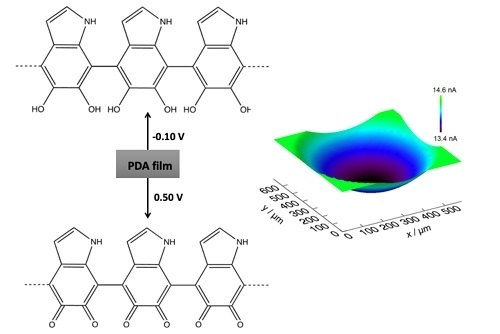
Micro-Structured Polydopamine Films via Pulsed Electrochemical Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electrochemical Measurements
2.3. AFM Measurements
2.4. SEM Imaging
2.5. IR Microspectroscopy
3. Results and Discussions
3.1. Optimization of the Deposition Process
3.2. Film Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ball, V. Physicochemical perspective on “polydopamine” and “poly(catecholamine)” films for their applications in biomaterial coatings (Review). Biointerphases 2014, 9, 030801. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.K.; Park, S.H.; Choi, J.W. Mussel-Inspired Coating and Adhesion for Rechargeable Batteries: A Review. ACS Appl. Mater. Interfaces 2018, 10, 7562–7573. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wei, W.Z.; Zeng, J.X.; Liu, X.Y.; Gao, Y.P. Application of a novel electrosynthesized polydopamine-imprinted film to the capacitive sensing of nicotine. Anal. Bioanal. Chem. 2006, 385, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Che, D.; Cheng, J.; Ji, Z.; Zhang, S.; Li, G.; Sun, Z.; You, J. Recent advances and applications of polydopamine-derived adsorbents for sample pretreatment. TrAC Trends Anal. Chem. 2017, 97, 1–14. [Google Scholar] [CrossRef]
- Singer, F.; Schlesak, M.; Mebert, C.; Höhn, S.; Virtanen, S. Corrosion Properties of Polydopamine Coatings Formed in One-Step Immersion Process on Magnesium. ACS Appl. Mater. Interfaces 2015, 7, 26758–26766. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Isoshima, T.; Nair, B.; Ito, Y. Template-Assisted Formation of Nanostructured Dopamine-Modified Polymers. Nanomaterials 2017, 7, 364. [Google Scholar] [CrossRef] [PubMed]
- Satyaprasad, A.; Jain, V.; Nema, S.K. Deposition of superhydrophobic nanostructured Teflon-like coating using expanding plasma arc. Appl. Surf. Sci. 2007, 253, 5462–5466. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Qin, R.; Chen, D. Surface-Engineered Polydopamine Particles as an Efficient Support for Catalytic Applications. Langmuir 2016, 32, 13675–13686. [Google Scholar] [CrossRef]
- Łuczak, T. Preparation and characterization of the dopamine film electrochemically deposited on a gold template and its applications for dopamine sensing in aqueous solution. Electrochim. Acta 2008, 53, 5725–5731. [Google Scholar] [CrossRef]
- Loget, G.; Wood, J.B.; Cho, K.; Halpern, A.R.; Corn, R.M. Electrodeposition of polydopamine thin films for DNA patterning and microarrays. Anal. Chem. 2013, 85, 9991–9995. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Stewart, M.H.; Trammell, S.A.; Susumu, K.; Delehanty, J.B.; Mei, B.C.; Melinger, J.S.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Quantum-dot/dopamine bioconjugates function as redox coupled assemblies for in vitro and intracellular pH sensing. Nat. Mater. 2010, 9, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cortez-Jugo, C.; Choi, G.H.; Björnmalm, M.; Dai, Y.; Yoo, P.J.; Caruso, F. Patterned Poly(dopamine) Films for Enhanced Cell Adhesion. Bioconjug. Chem. 2017, 28, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, L.; Li, J.; Yang, C.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. Uv-triggered dopamine polymerization: Control of polymerization, surface coating, and photopatterning. Adv. Mater. 2014, 26, 8029–8033. [Google Scholar] [CrossRef] [PubMed]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; Gracio, J.J.D.A.; Toniazzo, V.; Ruch, D. Dopamine-melanin film deposition depends on the used oxidant and buffer solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Stöckle, B.; Ng, D.Y.W.; Meier, C.; Paust, T.; Bischoff, F.; Diemant, T.; Behm, R.J.; Gottschalk, K.E.; Ziener, U.; Weil, T. Precise control of polydopamine film formation by electropolymerization. Macromol. Symp. 2014, 346, 73–81. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, B.C.; Li, Z.J.; Ren, K.F.; Jin, L.J.; Zhang, S.M.; Chang, H.; Sun, Y.X.; Ji, J. Electropolymerization of dopamine for surface modification of complex-shaped cardiovascular stents. Biomaterials 2014, 35, 7679–7689. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Deng, C.; Xie, Q.; Yang, Y.; Yao, S. Electrochemical quartz crystal impedance study on immobilization of glucose oxidase in a polymer grown from dopamine oxidation at an Au electrode for glucose sensing. Electrochim. Acta 2006, 51, 5478–5486. [Google Scholar] [CrossRef]
- Kang, X.; Cai, W.; Zhang, S.; Cui, S. Revealing the formation mechanism of insoluble polydopamine by using a simplified model system. Polym. Chem. 2017, 8, 860–864. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Ding, Y.; Weng, L.-T.; Yang, M.; Yang, Z.; Lu, X.; Huang, N.; Leng, Y. Insights into the Aggregation/Deposition and Structure of a Polydopamine Film. Langmuir 2014, 30, 12258–12269. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Herlinger, E.; Jameson, R.F.; Linert, W. Spontaneous autoxidation of dopamine. J. Chem. Soc. Perkin Trans. 2 1995, 2, 259. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.; Panzella, L.; Oscurato, S.; Salvatore, M.; Avolio, R.; Errico, M.; Maddalena, P.; Napolitano, A.; d’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Young, M.; Xu, F.; Cheng, G.; Cong, H. Properties of Electropolymerized Dopamine and Its Analogues. Langmuir 2019, 35, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Fan, F.R.F.; Kwak, J.; Lev, O. Scanning Electrochemical Microscopy. Introduction and Principles. Anal. Chem. 1989, 61, 132–138. [Google Scholar] [CrossRef]
- Polcari, D.; Dauphin-Ducharme, P.; Mauzeroll, J. Scanning Electrochemical Microscopy: A Comprehensive Review of Experimental Parameters from 1989 to 2015. Chem. Rev. 2016, 116, 13234–13278. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.; Knittel, P.; Kranz, C. Scanning electrochemical microscopy: An analytical perspective. Anal. Bioanal. Chem. 2018, 410, 307–324. [Google Scholar] [CrossRef]
- Wittstock, G.; Burchardt, M.; Pust, S.E.; Shen, Y.; Zhao, C. Scanning electrochemical microscopy for direct imaging of reaction rates. Angew. Chem. Int. Ed. 2007, 46, 1584–1617. [Google Scholar] [CrossRef]
- Bard, A.J.; Mirkin, M.V.; Unwin, P.R.; Wipf, D. SECM: 12. Theory and Experiment of the Feedback Mode with Finite Heterogeneous Electron-Transfer Kinetics and Arbitrary Substrate Size. J. Phys. Chem. 1992, 96, 1861–1868. [Google Scholar] [CrossRef]
- Cornut, R.; Lefrou, C. New analytical approximation of feedback approach curves with a microdisk SECM tip and irreversible kinetic reaction at the substrate. J. Electroanal. Chem. 2008, 621, 178–184. [Google Scholar] [CrossRef]
- Peltola, E.; Sainio, S.; Holt, K.B.; Palomäki, T.; Koskinen, J.; Laurila, T. Electrochemical Fouling of Dopamine and Recovery of Carbon Electrodes. Anal. Chem. 2018, 90, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Mandler, D. Micro- and Nanopatterning Using Scanning Electrochemical Microscopy. In Scanning Electrochemical Microscopy; Bard, A.J., Mirkin, M.V., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 490–521. ISBN 978-1-4398-3113-7. [Google Scholar]
- Mandler, D. A New Approach to the High Resolution Electrodeposition of Metals via the Feedback Mode of the Scanning Electrochemical Microscope. J. Electrochem. Soc. 1990, 137, 1079–1086. [Google Scholar] [CrossRef]
- Kranz, C.; Ludwig, M.; Gaub, H.E.; Schuhmann, W. Lateral deposition of polypyrrole lines by means of the scanning electrochemical microscope. Adv. Mater. 1995, 7, 38–40. [Google Scholar] [CrossRef]
- Hecht, E.; Liedert, A.; Ignatius, A.; Mizaikoff, B.; Kranz, C. Local detection of mechanically induced ATP release from bone cells with ATP microbiosensors. Biosens. Bioelectron. 2013, 44, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, W.; Kranz, C.; Wohlschläger, H.; Strohmeier, J. Pulse technique for the electrochemical deposition of polymer films on electrode surfaces. Biosens. Bioelectron. 1997, 12, 1157–1167. [Google Scholar] [CrossRef]
- Patel, K.; Singh, N.; Yadav, J.; Nayak, J.M.; Sahoo, S.K.; Lata, J.; Chand, D.; Kumar, S.; Kumar, R. Polydopamine films change their physicochemical and antimicrobial properties with a change in reaction conditions. Phys. Chem. Chem. Phys. 2018, 20, 5744–5755. [Google Scholar] [CrossRef]
- Bernsmann, F.; Voegel, J.C.; Ball, V. Different synthesis methods allow to tune the permeability and permselectivity of dopamine-melanin films to electrochemical probes. Electrochim. Acta 2011, 56, 3914–3919. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Perspectives on poly(dopamine). Chem. Sci. 2013, 4, 3796–3802. [Google Scholar] [CrossRef]
- Zigah, D.; Noël, J.-M.; Lagrost, C.; Hapiot, P. Charge Transfer between Electroactive Species Immobilized on Carbon Surfaces by Aryl Diazonium Reduction. SECM Investigations. J. Phys. Chem. C 2010, 114, 3075–3081. [Google Scholar] [CrossRef]
- Hauquier, F.; Matrab, T.; Kanoufi, F.; Combellas, C. Local direct and indirect reduction of electrografted aryldiazonium/gold surfaces for polymer brushes patterning. Electrochim. Acta 2009, 54, 5127–5136. [Google Scholar] [CrossRef]
- Chumillas, S.; Palomäki, T.; Zhang, M.; Laurila, T.; Climent, V.; Feliu, J.M. Analysis of catechol, 4-methylcatechol and dopamine electrochemical reactions on different substrate materials and pH conditions. Electrochim. Acta 2018, 292, 309–321. [Google Scholar] [CrossRef]
- Müller, M.; Keßler, B. Deposition from dopamine solutions at Ge substrates: An in situ ATR-FTIR study. Langmuir 2011, 27, 12499–12505. [Google Scholar] [CrossRef] [PubMed]






| Number of Pulse Cycles | Substrate Potential vs. Ag/AgCl Redox Mediator: [Fe(CN)6]4− | Substrate Potential mV vs. Ag/AgCl Redox Mediator: Ru(NH3)63+ | ||
|---|---|---|---|---|
| −0.1 V | 0.5 V | −0.1 V | 0.5 V | |
| 1 | 1.4331 ± 0.0402 | 2.3090 ± 0.0334 | 2.7222 ± 0.0211 | 1.6197 ± 0.0274 |
| 15 | 0.0257 ± 0.0034 | 0.0478 ± 0.0027 | 0.1617 ± 0.0133 | 0.0617 ± 0.0082 |
| 60 | 0.0009 ± 0.0003 | 0.0012 ± 0.0002 | 0.0068 ± 0.0012 | 0.0045 ± 0.0007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Daboss, S.; Blaimer, D.; Kranz, C. Micro-Structured Polydopamine Films via Pulsed Electrochemical Deposition. Nanomaterials 2019, 9, 242. https://doi.org/10.3390/nano9020242
Lin J, Daboss S, Blaimer D, Kranz C. Micro-Structured Polydopamine Films via Pulsed Electrochemical Deposition. Nanomaterials. 2019; 9(2):242. https://doi.org/10.3390/nano9020242
Chicago/Turabian StyleLin, Jing, Sven Daboss, Dominik Blaimer, and Christine Kranz. 2019. "Micro-Structured Polydopamine Films via Pulsed Electrochemical Deposition" Nanomaterials 9, no. 2: 242. https://doi.org/10.3390/nano9020242
APA StyleLin, J., Daboss, S., Blaimer, D., & Kranz, C. (2019). Micro-Structured Polydopamine Films via Pulsed Electrochemical Deposition. Nanomaterials, 9(2), 242. https://doi.org/10.3390/nano9020242