Enhanced Antifungal Activities of Eugenol-Entrapped Casein Nanoparticles against Anthracnose in Postharvest Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
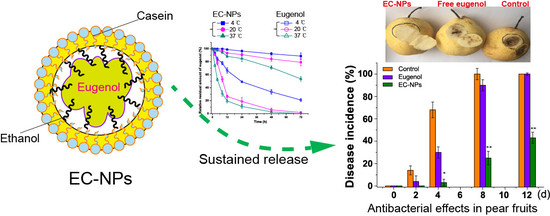
2.2. Preparation of Nanoparticles
2.3. Characterization of EC-NPs
2.4. Stability Assessments of EC-NPs
2.5. Cell Culture
2.6. Determination of Antifungal Activity
2.7. Effects of EC-NPs on Anthracnose Disease on Pear Fruit
3. Results and Discussion
3.1. Characterization of Eugenol-Entrapped Casein Nanoparticles
3.2. Release Assessment of Eugenol Nanoparticles
3.3. Antifungal Assessment of Eugenol Nanoparticles
3.4. EC-NPs as Preservative against Fruit Corruption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2019, 29, 1059–1075. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.E.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesalski, H.-K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Safety and efficacy. Nutrition 2009, 25, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Li, D.Y.; Zhang, H.F.; Xue, H.Z.; Pan, C.E.; Zhao, S.H.; Wang, L. Resveratrol inhibits invasion and metastasis of hepatocellular carcinoma cells. J. Anim. Vet. Adv. 2010, 9, 3117–3124. [Google Scholar] [CrossRef] [Green Version]
- Chai, L.Q.; Meng, J.H.; Gao, J.; Xu, Y.; Wang, X.W. Identification of a crustacean β-1, 3-glucanase related protein as a pattern recognition protein in antibacterial response. Fish Shellfish Immunol. 2018, 80, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Dianella, S. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar]
- Zhang, H.; Li, M. Transcriptional profiling of ESTs from the biocontrol fungus Chaetomium cupreum. Sci. World J. 2012, 2012, 1–7. [Google Scholar]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanodispersed eugenol has improved antimicrobial activity against Escherichia coli O157: H7 and Listeria monocytogenes in bovine milk. Int. J. Food Microbiol. 2013, 161, 53–59. [Google Scholar] [CrossRef]
- Chen, F.; Shi, Z.; Neoh, K.; Kang, E. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef]
- Garg, A.; Singh, S. Enhancement in antifungal activity of eugenol in immunosuppressed rats through lipid nanocarriers. Colloids Surf. B Biointerfaces 2011, 87, 280–288. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, C.; Xie, Z.; Liu, L.; Peng, C.; Xue, F. Botryoid-shaped nanoparticles-enhanced ELISA for ochratoxin A. Food Agric. Immunol. 2017, 28, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y. Eugenol nanoemulsion stabilized with zein and sodium caseinate by self-assembly. J. Agric. Food Chem. 2017, 65, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Moreira, R.G.; Elena, C.P. Poly (DL-lactide-co-glycolide)(PLGA) nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J. Food Sci. 2011, 76, N16–N24. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B Biointerfaces 2014, 114, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, M.; Li, W.; Zhao, L.; Wang, Z.; Yan, X.; Norde, W.; Li, Y. Pickering emulsions stabilized by whey protein nanoparticles prepared by thermal cross-linking. Colloids Surf. B: Biointerfaces 2015, 127, 96–104. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Sokolik, C.G.; Rina, B.S.B.; Gedanken, A.; Lellouche, J.P. Proteinaceous microspheres as a delivery system for carvacrol and thymol in antibacterial applications. Ultrason. Sonochem. 2018, 41, 288–296. [Google Scholar] [CrossRef]
- Dalgleish, D.G. On the structural models of bovine casein micelles—Review and possible improvements. Soft Matter 2011, 7, 2265–2272. [Google Scholar] [CrossRef]
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y.D. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocolloid. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Horne, D.S. Casein micelle structure: Models and muddles. Curr. Opin. Colloid Interface Sci. 2006, 11, 148–153. [Google Scholar] [CrossRef]
- Bachar, M.; Mandelbaum, A.; Portnaya, I.; Perlstein, H.; Chen, S.E.; Barenholz, Y.; Danino, D. Development and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled β-casein micelles. J. Control. Release 2012, 160, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Yao, R.; Qin, D.; Chen, Q.; Du, Q. Enhancement in antibacterial activities of eugenol-entrapped ethosome nanoparticles via strengthening its permeability and sustained release. J. Agric. Food Chem. 2019, 67, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, Z.; Wang, H.; Chen, S. Quantification of eugenol and bancroftione in Caryophylli Fructus using high-performance liquid chromatography. J. Chinses Pharm. Sci. 2010, 19, 459–463. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, L.; Yu, C.; Jin, P.; Qin, D.; Xu, Y.; Yin, J.; Liu, Z.; Du, Q. Enhanced anti-arthritic efficacy by nanoparticles of (-)-Epigallocatechin gallate-glucosamine-casein. J. Agric. Food Chem. 2019, 67, 6476–6486. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Long, L.; Xu, L.; Lindsey, K.; Zhu, L. Suppression of the homeobox gene HDTF1 enhances resistance to Verticillium dahliae and Botrytis cinerea in cotton. J. Integr. Plant Biol. 2016, 58, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Dhara, L.; Tripathi, A. Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing enterobacteriaceae by in vitro and molecular docking analysis. Eur. J. Integr. Med. 2013, 5, 527–536. [Google Scholar] [CrossRef]
- Shapira, A.; Markman, G.; Assaraf, Y.G.; Livney, Y.D. β-casein–based nanovehicles for oral delivery of chemotherapeutic drugs: Drug-protein interactions and mitoxantrone loading capacity. Nanomedicine 2010, 6, 547–555. [Google Scholar] [CrossRef]
- Huo, Q. Protein complexes/aggregates as potential cancer biomarkers revealed by a nanoparticle aggregation immunoassay. Colloids Surf. B Biointerfaces 2010, 78, 259–265. [Google Scholar] [CrossRef]
- Silva, L.M.; Hill, L.E.; Figueiredo, E.; Gomes, C.L. Delivery of phytochemicals of tropical fruit by-products using poly (DL-lactide-co-glycolide)(PLGA) nanoparticles: Synthesis, characterization, and antimicrobial activity. Food Chem. 2014, 165, 362–370. [Google Scholar] [CrossRef]
- Müller, R.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Del. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Wattanasatcha, A.; Rengpipat, S.; Supason, W. Thymol nanospheres as an effective anti-bacterial agent. Int. J. Pharm. 2012, 434, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Bédard, M.F.; Braun, D.; Sukhorukov, G.B.; Skirtach, A.G. Toward self-assembly of nanoparticles on polymeric microshells: Near-ir release and permeability. ACS Nano. 2008, 2, 1807–1816. [Google Scholar] [CrossRef]




| Eugenol (mg/mL) | Size (nm) | PDI | Zeta (mV) | EE (%) |
|---|---|---|---|---|
| 1 | 249.3 ± 3.3 | 0.261 ± 0.003 | −14.47 ± 0.31 | 90.4 ± 0.8 |
| 2 | 278.3 ± 5.1 | 0.270 ± 0.012 | −16.61 ± 0.11 | 91.1 ± 0.3 |
| 3 | 289.6 ± 1.8 | 0.283 ± 0.024 | −20.64 ± 0.23 | 87.1 ± 0.6 |
| 4 | 307.4 ± 2.5 | 0.284 ± 0.033 | −21.18 ± 0.67 | 86.3 ± 0.2 |
| 5 | 333.8 ± 4.8 | 0.311 ± 0.009 | −21.91 ± 0.37 | 70.1 ± 1.2 |
| 6 | 326.9 ± 6.3 | 0.333 ± 0.007 | −20.20 ± 0.26 | 67.1 ± 0.9 |
| Temperature | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Control | 307.4 ± 2.5 | 0.284 ± 0.033 | −21.18 ± 0.67 |
| 4 °C | 305.4 ± 1.9 | 0.289 ± 0.009 | −21.01 ± 0.13 |
| 20 °C | 310.3 ± 6.2 | 0.290 ± 0.008 | −17.21 ± 0.32 |
| 37 °C | 312.6 ± 4.2 | 0.303 ± 0.014 | −17.32 ± 0.24 |
| CEugenol a (ug/mL) | Mycelial Growth Inhibition (%) b | ||
|---|---|---|---|
| Native Eugenol | Casein-Eugenol Mixture | EC-NPs | |
| 1.00 | 0.00 | 0.00 | 0.00 |
| 4.03 | 1.88 ± 0.67 | 2.38 ± 1.02 | 1.47 ± 0.76 |
| 8.04 | 8.41 ± 1.35 | 7.32 ± 1.03 | 7.57 ± 0.87 |
| 10.06 | 9.41 ± 0.87 | 10.91 ± 2.17 | 9.24 ± 0.39 |
| 15.08 | 17.62 ± 2.23 | 15.62 ± 1.98 | 14.67 ± 2.21 |
| 20.11 | 24.97 ± 2.62 | 27.27 ± 2.01 | 31.48 ± 1.62 |
| 25.13 | 28.34 ± 3.12 | 30.34 ± 1.36 | 36.98 ± 0.28 |
| 30.16 | 38.70 ± 1.26 | 37.40 ± 3.72 | 44.40 ± 1.31 |
| 35.19 | 68.21 ± 1.67 | 69.93 ± 0.89 | 87.9 ± 2.34 |
| 40.21 | 87.43 ± 3.17 | 89.21 ± 2.34 | 100 ± 0.00 |
| 50.24 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhou, S.; Fan, C.; Du, Q.; Jin, P. Enhanced Antifungal Activities of Eugenol-Entrapped Casein Nanoparticles against Anthracnose in Postharvest Fruits. Nanomaterials 2019, 9, 1777. https://doi.org/10.3390/nano9121777
Xue Y, Zhou S, Fan C, Du Q, Jin P. Enhanced Antifungal Activities of Eugenol-Entrapped Casein Nanoparticles against Anthracnose in Postharvest Fruits. Nanomaterials. 2019; 9(12):1777. https://doi.org/10.3390/nano9121777
Chicago/Turabian StyleXue, Yang, Shitong Zhou, Chenyue Fan, Qizhen Du, and Peng Jin. 2019. "Enhanced Antifungal Activities of Eugenol-Entrapped Casein Nanoparticles against Anthracnose in Postharvest Fruits" Nanomaterials 9, no. 12: 1777. https://doi.org/10.3390/nano9121777
APA StyleXue, Y., Zhou, S., Fan, C., Du, Q., & Jin, P. (2019). Enhanced Antifungal Activities of Eugenol-Entrapped Casein Nanoparticles against Anthracnose in Postharvest Fruits. Nanomaterials, 9(12), 1777. https://doi.org/10.3390/nano9121777