A Novel Flower-Like Ag/AgCl/BiOCOOH Ternary Heterojunction Photocatalyst: Facile Construction and Its Superior Photocatalytic Performance for the Removal of Toxic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Photocatalysts Preparation
2.3. Characterization
2.4. Photocatalytic Tests
3. Results and Discussion
3.1. Structure and Morphology
3.2. Optical Properties
3.3. Photocatalytic Activity
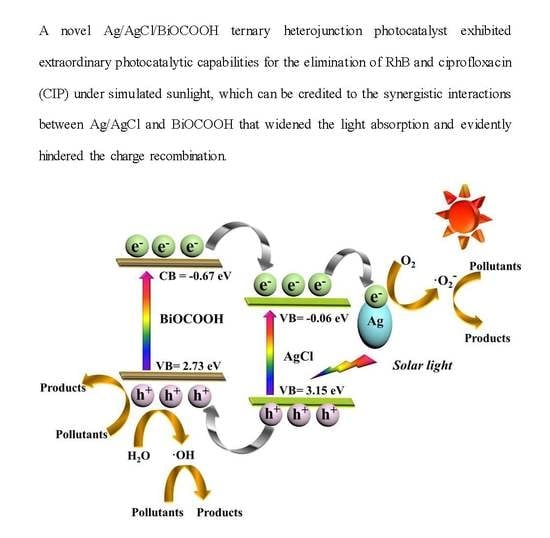
3.4. Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Jassby, D.; Cath, T.Y.; Buisson, H. The role of nanotechnology in industrial water treatment. Nat. Nanotechnol. 2018, 13, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, X.; Liu, J.; Zhang, L. Synthesis of Ta3N5/Bi2MoO6 core-shell fiber-shaped heterojunctions as efficient and easily recyclable photocatalysts. Environ. Sci. Nano 2017, 4, 1155–1167. [Google Scholar] [CrossRef]
- Liu, Z.; Leow, W.R.; Chen, X. Bio-inspired plasmonic photocatalysts. Small Methods 2019, 3, 1800295. [Google Scholar] [CrossRef]
- Cates, E.L. Photocatalytic water treatment: So where are we going with this? Environ. Sci. Technol. 2017, 51, 757–758. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Huang, J.; Feng, Z.; Chen, Z.; Zhu, Y. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J. Alloys Compd. 2019, 802, 196–209. [Google Scholar] [CrossRef]
- Helal, A.; Harraza, F.A.; Ismail, A.A.; Sami, T.M.; Ibrahim, I.A. Hydrothermal synthesis of novel heterostructured Fe2O3/Bi2S3 nanorods with enhanced photocatalytic activity under visible light. Appl. Catal. B Environ. 2017, 213, 18–27. [Google Scholar] [CrossRef]
- Kong, L.; Ambrosi, A.; Zafir, M.; Guan, J.; Pumera, M. Smart robots: Self-propelled 3D-printed Aircraft Carrier of light-powered smart micromachines for large-volume nitroaromatic explosives removal. Adv. Funct. Mater. 2019, 29, 190387. [Google Scholar]
- Yang, L.L.; Han, Q.F.; Wang, X.; Zhu, J.W. Highly efficient removal of aqueous chromate and organic dyes by ultralong HCOOBiO nanowires. Chem. Eng. J. 2015, 262, 169–178. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Cheng, G.; Lu, Z.; Tang, J.L.; Yu, X.L.; Chen, R. BiOCOOH hierarchical nanostructures: Shape-controlled solvothermal synthesis and photocatalytic degradation performances. Cryst. Eng. Comm. 2011, 13, 2381–2390. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Zhang, H.; Cheng, Q.; Cheng, X. Construction of BiOCOOH/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic degradation of amido black 10B. Sep. Purif. Technol. 2019, 210, 125–134. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Su, Y.; Shen, L.; Wang, F.; Liu, H.; Liu, Y.; Cai, Z.; Lv, W.; Liu, G. Accelerated photocatalytic degradation of diclofenac by a novel CQDs/BiOCOOH hybrid material under visible-light irradiation: Dechloridation, detoxicity, and a new superoxide radical model study. Chem. Eng. J. 2018, 332, 737–748. [Google Scholar] [CrossRef]
- Li, S.; Mo, L.; Liu, Y.; Zhang, H.; Ge, Y.; Zhou, Y. Ag2CO3 decorating BiOCOOH microspheres with enhanced full-spectrum photocatalytic activity for the degradation of toxic pollutants. Nanomaterials 2018, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Liu, Y.; Xu, K.; Liu, J. In situ anion exchange strategy to construct flower-like BiOCl/BiOCOOH p-n heterojunctions for efficiently photocatalytic removal of aqueous toxic pollutants under solar irradiation. J. Alloys Compd. 2019, 781, 582–588. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Jiang, W.; Liu, Y.; Ge, Y.; Liu, J. Facile construction of flower-like bismuth oxybromide/bismuth oxide formate p-n heterojunctions with significantly enhanced photocatalytic performance under visible light. J. Colloid Interface Sci. 2019, 548, 12–19. [Google Scholar] [CrossRef]
- Xu, B.Y.; An, Y.; Liu, Y.Y.; Huang, B.B.; Qin, X.Y.; Zhang, X.Y.; Dai, Y.; Whangbo, M.-H. An efficient visible-light photocatalyst made from a nonpolar layered semiconductor by grafting electron-withdrawing organic molecules to its surface. Chem. Commun. 2016, 52, 13507–13510. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Niu, J.; Chen, M.; Teng, F. Preparation of Bi2MoO6–BiOCOOH plate-on-plate heterojunction photocatalysts with significantly improved photocatalytic performance under visible light irradiation. J. Taiwan Inst. Chem. Eng. 2019, 97, 326–335. [Google Scholar] [CrossRef]
- Ye, R.; Zhao, J.; Wickemeyer, B.B.; Toste, F.D.; Somorjai, G.A. Foundations and strategies of the construction of hybrid catalysts for optimized performances. Nature Catal. 2018, 1, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Liu, X. One-pot synthesis of ternary Ag2CO3/Ag/AgCl photocatalyst in natural geothermal water with enhanced photocatalytic activity under visible light irradiation. J. Hazard. Mater. 2014, 280, 260–268. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Z.; Yang, C.; Zhou, W.; Jiao, S.; Zhu, H. Hierarchically plasmonic Z-scheme photocatalyst of Ag/AgCl nanocrystals decorated mesoporous single-crystalline metastable Bi20TiO32 nanosheets. J. Phys. Chem. C 2013, 117, 5132–5141. [Google Scholar] [CrossRef]
- Yang, S.-F.; Niu, C.-G.; Huang, D.-W.; Zhang, H.; Liang, C.; Zeng, G.-M. SrTiO3 nanocubes decorated with Ag/AgCl nanoparticles as photocatalysts with enhanced visible-light photocatalytic activity towards the degradation of dyes, phenol and bisphenol A. Environ. Sci. Nano 2017, 4, 585. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Li, M.; Zhang, Q.; Wang, Z.; Dai, Y.; Zhang, X.; Liu, Y.; Whangbo, M.-H.; Huang, B. Adsorption of gaseous ethylene via induced polarization on plasmonic photocatalyst Ag/AgCl/TiO2 and subsequent photodegradation. Appl. Catal. B 2018, 220, 356–361. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Gao, C.; Dong, F.; Chen, X. Ternary Ag/AgCl-(BiO)2CO3 composites as high-performance visible-light plasmonic photocatalysts. Catal. Today 2017, 284, 67–76. [Google Scholar] [CrossRef]
- Jiang, Z.; Pan, J.; Wang, B.; Li, C. Two dimensional Z-scheme AgCl/Ag/CaTiO3 nano-heterojunctions for photocatalytic hydrogen production enhancement. Appl. Surf. Sci. 2018, 436, 519–526. [Google Scholar] [CrossRef]
- Ao, Y.; Bao, J.; Wang, P.; Wang, C. A novel heterostructured plasmonic photocatalyst with high photocatalytic activity: Ag@AgCl nanoparticles modified titanium phosphate nanoplates. J. Alloys Compd. 2017, 698, 410–419. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Zhou, Y.; Liu, Y.; Mo, L. Hierarchical architectures of bismuth molybdate nanosheets onto nickel titanate nanofibers: Facile synthesis and efficient photocatalytic removal of tetracycline hydrochloride. J. Colloid Interface Sci. 2018, 521, 42–49. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Liu, J.; Wang, Z. Facile synthesis of flower-like Ag3VO4/Bi2WO6 heterojunction with enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2017, 501, 156–163. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Fung, C.S.L.; Rather, R.A.; Lo, I.M.C. One-pot hydrothermal synthesis of g-C3N4/Ag/AgCl/BiVO4 micro-flower composite for the visible light degradation of ibuprofen. Chem. Eng. J. 2018, 341, 248–261. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Zhou, Y.; Liu, J.; Wang, Z. Facile synthesis of cerium oxide nanoparticles decorated flower-like bismuth molybdate for enhanced photocatalytic activity toward organic pollutant degradation. J. Colloid Interface Sci. 2018, 530, 171–178. [Google Scholar] [CrossRef]
- Zheng, J.; Chang, F.; Jiao, M.; Xu, Q.; Deng, B.; Hu, X. A visible-light-driven heterojuncted composite WO3/Bi12O17Cl2: Synthesis, characterization, and improved photocatalytic performance. J. Colloid Interface Sci. 2018, 510, 20–31. [Google Scholar] [CrossRef]
- Regulska, E.; Breczko, J.; Basa, A. Pristine and graphene-quantum-dots-decorated spinel nickel aluminate for water remediation from dyes and toxic pollutants. Water 2019, 11, 953. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Xu, Y.; Feng, Z.; Huang, J. Defect-assisted surface modification enhances the visible light photocatalytic performance of g-C3N4@C-TiO2 direct Z-scheme heterojunction. Chin. J. Catal. 2019, 40, 424–443. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Huang, D.-W.; Zhang, L.; Liang, C.; Zeng, G.-M. Study of the photocatalytic degradation pathway of norfloxacin and mineralization activity using a novel ternary Ag/AgCl-CeO2 photocatalyst. J. Catal. 2017, 355, 73–86. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Zhang, J.; Xu, K.; Wang, Z. In situ construction of WO3 nanoparticles decorated Bi2MoO6 microspheres for boosting photocatalytic degradation of refractory pollutants. J. Colloid Interface Sci. 2019, 556, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Wu, F.; Zheng, J.; Cheng, W.; Yan, W.; Deng, B.; Hu, X. In-situ establishment of binary composites a-Fe2O3/Bi12O17Cl2 with both photocatalytic and photo-Fenton features. Chemosphere 2018, 210, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Wang, X. Enhanced visible light photocatalytic activity of BiOI/BiOCOOH composites synthesized via ion exchange strategy. RSC Adv. 2015, 5, 7589–7596. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, C.; Ke, J.; Zhou, L.; Zeng, G. Ag/AgCl/Bi2MoO6 composite nanosheets: A plasmonic Z-scheme visible light photocatalyst. Catal. Commun. 2015, 59, 30–34. [Google Scholar] [CrossRef]
- Li, X.; Fang, S.; Ge, L.; Han, C.; Qiu, P.; Liu, W. Synthesis of flower-like Ag/AgCl-Bi2MoO6 plasmonic photocatalysts with enhanced visible-light photocatalytic performance. Appl. Catal. B 2015, 176–177, 62–69. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A.; Abedi, M. Decoration of carbon dots and AgCl over g-C3N4 nanosheets: Novel photocatalysts with substantially improved activity under visible light. Sep. Purif. Technol. 2018, 199, 64–77. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xue, B.; Wu, G.; Liu, Y.; Zhang, H.; Ma, D.; Zuo, J. A Novel Flower-Like Ag/AgCl/BiOCOOH Ternary Heterojunction Photocatalyst: Facile Construction and Its Superior Photocatalytic Performance for the Removal of Toxic Pollutants. Nanomaterials 2019, 9, 1562. https://doi.org/10.3390/nano9111562
Li S, Xue B, Wu G, Liu Y, Zhang H, Ma D, Zuo J. A Novel Flower-Like Ag/AgCl/BiOCOOH Ternary Heterojunction Photocatalyst: Facile Construction and Its Superior Photocatalytic Performance for the Removal of Toxic Pollutants. Nanomaterials. 2019; 9(11):1562. https://doi.org/10.3390/nano9111562
Chicago/Turabian StyleLi, Shijie, Bing Xue, Genying Wu, Yanping Liu, Huiqiu Zhang, Deyun Ma, and Juncheng Zuo. 2019. "A Novel Flower-Like Ag/AgCl/BiOCOOH Ternary Heterojunction Photocatalyst: Facile Construction and Its Superior Photocatalytic Performance for the Removal of Toxic Pollutants" Nanomaterials 9, no. 11: 1562. https://doi.org/10.3390/nano9111562
APA StyleLi, S., Xue, B., Wu, G., Liu, Y., Zhang, H., Ma, D., & Zuo, J. (2019). A Novel Flower-Like Ag/AgCl/BiOCOOH Ternary Heterojunction Photocatalyst: Facile Construction and Its Superior Photocatalytic Performance for the Removal of Toxic Pollutants. Nanomaterials, 9(11), 1562. https://doi.org/10.3390/nano9111562