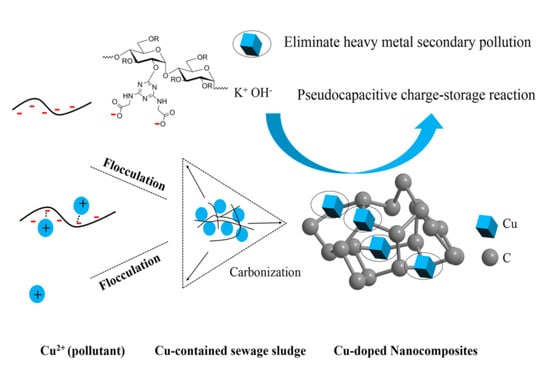
Cu-Doped Porous Carbon Derived from Heavy Metal-Contaminated Sewage Sludge for High-Performance Supercapacitor Electrode Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flocculation Experiment
2.3. Fabrication of Cu-Doped Carbon Materials Using Cu(II)-Containing Sludge
2.4. Characterization Methods
2.5. Electrochemical Measurements
3. Results
3.1. Removal of Cu(II) from Aqueous Solution
3.2. Characterization of SFC-x
3.3. Electrochemical Performance of SFC-x
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xue, Y.; Wang, C.; Hu, Z.; Zhou, Y.; Xiao, Y.; Wang, T. Pyrolysis of sewage sludge by electromagnetic induction: Biochar properties and application in adsorption removal of Pb(II), Cd(II) from aqueous solution. Waste Manag. 2019, 89, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agrawal, M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-N.; Nie, C.-C.; Zhang, H.; Lyu, X.-J.; Qiu, J.; Li, L. Recovery of metals in waste printed circuit boards by flotation technology with soap collector prepared by waste oil through saponification. Waste Manag. 2019, 89, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, X.; Luo, M.; Yang, P.; Zhou, X. Fast one-pot microwave preparation and plasma modification of porous carbon from waste lignin for energy storage application. Waste Manag. 2019, 89, 129–140. [Google Scholar] [CrossRef]
- Tran, C.; Lawrence, D.; Richey, F.W.; Dillard, C.; Elabd, Y.A.; Kalra, V. Binder-free three-dimensional high energy density electrodes for ionic-liquid supercapacitors. Chem. Commun. 2015, 51, 13760–13763. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Wang, X.; Shen, G. Intercalation pseudo-capacitive TiNb2O7@carbon electrode for high-performance lithium ion hybrid electrochemical supercapacitors with ultrahigh energy density. Nano Energy 2015, 15, 104–115. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Wang, S.; Ren, Z.; Yu, J. Transition metal doped MnO2 nanosheets grown on internal surface of macroporous carbon for supercapacitors and oxygen reduction reaction electrocatalysts. Appl. Mater. Today 2016, 3, 63–72. [Google Scholar] [CrossRef]
- Tian, D.; Lu, X.; Zhu, Y.; Li, M.; Wang, C. Fabrication of two-dimensional metal-organic frameworks on electrospun nanofibers and their derived metal doped carbon nanofibers for an advanced asymmetric supercapacitor with a high energy density. J. Power Sources 2019, 413, 50–58. [Google Scholar] [CrossRef]
- Wu, Z.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.; Müllen, K. Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, H.; Fang, L.; Mu, Y.; Wang, Y. Facile preparation of novel dandelion-like Fe-doped NiCo2O4 microspheres@nanomeshes for excellent capacitive property in asymmetric supercapacitors. J. Power Sources 2016, 327, 135–144. [Google Scholar] [CrossRef]
- Shen, L.; Yu, L.; Wu, H.B.; Yu, X.-Y.; Zhang, X.; Lou, X.W. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 2015, 6, 6694. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Yu, H.; Huang, X.; Bian, T.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Wu, L.; Tung, C.; Zhang, T. Well-Dispersed ZIF-Derived Co,N-Co-doped carbon nanoframes through mesoporous-silica-protected calcination as efficient oxygen reduction electrocatalysts. Adv. Mater. 2016, 28, 1668–1674. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Behm, M.; Irvine, J.T.S. Influence of structure and composition upon performance of tin phosphate based negative electrodes for lithium batteries. Electrochim. Acta 2002, 47, 1727–1738. [Google Scholar] [CrossRef]
- Guibal, E.; Roussy, J. Coagulation and flocculation of dye-containing solutions using a biopolymer (Chitosan). React. Funct. Polym. 2007, 67, 33–42. [Google Scholar] [CrossRef]
- Jia, S.; Yang, Z.; Yang, W.; Zhang, T.; Zhang, S.; Yang, X.; Dong, Y.; Wu, J.; Wang, Y. Removal of Cu(II) and tetracycline using an aromatic rings-functionalized chitosan-based flocculant: Enhanced interaction between the flocculant and the antibiotic. Chem. Eng. J. 2016, 283, 495–503. [Google Scholar] [CrossRef]
- Shi, Y.; Ju, B.; Zhang, S. Flocculation behavior of a new recyclable flocculant based on pH responsive tertiary amine starch ether. Carbohydr. Polym. 2012, 88, 132–138. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Giovanni, M.; Poh, H.L.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Šaněk, F.; Khezri, B.; Webster, R.D.; Pumera, M. Noble metal (Pd, Ru, Rh, Pt, Au, Ag) doped graphene hybrids for electrocatalysis. Nanoscale 2012, 4, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of materials and fabrication methods. J. Energy Eng.-ASCE 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, J.; Li, S.; Meng, Y.; Liu, J. Three-dimensional nitrogen doped holey reduced graphene oxide framework as metal-free counter electrodes for high performance dye-sensitized solar cells. J. Power Sources 2016, 308, 44–51. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Li, L.; Xi, Y.; Li, J.; Lu, M.; Cao, J.; Han, W. Self-assembly of biomass microfibers into 3D layer-stacking hierarchical porous carbon for high performance supercapacitors. Electrochim. Acta 2018, 286, 264–270. [Google Scholar] [CrossRef]
- Shi, R.; Han, C.; Li, H.; Xu, L.; Zhang, T.; Li, J.; Lin, Z.; Wong, C.; Kang, F.; Li, B. NaCl-templated synthesis of hierarchical porous carbon with extremely large specific surface area and improved graphitization degree for high energy density lithium ion capacitors. J. Mater. Chem. A 2018, 6, 17057–17066. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Xu, J.; Cheng, G.; Yang, W.; Kou, T.; Zhang, Z.; Ding, Y. 3D binder-free Cu2O@Cu nanoneedle arrays for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 18229–18235. [Google Scholar] [CrossRef]
- Sun, S. Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale 2015, 7, 10850–10882. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 nanowire@mnO2 ultrathin nanosheet core/shell arrays: A new class of high-performance pseudocapacitive materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef]
- Teng, S.; Siegel, G.; Prestgard, M.C.; Wang, W.; Tiwari, A. Synthesis and characterization of copper-infiltrated carbonized wood monoliths for supercapacitor electrodes. Electrochim. Acta 2015, 161, 343–350. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Wang, Y.; Zhang, J.; Yan, X.; Lei, T.; Peng, Z.; Zhou, Z.; Lv, H. Facile preparation of S-doped Cu/C core-shell composite for high-performance anode of pseudocapacitors. Electrochim. Acta 2018, 259, 1030–1036. [Google Scholar] [CrossRef]
- Vidhyadharan, B.; Misnon, I.I.; Aziz, R.A.; Padmasree, K.P.; Yusoff, M.M.; Jose, R. Superior supercapacitive performance in electrospun copper oxide nanowire electrodes. J. Mater. Chem. A 2014, 2, 6578–6588. [Google Scholar] [CrossRef]
- Wang, G.; Huang, J.; Chen, S.; Gao, Y.; Cao, D. Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam. J. Power Sources 2011, 196, 5756–5760. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Zhao, H.; Chen, D.; Liu, F.; Xiang, J.; Hu, Z.; Li, Y. Facile shape control of Co3O4 and the effect of the crystal plane on electrochemical performance. Adv. Mater. 2012, 24, 5762–5766. [Google Scholar] [CrossRef] [PubMed]
- Pendashteh, A.; Mousavi, M.F.; Rahmanifar, M.S. Fabrication of anchored copper oxide nanoparticles on graphene oxide nanosheets via an electrostatic coprecipitation and its application as supercapacitor. Electrochim. Acta 2013, 88, 347–357. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, M.; Wang, T.; Li, F.; Zhang, Y.X. Construction of unique cupric oxide–manganese dioxide core–shell arrays on a copper grid for high-performance supercapacitors. J. Mater. Chem. A 2016, 4, 10786–10793. [Google Scholar] [CrossRef]
- Nara, H.; Morita, K.; Mukoyama, D.; Yokoshima, T.; Momma, T.; Osaka, T. Impedance analysis of LiNi1/3Mn1/3Co1/3O2 cathodes with different secondary-particle size distribution in Lithium-ion Battery. Electrochim. Acta 2017, 241, 323–330. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Cao, G.; Wu, F.; Yang, Y. Sustainable nitrogen-doped porous carbon with high surface areas prepared from gelatin for supercapacitors. J. Mater. Chem. A 2012, 22, 19088–19093. [Google Scholar] [CrossRef]
- Wang, K.; Dong, X.; Zhao, C.; Qian, X.; Xu, Y. Facile synthesis of Cu2O/CuO/RGO nanocomposite and its superior cyclability in supercapacitor. Electrochim. Acta 2015, 152, 433–442. [Google Scholar] [CrossRef]
- Dong, X.; Wang, K.; Zhao, C.; Qian, X.; Chen, S.; Li, Z.; Liu, H.; Dou, S. Direct synthesis of RGO/Cu2O composite films on Cu foil for supercapacitors. J. Alloy. Compd. 2014, 586, 745–753. [Google Scholar] [CrossRef]
- Yu, L.; Jin, Y.; Li, L.; Ma, J.; Wang, G.; Geng, B.; Zhang, X. 3D porous gear-like copper oxide and their high electrochemical performance as supercapacitors. CrystEngComm 2013, 15, 7657–7662. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Yu, F.; Liu, L.; Jia, X.; Lv, Y.; Chen, L.; Xu, Y.; Shi, Y.; Guo, X. Cu-Doped Porous Carbon Derived from Heavy Metal-Contaminated Sewage Sludge for High-Performance Supercapacitor Electrode Materials. Nanomaterials 2019, 9, 892. https://doi.org/10.3390/nano9060892
Tan Z, Yu F, Liu L, Jia X, Lv Y, Chen L, Xu Y, Shi Y, Guo X. Cu-Doped Porous Carbon Derived from Heavy Metal-Contaminated Sewage Sludge for High-Performance Supercapacitor Electrode Materials. Nanomaterials. 2019; 9(6):892. https://doi.org/10.3390/nano9060892
Chicago/Turabian StyleTan, Zhouliang, Feng Yu, Liu Liu, Xin Jia, Yin Lv, Long Chen, Yisheng Xu, Yulin Shi, and Xuhong Guo. 2019. "Cu-Doped Porous Carbon Derived from Heavy Metal-Contaminated Sewage Sludge for High-Performance Supercapacitor Electrode Materials" Nanomaterials 9, no. 6: 892. https://doi.org/10.3390/nano9060892
APA StyleTan, Z., Yu, F., Liu, L., Jia, X., Lv, Y., Chen, L., Xu, Y., Shi, Y., & Guo, X. (2019). Cu-Doped Porous Carbon Derived from Heavy Metal-Contaminated Sewage Sludge for High-Performance Supercapacitor Electrode Materials. Nanomaterials, 9(6), 892. https://doi.org/10.3390/nano9060892