Immobilization of Polyiodide Redox Species in Porous Carbon for Battery-Like Electrodes in Eco-Friendly Hybrid Electrochemical Capacitors
Abstract
:1. Introduction
2. Experimental
2.1. Materials for Electrodes and Electrolytes
2.2. Assembling of Hybrid Cells and Physicochemical Investigation on Electrodes
2.3. Electrochemical Characterization of Hybrid Cells
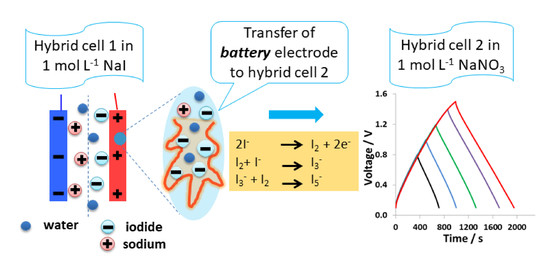
3. Results and Discussion
3.1. Hybrid Cell 1 with ACCpristine(+)/KAC(-) and 1 mol L−1 Sodium Iodide
3.2. Nanostructuration of Battery-Like Electrode Owing to Carbon/Iodide Interface
3.3. Design and Performance of Hybrid Cell 2 with ACCiodide(+)/KAC (-)Electrodes and 1 mol L−1 Sodium Nitrate Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic, Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Miller, J.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar]
- Hamilton, T. Next Stop: Ultracapacitor Buses. MIT Technology Review, 13 April 2013. [Google Scholar]
- Maxwell Ultracapacitor Transportation Solutions. Maxwell Technologies, 6 February 2014.
- Ernst, K. Mazda’s regenerative braking system switches batteries for capacitors. Motor Authority, 2011. [Google Scholar]
- Wang, J.; Yang, Z.; Liu, S.; Zhang, Q.; Han, Y.A. comprehensive overview of hybrid construction machinery. Adv. Mech. Eng. 2016, 8, 1–15. [Google Scholar] [CrossRef]
- Pell, W.; Conway, B. Peculiarities and requirements of asymmetric capacitor devices based on combination of capacitor and battery-type electrode. J. Power Sources 2004, 136, 334–345. [Google Scholar] [CrossRef]
- Bichat, M.; Raymundo- Piñero, E.; Béguin, F. High voltage supercapacitor built with seaweed carbons in neutral aqueous electrolyte. Carbon 2010, 48, 4351–4361. [Google Scholar] [CrossRef]
- Gao, Q.; Demarconnay, L.; Raymundo-Piñero, E.; Béguin, F. Exploring the large voltage range of carbon/carbon supercapacitors in aqueous lithium sulfate electrolyte. Energy & Environ. Sci 2012, 5, 9611–9617. [Google Scholar]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy & Environ. Sci. 2012, 5, 5842–5850. [Google Scholar]
- Chae, J.; Chen, G. 19 V aqueous carbon-carbon supercapacitors with unequal electrode capacitances. Electrochim. Acta 2012, 86, 248–254. [Google Scholar]
- Lota, G.; Frackowiak, E. Striking capacitance of carbon/iodide interface. Electrochem. Commun. 2009, 11, 87–90. [Google Scholar] [CrossRef]
- Abbas, Q.; Babuchowska, P.; Frackowiak, E.; Béguin, F. Sustainable AC/AC hybrid electrochemical capacitors in aqueous electrolyte approaching the performance of organic systems. J. Power Sources 2016, 326, 652–659. [Google Scholar] [CrossRef]
- Roldán, S.; Granda, M.; Menéndez, R.; Santamaría, R.; Blanco, C. Mechanisms of energy storage in carbon-based supercapacitors modified with a quinoid redox-active electrolyte. J. Phys. Chem C 2011, 115, 17606–17611. [Google Scholar]
- Chun, S.-E.; Evanko, B.; Wang, X.; Vonlanthen, D.; Ji, X.; Stucky, G.D.; Boettcher, S.W. Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge. Nat. Commun. 2015, 6, 7818. [Google Scholar] [CrossRef] [PubMed]
- Roldán, S.; Blanco, C.; Granda, M.; Menendez, R.; Santamaría, R. Towards a further generation of high-energy carbon-based capacitors by using redox-active electrolytes. Angew. Chem. Int. Ed. Engl. 2011, 50, 1699–1701. [Google Scholar]
- Chen, L.; Bai, H.; Huanga, Z.; Li, L. Mechanism investigations and suppression of self-discharge in active electrolyte enhanced supercapacitors. Energy Environ. Sci. 2014, 7, 1750–1759. [Google Scholar] [CrossRef]
- Lee, J.; Srimuk, P.; Fleischmann, S.; Ridder, A.; Zeiger, M.; Presser, V. Nanoconfinement of redox reactions enables rapid zinc iodide energy storage with high efficiency. J. Mater. Chem. A 2017, 5, 12520–12527. [Google Scholar] [CrossRef]
- Menzel, J.; Fic, K.; Frackowiak, E. Hybrid aqueous capacitors with improved energy/power performance. Prog. Nat. Sci.: Mater. Int. 2015, 25, 642–649. [Google Scholar] [CrossRef] [Green Version]
- Przygocki, P.; Abbas, Q.; Babuchowska, P.; Béguin, F. Confinement of iodides in carbon porosity to prevent from positive electrode oxidation in high voltage aqueous hybrid electrochemical capacitors. Carbon 2017, 125, 391–400. [Google Scholar] [CrossRef]
- Przygocki, P.; Abbas, Q.; Béguin, F. Capacitance enhancement of hybrid electrochemical capacitor with asymmetric carbon electrodes configuration in neutral aqueous electrolyte. Electrochim. Acta 2018, 269, 640–648. [Google Scholar] [CrossRef]
- Przygocki, P.; Abbas, Q.; Gorska, B.; Béguin, F. High-energy hybrid electrochemical capacitor operating down to −40 °C with aqueous redox electrolyte based on choline salts. J. Power Sources 2019, 427, 283–292. [Google Scholar] [CrossRef]
- Gorska, B.; Frackowiak, E.; Béguin, F. Redox active electrolytes in carbon/carbon electrochemical capacitors. Curr. Opin. Electrochem. 2018, 9, 95–105. [Google Scholar] [CrossRef]
- Lota, G.; Fic, K.; Frackowiak, E. Alkali metal iodide/carbon interface as a source of pseudocapacitance. Electrochem. Commun. 2011, 13, 38–41. [Google Scholar] [CrossRef]
- Frąckowiak, E.; Meller, M.; Menzel, J.; Gastol, D.; Fic, K. Redox-active electrolyte for supercapacitor applications. Faraday Discuss. 2014, 172, 179–198. [Google Scholar]
- Abbas, Q.; Béguin, F. Influence of iodide/iodine redox system on the slef-discharge of AC/AC electrochemical capacitors in salt aqueous electrolyte. Prog. Nat. Sci.: Mater. Int. 2015, 25, 622–630. [Google Scholar] [CrossRef]
- Reber, D.; Kühnel, R.-S.; Battaglia, C. High-voltage aqueous supercapacitors based on NaTFSI. Sustain. Energy Fuels 2017, 1, 2155–2161. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Zhou, X.; Chen, G. unequalisation of electrode capacitances for enhanced energy capacity in asymmetrical supercapacitors. Energy Environ. Sci. 2010, 3, 1499–1502. [Google Scholar]
- Savastano, M.; Bazzicalupi, C.; Garcia, C.; Gellini, C.; Dolores Lopez de la Torre, M.; Mariani, P.; Pichierri, F.; Bianchi, A.; Melguizo, M. Iodide and triiodide anion complexes involving anion-π interactions with a tetrazine-based receptor. Dalton Trans. 2017, 46, 4518–4529. [Google Scholar] [CrossRef]
- Teitelbaum, R.; Ruby, S.; Marks, T. Charge transfer and partial oxidation in the conductive hydrocarbon-iodine complex “2perylene.cntdot.3I2”. J. Am. Chem. Soc. 1979, 101, 7568–7573. [Google Scholar] [CrossRef]
- Danten, Y.; Guillot, B.; Guissani, Y. Investigation of charge transfer complexes by computer simulation. I. iodine in benzene solution. J. Chem. Phys. 1992, 96, 3782–3794. [Google Scholar] [CrossRef]
- Simek, P.; Klimova, K.; Sedmidubsky, D.; Jankovsky, O.; Pumera, M.; Sofer, Z. Towards graphene iodide: Iodination of graphite oxide. Nanoscale 2015, 7, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Kalita, G.; Wakita, K.; Takahashi, M.; Umeno, M. Iodine doping in solid precursor-based CVD growth graphene film. J. Mater. Chem. 2011, 21, 15209–15213. [Google Scholar] [CrossRef]
- Michel, T.; Alvarez, L.; Sauvajol, J.; Almairac, R.; Aznar, R.; Mathon, O.; Bantignies, J.; Flahaut, E. Structural elective charge transfer in iodine-doped carbon nanotubes. J. Phys. Chem. Solids 2006, 67, 1190–1192. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Eklund, P. Phonons in carbon nanotubes. Adv. Phys. 2000, 49, 705–814. [Google Scholar] [CrossRef]
- Barpanda, P.; Fanchini, G.; Amatucci, G. Physical and electrochemical properties of iodine-modified activated carbons. J. Electrochem. Soc. 2007, 154, A467–A476. [Google Scholar] [CrossRef]
- He, Y.; Zhuang, X.; Lei, C.; Lei, L.; Hou, Y.; Mai, Y.; Feng, X. Porous carbon nanosheets: Synthesis strategies and electrochemical energy related applications. Nano Today 2019, 24, 103–119. [Google Scholar] [CrossRef]
- Sengottaiyan, C.; Jayavel, R.; Bairi, P.; Shresta, G.; Ariga, K.; Shrestha, L. Cobalt oxide/reduced graphene oxide composite enhanced electrochemical supercapacitance performance. Bull. Chem. Soc. Jpn. 2017, 90, 955–962. [Google Scholar] [CrossRef]
- Feng, D.; Lei, T.; Lukatskaya, M.; Park, K.; Huang, Z.; Lee, M.; Shaw, L.; Chen, S.; Yakovenko, A.; Kulkarni, A.; et al. Robust and conductive two-dimensional metal-organic frameworks with exceptionally high volumetric and area capacitance. Nat. Energy 2018, 3, 30–36. [Google Scholar] [CrossRef]
- Huang, J.; Wei, Z.; Liano, J.; Ni, W.; Wang, C.; Ma, J. Molybdenum and tungsten chalcogenides for lithium/sodium-ion batteries: Beyond MoS2. J. Energy Chem. 2019, 33, 100–124. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Stauss, S.; Honma, I. Biocompatible Batteries–Materials and Chemistry, fabrication, applications, and future prospects. Bull. Chem. Soc. Jpn. 2018, 91, 492–505. [Google Scholar] [CrossRef]
- Lee, J.; Srimuk, P.; Carpier, S.; Choi, J.; Zornitta, R.; Aslan, M.; Presser, V. Confined redox reactions of iodide in carbon nanopores for fast and energy-efficient desalination of brackish water and seawater. ChemSusChem 2018, 11, 3460–3472. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ishii, Y.; Kawasaki, S. Safe, economical and fast-charging secondary batteries using single-walled carbon nanotubes. Jpn. J. Appl. Phys. 2019, 58, SAAE02. [Google Scholar] [CrossRef]
- Kisu, K.; Iwama, E.; Naoi, W.; Simon, P.; Naoi, K. Electrochemical kinetics of nanostructure LiFePO4/graphitic carbon electrodes. Electrochem. Commun. 2016, 72, 10–14. [Google Scholar] [CrossRef]
- Laehäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate methods for evaluating the efficiency and capacitance behavior of different types of supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar]
- Balducci, A.; Bélanger, D.; Brousse, T.; Long, J.-W.; Sugimoto, W. Perspective–A guideline for reporting performance metrics with electrochemical capacitors: From electrode materials to full devices. J. Electrochem. Soc. 2017, 164, A1487–A1488. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas D’Equilibres Electrochimiques; Gauthier-Villars: Paris, France, 1963; p. 614. [Google Scholar]
- Gao, Q. Optimizing carbon/carbon supercapacitors in aqueous alkali sulfates electrolytes. J. Energy Chem. 2019, 38, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Abbas, Q.; Gollas, B.; Presser, V. Reduced Faradaic contributions and fast charging of nanoporous carbon electrodes in a concentrated sodium nitrate aqueous electrolyte for supercapacitors. Energy Technol. 2019, 7, 1900430. [Google Scholar] [CrossRef]
- Lee, J.; Srimuk, P.; Fleischmann, S.; Su, X.; Alan Hatton, T.; Presser, V. Redox-electrolytes for non-flow electrochemical energy storage: A critical review and best practice. Prog. Mater. Sci. 2019, 101, 46–89. [Google Scholar] [CrossRef]
- Niu, J.; Conway, B.E.; Pell, W.G. Comparative studies of self-discharge by potential decay and float-current measurements at C double-layer capacitor and battery electrodes. J. Power Sources 2004, 134, 332–343. [Google Scholar] [CrossRef]
- Niu, J.; Pell, W.G.; Conway, B.E. Requirements for performance characterization of C double-layer supercapacitors: Applications to a high surface-area C-cloth material. J. Power Sources 2006, 156, 725–740. [Google Scholar] [CrossRef]








| 2 mV s−1 | 5 mV s−1 | 10 mV s−1 | 50 mV s−1 | |
|---|---|---|---|---|
| Capacitance (F g−1) | 72 | 65 | 59 | 35 |
| Energy Efficiency (%) | 92 | 97 | 97 | 98 |
| 0.1 A g−1 | 0.25 A g−1 | 0.5 A g−1 | 1.0 A g−1 | |
|---|---|---|---|---|
| Capacitance (F g−1) | 74 | 71 | 63 | 54 |
| Energy Efficiency (%) | 68 | 79 | 82 | 82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Q.; Fitzek, H.; Schröttner, H.; Dsoke, S.; Gollas, B. Immobilization of Polyiodide Redox Species in Porous Carbon for Battery-Like Electrodes in Eco-Friendly Hybrid Electrochemical Capacitors. Nanomaterials 2019, 9, 1413. https://doi.org/10.3390/nano9101413
Abbas Q, Fitzek H, Schröttner H, Dsoke S, Gollas B. Immobilization of Polyiodide Redox Species in Porous Carbon for Battery-Like Electrodes in Eco-Friendly Hybrid Electrochemical Capacitors. Nanomaterials. 2019; 9(10):1413. https://doi.org/10.3390/nano9101413
Chicago/Turabian StyleAbbas, Qamar, Harald Fitzek, Hartmuth Schröttner, Sonia Dsoke, and Bernhard Gollas. 2019. "Immobilization of Polyiodide Redox Species in Porous Carbon for Battery-Like Electrodes in Eco-Friendly Hybrid Electrochemical Capacitors" Nanomaterials 9, no. 10: 1413. https://doi.org/10.3390/nano9101413
APA StyleAbbas, Q., Fitzek, H., Schröttner, H., Dsoke, S., & Gollas, B. (2019). Immobilization of Polyiodide Redox Species in Porous Carbon for Battery-Like Electrodes in Eco-Friendly Hybrid Electrochemical Capacitors. Nanomaterials, 9(10), 1413. https://doi.org/10.3390/nano9101413