaging with Therapeutic Functions
3.2. Magnetic Resonance Imaging (MRI)
3.2.1. LDH Nanohybrids for MRI
3.2.2. LDH Nanohybrids for MRI with Therapeutic Functions
3.3. Multimodal Imaging
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2006; Volume 119, pp. 1–87. [Google Scholar]
- Park, D.H.; Hwang, S.J.; Oh, J.M.; Yang, J.H.; Choy, J.H. Polymer–inorganic supramolecular nanohybrids for red, white, green, and blue applications. Prog. Polym. Sci. 2013, 38, 1442–1486. [Google Scholar] [CrossRef]
- Oh, J.M.; Park, D.H.; Choi, S.J.; Choy, J.H. LDH nanocontainers as bio-reservoirs and drug delivery carriers. Recent Pat. Nanotech. 2012, 6, 200–217. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Oh, J.M.; Park, D.W.; Choy, J.H. Integrated bio-inorganic hybrid systems for nano-forensics. Chem. Soc. Rev. 2011, 40, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Cho, J.; Kwon, O.J.; Yun, C.O.; Choy, J.H. Biodegradable Inorganic Nanovector: Passive versus Active Tumor Targeting in siRNA Transportation. Angew. Chem. Int. Ed. 2016, 55, 4582–4586. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, J.E.; Oh, J.M.; Shul, Y.G.; Choy, J.H. DNA Core@Inorganic Shell. J. Am. Chem. Soc. 2010, 132, 16735–16736. [Google Scholar] [CrossRef]
- Choy, J.H.; Kwak, S.Y.; Park, J.S.; Jeong, Y.J.; Portier, J. Intercalative nanohybrids of nucleoside monophosphates and DNA in layered metal hydroxide. J. Am. Chem. Soc. 1999, 121, 1399–1400. [Google Scholar] [CrossRef]
- Oh, J.M.; Choi, S.J.; Kim, S.T.; Choy, J.H. Cellular Uptake Mechanism of an Inorganic Nanovehicle and Its Drug Conjugates: Enhanced Efficacy Due To Clathrin-Mediated Endocytosis. Bioconjugate Chem. 2006, 17, 1411–1417. [Google Scholar] [CrossRef]
- Wang, L.; Xing, H.; Zhang, S.; Ren, Q.; Pan, L.; Zhang, K.; Bu, W.; Zheng, X.; Zhou, L.; Peng, W.; et al. A Gd-doped Mg-Al-LDH/Au nanocomposite for CT/MR bimodal imagings and simultaneous drug delivery. Biomaterials 2013, 34, 3390–3401. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, K.L.; Chen, S.; Li, S.H.; Wang, L.L.; Wang, L.P.; Liu, R.; Gao, J.; Yang, H.H. Manganese-iron layered double hydroxide: A theranostic nanoplatform with pH-responsive MRI contrast enhancement and drug release. J. Mater. Chem. B 2017, 5, 3629–3633. [Google Scholar] [CrossRef]
- Xie, W.S.; Guo, Z.H.; Cao, Z.B.; Gao, Q.; Wang, D.; Boyer, C.; Kallavaris, M.; Sun, X.D.; Wang, X.M.; Zhao, L.Y.; et al. Manganese-based magnetic layered double hydroxide nanoparticle: A pH-sensitive and concurrently enhanced T1/T2-weighted dual-mode magnetic resonance imaging contrast agent for accurate cancer diagnosis. ACS Biomater. Sci. Eng. 2019, 5, 2555–2562. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Bulte, J.W. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 2013, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, C.H.; Ko, H.J.; Suh, J.S.; Yoon, H.G.; Lee, K.; Huh, Y.M.; Haam, S. Multifunctional magneto-polymeric nanohybrids for targeted detection and synergistic therapeutic effects on breast cancer. Angew. Chem. Int. Ed. 2007, 46, 8836–8839. [Google Scholar] [CrossRef]
- Weissleder, R. Molecular Imaging in Cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Peng, X.H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In Vivo Tumor Targeting and Spectroscopic Detection with Surface-Enhanced Raman Nanoparticle Tags. Nat. Biotechnol. 2007, 26, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Michalet, X.; Pinaud, F.F.; Bentoilla, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.W.; Jin, R.; Mirkin, C.A. Nanoparticles with Raman Spectroscopic Fingerprints for DNA and RNA Detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [Green Version]
- Schwab, K. The Fourth Industrial Revolution; World Economic Forum: Cologny, Switzerland, 2016. [Google Scholar]
- Wei, P.R.; Cheng, S.H.; Liao, W.N.; Kao, K.C.; Weng, C.F.; Lee, C.H. Synthesis of chitosan-coated near-infrared layered double hydroxide nanoparticles for in vivo optical imaging. J. Mater. Chem. 2012, 22, 5503. [Google Scholar] [CrossRef]
- Chung, H.E.; Park, D.H.; Choy, J.H.; Choi, S.J. Intracellular trafficking pathway of layered double hydroxide nanoparticles in human cells: Size-dependent cellular delivery. Appl. Clay. Sci. 2012, 65, 24–30. [Google Scholar] [CrossRef]
- Choy, J.H.; Kwak, S.Y.; Jeong, Y.J.; Park, J.S. Inorganic Layered Double Hydroxides as Nonviral Vectors. Angew. Chem. 2000, 39, 4041–4045. [Google Scholar] [CrossRef]
- Hakeem, A.; Zhan, G.T.; Xu, Q.B.; Yong, T.Y.; Gan, L.; Yang, X.L. Facile synthesis of pH-responsive doxorubicin-loaded layered double hydroxide for efficient cancer therapy. J. Mater. Chem. B 2018, 6, 5768–5774. [Google Scholar] [CrossRef]
- Hu, T.Y.; He, J.; Zhang, S.M.; Mei, X.; Zhang, W.K.; Liang, R.Z.; Wei, M.; Evans, D.G.; Duan, X. An ultrathin photosensitizer for simultaneous fluorescence imaging and photodynamic therapy. Chem. Commun. 2018, 54, 5760–5763. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Zuo, H.L.; Zhang, E.Q.; Li, L.; Henrich-Noack, P.; Cooper, H.M.; Qian, Y.J.; Xu, Z.P. Brain targeting delivery facilitated by ligand-functionalized layered double hydroxide nanoparticles. ACS Appl. Mater. Interfaces 2018, 24, 20326–20333. [Google Scholar] [CrossRef]
- Li, B.; Gu, Z.; Kurniawan, N.; Chen, W.; Xu, Z.P. Manganese-Based Layered Double Hydroxide Nanoparticles as a T1-MRI Contrast Agent with Ultrasensitive pH Response and High Relaxivity. Adv. Mater. 2017, 29, 1700373. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, J.M.; Lee, J.S.; Kim, T.J.; Choy, J.H. Gadolinium (III) Diethylenetriamine Pentaacetic Acid/Layered Double Hydroxide Nanohybrid as Novel T1-Magnetic Resonant Nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 5181–5184. [Google Scholar] [CrossRef]
- Li, B.; Tang, J.; Chen, W.; Hao, G.; Kurniawan, N.; Gu, Z.; Xu, Z.P. Novel theranostic nanoplatform for complete mice tumor elimination via MR imaging-guided acid-enhanced photothermo-/chemo-therapy. Biomaterials 2018, 177, 40–51. [Google Scholar] [CrossRef]
- Xu, Z.P.; Kurniawan, N.D.; Bartlett, P.F.; Lu, G.Q. Enhancement of Relaxivity Rates of Gd–DTPA Complexes by Intercalation into Layered Double Hydroxide Nanoparticles. Chem. Eur. J. 2007, 13, 2824–2830. [Google Scholar] [CrossRef]
- Guan, S.; Weng, Y.; Li, M.; Liang, R.; Sun, C.; Qu, X.; Zhou, S. An NIR-sensitive layered supramolecular nanovehicle for combined dual-modal imaging and synergistic therapy. Nanoscale 2017, 9, 10367–10374. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.; Wang, W.; Yan, L.; Hu, T.; Liang, R.; Yan, D.; Wei, M.; Evans, D.; Duan, X. Hydrotalcite monolayer toward high performance synergistic dual-modal imaging and cancer therapy. Biomaterials 2018, 165, 14–24. [Google Scholar] [CrossRef]
- Choi, J.; Jun, Y.; Yeon, S.I.; Kim, H.C.; Shin, J.S.; Cheon, J. Biocompatible Heterostructured Nanoparticles for Multimodal Biological Detection. J. Am. Chem. Soc. 2006, 128, 15982–15983. [Google Scholar] [CrossRef]
- Guan, S.; Liang, R.; Li, C.; Wei, M. A supramolecular material for dual-modal imaging and targeted cancer therapy. Talanta 2017, 165, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Guan, S.; Lu, H.; Meng, X.; Kaassis, A.Y.; Ren, X.; Qu, X.; Sun, C.; Xie, Z.; Zhou, S. Confinement of carbon dots localizing to the ultrathin layered double hydroxides toward simultaneous triple-mode bioimaging and photothermal therapy. Talanta 2018, 184, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Choi, S.J.; Lee, G.E.; Kim, J.E.; Choy, J.H. Inorganic Metal Hydroxide Nanoparticles for Targeted Cellular Uptake Through Clathrin-Mediated Endocytosis. Chem. Asian. J. 2009, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.G.; Kim, J. Functional mesoporous silica nanoparticles for bio-imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 11, e1515. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Usman, M.S.; Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Masarudin, M.J.; Saad, F.F.A. Synthesis and characterization of protocatechuic acid-loaded gadolinium-layered double hydroxide and gold nanocomposite for theranostic application. Appl. Nanosci. 2018, 8, 973–986. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Cheon, J.; Lee, J.H. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc. Chem. Res. 2008, 41, 1630–1640. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.C.; Nah, H.; Woo, S.; Oh, J.; Kim, K.M.; Cheon, G.J.; Chang, Y.; Yoo, J.; Cheon, J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chem. Int. Ed. 2008, 47, 6259–6262. [Google Scholar] [CrossRef]
- Yang, J.; Lim, E.M.; Lee, H.J.; Park, J.; Lee, S.C.; Lee, K.; Yoon, H.G.; Suh, J.S.; Huh, Y.M.; Haam, S. Fluorescent magnetic nanohybrids as multimodal imaging agents for human epithelial cancer detection. Biomaterials 2008, 29, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.H.; Choi, Y.; Kim, S.; Cheon, J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rev. 2015, 44, 4501–4516. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Lee, B.I.; Lee, K.S.; Im, G.H.; Byeon, S.H.; Lee, J.H.; Lee, I.S. Surface Modification of Exfoliated Layered Gadolinium Hydroxide for the Development of Multimodal Contrast Agents for MRI and Fluorescence Imaging. Adv. Funct. Mater. 2009, 19, 3375–3380. [Google Scholar] [CrossRef]
- Park, D.H.; Choi, G.; Choy, J.H. Bio-Layered Double Hydroxides Nanohybrids for Theranostics Applications. In Photofunctional Layered Materials; Structure and Bonding; Springer: Cham, Switzerland, 2015; Volume 166, pp. 137–175. [Google Scholar]
- Oh, J.M.; Hwang, S.H.; Choy, J.H. The effect of synthetic conditions on tailoring the size of hydrotalcite particles. Solid State Ion. 2002, 151, 285–291. [Google Scholar] [CrossRef]
- Choy, J.H.; Kwak, S.Y.; Park, J.S.; Jeong, Y.J. Cellular uptake behavior of [γ-32P] labeled ATP–LDH nanohybrids. J. Mater. Chem. 2001, 11, 1671–1674. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, W.J.; Lee, J.Y.; Paek, S.M.; Oh, J.M. Isomorphous substitution of divalent metal ions in layered double hydroxides through a soft chemical hydrothermal reaction. Dalton Trans. 2014, 43, 10430. [Google Scholar] [CrossRef]
- Jung, Y.; Ji, E.; Capasso, A.; Lee, G.H. Recent Progresses in the Growth of Two-dimensional Transition Metal Dichalcogenides. J. Korean Ceram. Soc. 2019, 56, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Oh, J.M.; Choi, S.J.; Lee, G.E.; Han, S.H.; Choy, J.H. Inorganic drug-delivery nanovehicle conjugated with Cancer-Cell-Specific ligand. Adv. Funct. Mater. 2009, 19, 1617–1624. [Google Scholar] [CrossRef]
- Wei, P.R.; Kuthati, Y.; Kankala, R.K.; Lee, C.H. Synthesis and Characterization of Chitosan-Coated Near-Infrared (NIR) Layered Double Hydroxide-Indocyanine Green Nanocomposites for Potential Applications in Photodynamic Therapy. Int. J. Mol. Sci. 2015, 16, 20943–20968. [Google Scholar] [CrossRef]
- Arratia-Quijada, J.; Jiménez, C.S.; Gurinov, A.; Pérez Centeno, A.; Ceja Andrade, I.; Carbajal Arízaga, G.G. Dysprosium-containing layered double hydroxides nanoparticles intercalated with biologically active species as an approach for theranostic systems. Mater. Sci. Eng. B 2016, 203, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Bandgar, S.S.; Yadav, H.M.; Shirguppikar, S.S.; Shinde, M.A.; Shejawal, R.V.; Kolekar, T.V.; Bamane, S.R. Enhanced Hemolytic Biocompatibility of Hydroxyapatite by Chromium (Cr3+) Doping in Hydroxyapatite Nanoparticles Synthesized by Solution Combustion Method. J. Korean Ceram. Soc. 2017, 54, 158–166. [Google Scholar] [CrossRef]
- Viruete, A.; Carbajal-Arízaga, G.G.; Hernández Gutiérrez, R.; Oaxaca Camacho, A.R.; Arratia-Quijada, J. Passive targeting effect of Dy-doped LDH nanoparticles hybridized with folic acid and gallic acid on HEK293 human kidney cells and HT29 human cells. J. Nanoparticle Res. 2018, 20, 333. [Google Scholar] [CrossRef]
- Peng, L.; Mei, X.; He, J.; Xu, J.; Zhang, W.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Monolayer Nanosheets with an Extremely High Drug Loading toward Controlled Delivery and Cancer Theranostics. Adv. Mater. 2018, 30, 1707389. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Tahriri, M.; Moztarzadeh, F.; Bader, R.; Tayebi, L. Nanostructured Hydroxyapatite for Biomedical Applications: From Powder to Bioceramic. J. Korean Ceram. Soc. 2018, 55, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, W.; Li, B.; Xu, K.; Cooper, H.; Gu, Z.; Xu, Z.P. MnAl Layered Double Hydroxide Nanoparticles as a Dual-Functional Platform for Magnetic Resonance Imaging and siRNA Delivery. Chem. Eur. J. 2017, 23, 14299–14306. [Google Scholar] [CrossRef]
- Son, Y.J.; Lee, I.C.; Jo, H.H.; Chung, T.J.; Oh, K.S. Setting Behavior and Drug Release from Brushite Bone Cement prepared with Granulated Hydroxyapatite and β-Tricalcium Phosphate. J. Korean Ceram. Soc. 2019, 56, 56–64. [Google Scholar] [CrossRef]
- Dutta, K.; Pramanik, A. Synthesis of a novel cone-shaped CaAl-layered double hydroxide (LDH): Its potential use as a reversible oil sorbent. Chem. Commun. 2013, 49, 6427–6429. [Google Scholar] [CrossRef]
- Darder, M.; López-Blanco, M.; Aranda, P.; Leroux, F.; Ruiz-Hitzky, E. Bio-Nanocomposites Based on Layered Double Hydroxides. Chem. Mater. 2005, 17, 1969–1977. [Google Scholar] [CrossRef]
- Song, X.R.; Wang, X.; Yu, S.X.; Cao, J.; Li, S.H.; Li, J.; Liu, G.; Yang, H.H.; Chen, X. Co₉ Se₈ nanoplates as a new theranostic platform for photoacoustic/magnetic resonance dual-modal-imaging-guided chemo-photothermal combination therapy. Adv. Mater. 2015, 27, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 2014, 26, 3433–3440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, J.; Gu, X.; Gong, H.; Shi, X.; Liu, T.; Wang, C.; Wang, X.; Liu, G.; Xing, H.; et al. PEGylated WS(2) nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv. Mater. 2014, 26, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef]
- Chen, M.; Tang, S.; Guo, Z.; Wang, X.; Mo, S.; Huang, X.; Liu, G.; Zheng, N. Core–Shell Pd@Au Nanoplates as Theranostic Agents for In-Vivo Photoacoustic Imaging, CT Imaging, and Photothermal Therapy. Adv. Mater. 2014, 26, 8210–8216. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, C.X.; Chen, L.G.; Yan, X.P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat. Commun. 2017, 8, 14998. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, L.; Tian, L.; Zhao, S.; Zhang, X.; Li, H. Synergistic photothermal/photodynamic suppression of prostatic carcinoma by targeted biodegradable MnO2 nanosheets. Drug Deliv. 2019, 26, 661–672. [Google Scholar] [CrossRef]
- Choi, G.; Jeon, I.R.; Piao, H.; Choy, J.H. Highly Condensed Boron Cage Cluster Anions in 2D Carrier and Its Enhanced Antitumor Efficiency for Boron Neutron Capture Therapy. Adv. Funct. Mater. 2017, 28, 1704470. [Google Scholar] [CrossRef]
- Wang, D.; Ge, N.; Yang, T.; Peng, F.; Qiao, Y.; Li, Q.; Liu, X. NIR-Triggered Crystal Phase Transformation of NiTi-Layered Double Hydroxides Films for Localized Chemothermal Tumor Therapy. Adv. Sci. 2018, 5, 1700782. [Google Scholar] [CrossRef]
- Mei, X.; Liang, R.; Peng, L.; Hu, T.; Wei, M. Layered double hydroxide bio-composites toward excellent systematic anticancer therapy. J. Mater. Chem. B 2017, 5, 3212–3216. [Google Scholar] [CrossRef]
- Gao, R.; Mei, X.; Yan, D.; Liang, R.; Wei, M. Nano-photosensitizer based on layered double hydroxide and isophthalic acid for singlet oxygenation and photodynamic therapy. Nat. Commun. 2018, 9, 2798. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Z.; Xu, Z.; Chen, X.; Zhu, G. A Cisplatin-Loaded Immunochemotherapeutic Nanohybrid Bearing Immune Checkpoint Inhibitors for Enhanced Cervical Cancer Therapy. Angew. Chem. 2018, 130, 3484–3488. [Google Scholar] [CrossRef] [Green Version]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2017, 28, 1703868. [Google Scholar] [CrossRef]
- Choi, G.; Eom, S.; Vinu, A.; Choy, J.H. 2D Nanostructured Metal Hydroxides with Gene Delivery and Theranostic Functions; A Comprehensive Review. Chem. Rec. 2018, 18, 1–22. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Han, J.S.; An, G.S.; Park, B.G.; Choi, S.C. The Influence of Functionalization of the Fe3O4 Nanoparticle on its Dispersion Property. J. Korean Ceram. Soc. 2018, 55, 80–84. [Google Scholar] [CrossRef]
- Falin, A.; Cai, Q.; Santos, E.J.G.; Scullion, D.; Qian, D.; Zhang, R.; Yang, Z.; Huang, S.; Watanabe, K.; Taniguchi, T.; et al. Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat. Commun. 2017, 8, 15815. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Jia, X.; Cai, S.; Liang, W.; Qin, Y.; Yang, R.; Wang, C. CpG loaded MoS2 nanosheets as multifunctional agents for photothermal enhanced cancer immunotherapy. Nanoscale 2017, 9, 5927–5934. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, Z.; Li, H.; Li, F.; Fan, C.; Zhang, H. Single-Layer MoS2-Based Nanoprobes for Homogeneous Detection of Biomolecules. J. Am. Chem. Soc. 2013, 135, 5998–6001. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z.; et al. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef] [PubMed]










| LDH Host | Contrast Agents | Therapeutic Agents | Molecular Engineering | Applications | Key Feature | References |
|---|---|---|---|---|---|---|
| MgAl | FITC | siRNA | Silane coupling, self-assembly, size control | Fluorescence imaging, gene-therapy | Selective tumor targeting conjugated with FA, siRNA-based gene-therapy in vitro and in vivo | [6] |
| MgAl | FITC | MTX | Intercalation (coprecipitation and ion exchange), size control | Fluorescence imaging, chemo-therapy | Intercellular uptake mechanism: clathrin-mediated endocytosis | [9] |
| MgAl | ICG | Intercalation, covalent coating | NIRF imaging | Organ-specific drug delivery system | [20] | |
| MgAl | FITC | Silane coupling, size control | Fluorescence imaging | Intracellular fate and trafficking mechanism: endolysosomal escape for 100 nm nanoparticles | [21] | |
| MgAl | FITC | DNA, adenosine triphosphate | Intercalation (ion exchange) | Fluorescence imaging, gene-therapy | Gene delivery system with high transfection efficiency | [22] |
| MgAl | Cy5 | DOX | A base-catalyzed coprecipitation | Fluorescence imaging, chemo-therapy | Internalization into cancer cells mechanism: macropinocytosis, clathrin- and lipid raft/caveolae-mediated endocytosis | [23] |
| MgAl | CDs, ICG | ICG | Self-assembly, ultrathin LDHs | Fluorescence imaging, photoacoustic imaging, two-photon imaging, PTT | Multifunctional theranostic nanocarrier system for the cancer treatment | [34] |
| MgAl | FITC | Silane coupling, size control | Fluorescence imaging | Targeted cellular uptake mechanism: particle size dependant clathrin-mediated endocytosis | [35] | |
| MgAl | FITC, ICG | ICG | Intercalation (ion exchange), covalent coating | Fluorescence imaging, PDT | High photo-toxicity of PDT due to the enhanced protection against photo and thermal degradations | [53] |
| MgAl | Cy7, FITC | Self-assembly | Fluorescence imaging, brain targeting | Enhanced brain cell targeting and cellular transportation for efficient brain disease treatment (ligand-modified LDH) | [25] | |
| GdMgAl | Gd3+, Au NPs | DOX | Substitution, self-assembly | MRI, CT, chemo-therapy | Selective cancer targeting in vivo through EPR effect | [10] |
| GdMgAl | Gd3+, ICG | DOX, ICG | Co-intercalation | MRI, fluorescence imaging, chemo-therapy, PTT | Multifunctional theranostic nano-systems for the cancer treatment | [30] |
| GdMgAl | Gd3+, ICG | DOX, ICG | MLDH, a novel bottom-up method | MRI, NIRF imaging, chemo-therapy, PTT, PDT | An ultrahigh drug loading content (LC): 797.36%, an encapsulation efficiency (EE): 99.67% | [57] |
| MnMgAl | Mn2+ | Coprecipitation, isomorphic substitution | MRI | pH-ultrasensitive T1-MRI performance (even with pH 6.5–7.0, i.e., the pH range in a tumor microenvironment) | [26] | |
| MnMgAl | Mn2+, IO NPs | Coprecipitation, isomorphic substitution, self-assembly | MRI (T1/T2) | Enhanced T1/T2 MRI signals both in vitro and in vivo | [12] | |
| MnAl | Mn2+ | siRNA | Coprecipitation, self-assembly | MRI, gene-therapy | An effective anticancer drug/gene delivery system, T1-weighted MRI in brain cancer theranostics | [60] |
| MnFe | Mn2+ | MTX | Coprecipitation, | MRI, chemo-therapy | The first work on MnFe-LDH | [11] |
| ZnAl | Gd-DTPA | Coprecipitation, size control | MRI | Similar T1-weighted MR contrast effect, a suitable particle size for in vivo | [27] | |
| DyZnAl | Dy3+ | Folate, ibuprofen and gallate ions | Coprecipitation, intercalation | MRI, drug delivery system | Theranostic materials with luminescent and magnetic properties | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Park, D.-H. Functional Layered Double Hydroxide Nanohybrids for Biomedical Imaging. Nanomaterials 2019, 9, 1404. https://doi.org/10.3390/nano9101404
Jin W, Park D-H. Functional Layered Double Hydroxide Nanohybrids for Biomedical Imaging. Nanomaterials. 2019; 9(10):1404. https://doi.org/10.3390/nano9101404
Chicago/Turabian StyleJin, Wenji, and Dae-Hwan Park. 2019. "Functional Layered Double Hydroxide Nanohybrids for Biomedical Imaging" Nanomaterials 9, no. 10: 1404. https://doi.org/10.3390/nano9101404
APA StyleJin, W., & Park, D. -H. (2019). Functional Layered Double Hydroxide Nanohybrids for Biomedical Imaging. Nanomaterials, 9(10), 1404. https://doi.org/10.3390/nano9101404