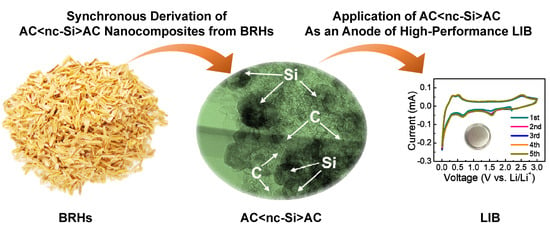
Activated Carbon-Decorated Spherical Silicon Nanocrystal Composites Synchronously-Derived from Rice Husks for Anodic Source of Lithium-Ion Battery
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of AC-Decorated nc-Si
2.2. Characterization of Material Properties
2.3. Analyses of Electrochemical Performances
3. Results and Discussion
3.1. Morphological Properties
3.2. Microstructural Properties
3.3. Crystallographic Characteristics
3.4. Textural Characteristics
3.5. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on advanced materials for Li-ion batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Hwang, T.H.; Je, S.H.; Buyukcakir, O.; Choi, J.W.; Coskun, A. Elemental-sulfur-mediated facile synthesis of a covalent triazine framework for high-performance lithium–sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010, 9, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nano. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Favors, Z.; Ionescu, R.; Ye, R.; Bay, H.H.; Ozkan, M.; Ozkan, C.S. Monodisperse porous silicon spheres as anode materials for lithium ion batteries. Sci. Rep. 2015, 5, 8781. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, G.; Liu, N.; Carney, T.J.; Yang, Y.; Cui, Y. Engineering empty space between Si nanoparticles for lithium-ion battery anodes. Nano Lett. 2012, 12, 904–909. [Google Scholar] [CrossRef]
- Hu, Y.-S.; Demir-Cakan, R.; Titirici, M.-M.; Müller, J.-O.; Schlögl, R.; Antonietti, M.; Maier, J. Superior storage performance of a Si@SiOx/C nanocomposite as anode material for lithium-ion batteries. Angew. Chem. Int. Ed. 2008, 47, 1645–1649. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.; Jang, H.Y.; Jung, I.; Liu, L.; Park, S. Synthesis of embossing Si nanomesh and its application as an anode for lithium ion batteries. J. Power Sources 2017, 362, 270–277. [Google Scholar] [CrossRef]
- Xiao, Q.; Fan, Y.; Wang, X.; Susantyoko, R.A.; Zhang, Q. A multilayer Si/CNT coaxial nanofiber LIB anode with a high areal capacity. Energy Environ. Sci. 2014, 7, 655–661. [Google Scholar] [CrossRef]
- Liu, J.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. Energy storage materials from nature through nanotechnology: A sustainable route from reed plants to a silicon anode for lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 9632–9636. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yu, Y.; Kung, H.H.; Wang, B.; Jiang, J. Si@SiOx/graphene hydrogel composite anode for lithium-ion battery. J. Power Sources 2016, 306, 42–48. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, H.; Yang, Y.; Wang, Z.; Li, X.; Zhou, R.; Peng, W. Facile synthesis of silicon/carbon nanospheres composite anode materials for lithium-ion batteries. Mater. Lett. 2016, 168, 138–142. [Google Scholar] [CrossRef]
- Jiao, L.-S.; Liu, J.-Y.; Li, H.-Y.; Wu, T.-S.; Li, F.; Wang, H.-Y.; Niu, L. Facile synthesis of reduced graphene oxide-porous silicon composite as superior anode material for lithium-ion battery anodes. J. Power Sources 2016, 315, 9–15. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; You, Y.; Xin, S.; Yin, Y.-X.; Zhang, J.; Wang, P.; Zheng, X.-s.; Cao, F.-F.; Guo, Y.-G. Rice husk-derived hierarchical silicon/nitrogen-doped carbon/carbon nanotube spheres as low-cost and high-capacity anodes for lithium-ion batteries. Nano Energy 2016, 25, 120–127. [Google Scholar] [CrossRef]
- Wang, L.; Gao, B.; Peng, C.; Peng, X.; Fu, J.; Chu, P.K.; Huo, K. Bamboo leaf derived ultrafine Si nanoparticles and Si/C nanocomposites for high-performance Li-ion battery anodes. Nanoscale 2015, 7, 13840–13847. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, H.; Qi, H.; Gao, X.; Liang, J.; Liang, C. Rice husk as the source of silicon/carbon anode material and stable electrochemical performance. ChemistrySelect 2018, 3, 5439–5444. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, H.; Qi, H.; Liang, J.; Liang, C. High performance of porous silicon/carbon/RGO network derived from rice husks as anodes for lithium-ion batteries. New J. Chem. 2018, 42, 19811–19817. [Google Scholar] [CrossRef]
- Toçoğlu, U.; Hatipoğlu, G.; Alaf, M.; Kayış, F.; Akbulut, H. Electrochemical characterization of silicon/graphene/MWCNT hybrid lithium-ion battery anodes produced via RF magnetron sputtering. Appl. Surf. Sci. 2016, 389, 507–513. [Google Scholar] [CrossRef]
- Tao, H.; Xiong, L.; Zhu, S.; Yang, X.; Zhang, L. Flexible binder-free reduced graphene oxide wrapped Si/carbon fibers paper anode for high-performance lithium ion batteries. Int. J. Hydrogen Energy 2016, 41, 21268–21277. [Google Scholar] [CrossRef]
- Tong, Y.; Xu, Z.; Liu, C.; Zhang, G. a.; Wang, J.; Wu, Z.G. Magnetic sputtered amorphous Si/C multilayer thin films as anode materials for lithium ion batteries. J. Power Sources 2014, 247, 78–83. [Google Scholar] [CrossRef]
- Wang, J.; Meng, X.; Fan, X.; Zhang, W.; Zhang, H.; Wang, C. Scalable synthesis of defect abundant Si nanorods for high-performance Li-ion battery anodes. ACS Nano 2015, 9, 6576–6586. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-L.; Zhang, B.; Kim, J.-K. Electrospun carbon nanofiber anodes containing monodispersed Si nanoparticles and graphene oxide with exceptional high rate capacities. Nano Energy 2014, 6, 27–35. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Xiao, C.; Du, N.; Shi, X.; Zhang, H.; Yang, D. Large-scale synthesis of Si@C three-dimensional porous structures as high-performance anode materials for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 20494–20499. [Google Scholar] [CrossRef]
- Chang, J.; Huang, X.; Zhou, G.; Cui, S.; Hallac, P.B.; Jiang, J.; Hurley, P.T.; Chen, J. Multilayered Si nanoparticle/reduced graphene oxide hybrid as a high-performance lithium-ion battery anode. Adv. Mater. 2014, 26, 758–764. [Google Scholar] [CrossRef]
- Yang, R.; Buonassisi, T.; Gleason, K.K. Organic vapor passivation of silicon at room temperature. Adv. Mater. 2013, 25, 2078–2083. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhang, A.; Lu, Y.; Zhou, C. Scalable preparation of porous silicon nanoparticles and their application for lithium-ion battery anodes. Nano Res. 2013, 6, 174–181. [Google Scholar] [CrossRef]
- Vlad, A.; Reddy, A.L.M.; Ajayan, A.; Singh, N.; Gohy, J.-F.; Melinte, S.; Ajayan, P.M. Roll up nanowire battery from silicon chips. Proc. Natl. Acad. Sci. USA 2012, 109, 15168–15173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, A.M.; Lieber, C.M. A laser ablation method for the synthesis of crystalline semiconductor nanowires. Science 1998, 279, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, H.; Pan, W.; Gong, J.; Khalid, B.; Zhong, M.; Wu, H. SiOx nanodandelion by laser ablation for anode of lithium-ion battery. Small 2015, 11, 6009–6012. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gong, K. Silicon-based materials from rice husks and their applications. Ind. Eng. Chem. Res. 2001, 40, 5861–5877. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Athinarayanan, J.; Periasamy, V.S. Biocompatibility assessment of rice husk-derived biogenic silica nanoparticles for biomedical applications. Mater. Sci. Eng. C 2015, 47, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Xing, A.; Tian, S.; Tang, H.; Losic, D.; Bao, Z. Mesoporous silicon engineered by the reduction of biosilica from rice husk as a high-performance anode for lithium-ion batteries. RSC Adv. 2013, 3, 10145–10149. [Google Scholar] [CrossRef]
- Liu, N.; Huo, K.; McDowell, M.T.; Zhao, J.; Cui, Y. Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes. Sci. Rep. 2013, 3, 1919. [Google Scholar] [CrossRef]
- Marchal, J.C.; Krug Iii, D.J.; McDonnell, P.; Sun, K.; Laine, R.M. A low cost, low energy route to solar grade silicon from rice hull ash (RHA), a sustainable source. Green Chem. 2015, 17, 3931–3940. [Google Scholar] [CrossRef]
- Basu, P.K.; King, C.J.; Lynn, S. Manufacture of silicon tetrachloride from rice hulls. AIChE J. 1973, 19, 439–445. [Google Scholar] [CrossRef]
- Pavarajarn, V.; Precharyutasin, R.; Praserthdam, P. Synthesis of silicon nitride fibers by the carbothermal reduction and nitridation of rice husk ash. J. Am. Ceram. Soc. 2010, 93, 973–979. [Google Scholar] [CrossRef]
- Sujirote, K.; Leangsuwan, P. Silicon carbide formation from pretreated rice husks. J. Mater. Sci. 2003, 38, 4739–4744. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: A comprehensive utilization strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, H.; Kim, Y.A.; Yang, K.-S.; Cruz-Silva, R.; Toda, I.; Yamada, T.; Terrones, M.; Endo, M.; Hayashi, T.; Saitoh, H. Rice husk-derived graphene with nano-sized domains and clean edges. Small 2014, 10, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Lee, H.; Jung, H.; Kim, A.; Ahmed, A.T.A.; Inamdar, A.I.; Kim, H.; Lee, S.; Im, H.; Young Kim, D. Ultrathin graphene nanosheets derived from rice husks for sustainable supercapacitor electrodes. New J. Chem. 2017, 41, 13792–13797. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, N.; Liu, D.; Xu, J.; Sha, J.; Yin, J.; Tan, X.; Yang, H.; Lu, H.; Lin, H. Direct carbonization of rice husk to prepare porous carbon for supercapacitor applications. Energy 2017, 128, 618–625. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Gao, W.; Wang, Z.; Ma, Y.; Wang, Z. Simultaneous preparation of silica and activated carbon from rice husk ash. J. Clean. Prod. 2012, 32, 204–209. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Zhu, Y.; An, D.; Gao, W.; Wang, Z.; Ma, Y.; Wang, Z. A sustainable route for the preparation of activated carbon and silica from rice husk ash. J. Hazard. Mater. 2011, 186, 1314–1319. [Google Scholar] [CrossRef]
- Jung, D.S.; Ryou, M.-H.; Sung, Y.J.; Park, S.B.; Choi, J.W. Recycling rice husks for high-capacity lithium battery anodes. Proc. Natl. Acad. Sci. USA 2013, 110, 12229–12234. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Xi, Y.; Chen, S.; Li, D.; She, X.; Sun, J.; Han, W.; Yang, D.; Guo, S. Prolifera-green-tide as sustainable source for carbonaceous aerogels with hierarchical pore to achieve multiple energy storage. Adv. Funct. Mater. 2016, 26, 8487–8495. [Google Scholar] [CrossRef]
- Yu, W.; Wang, H.; Liu, S.; Mao, N.; Liu, X.; Shi, J.; Liu, W.; Chen, S.; Wang, X. N, O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes. J. Mater. Chem. A 2016, 4, 5973–5983. [Google Scholar] [CrossRef]
- Kim, J.M.; Guccini, V.; Seong, K.-d.; Oh, J.; Salazar-Alvarez, G.; Piao, Y. Extensively interconnected silicon nanoparticles via carbon network derived from ultrathin cellulose nanofibers as high performance lithium ion battery anodes. Carbon 2017, 118, 8–17. [Google Scholar] [CrossRef]
- Xiao, K.; Ding, L.-X.; Chen, H.; Wang, S.; Lu, X.; Wang, H. Nitrogen-doped porous carbon derived from residuary shaddock peel: a promising and sustainable anode for high energy density asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 372–378. [Google Scholar] [CrossRef]
- Lv, W.; Wen, F.; Xiang, J.; Zhao, J.; Li, L.; Wang, L.; Liu, Z.; Tian, Y. Peanut shell derived hard carbon as ultralong cycling anodes for lithium and sodium batteries. Electrochim. Acta 2015, 176, 533–541. [Google Scholar] [CrossRef]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Ahmad, W.; bahrani, M.R.A.; Yang, Z.; Khan, J.; Jing, W.; Jiang, F.; Chu, L.; Liu, N.; Li, L.; Gao, Y. Extraction of nano-silicon with activated carbons simultaneously from rice husk and their synergistic catalytic effect in counter electrodes of dye-sensitized solar cells. Sci. Rep. 2016, 6, 39314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekar, S.; Lee, Y.; Kim, D.Y.; Lee, S. Substantial LIB anode performance of graphitic carbon nanoflakes derived from biomass green-tea waste. Nanomaterials 2019, 9, 871. [Google Scholar] [CrossRef]
- Tang, Y.; Yuan, S.; Guo, Y.; Huang, R.; Wang, J.; Yang, B.; Dai, Y. Highly ordered mesoporous Si/C nanocomposite as high performance anode material for Li-ion batteries. Electrochim. Acta 2016, 200, 182–188. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K. Flexible fiber supercapacitor using biowaste-derived porous carbon. Chem. Electro. Chem. 2015, 2, 1111–1116. [Google Scholar]
- Ren, Y.; Zhang, J.; Xu, Q.; Chen, Z.; Yang, D.; Wang, B.; Jiang, Z. Biomass-derived three-dimensional porous N-doped carbonaceous aerogel for efficient supercapacitor electrodes. RSC Adv. 2014, 4, 23412–23419. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ding, C.X.; Zhang, L.C.; Xu, H.W.; Zhang, D.W.; Cheng, T.; Chen, C.H. A novel carbon–silicon composite nanofiber prepared via electrospinning as anode material for high energy-density lithium ion batteries. J. Power Sources 2010, 195, 5052–5056. [Google Scholar] [CrossRef]
- Sun, L.; Su, T.; Xu, L.; Du, H.-B. Preparation of uniform Si nanoparticles for high-performance Li-ion battery anodes. Phy. Chem. Chem. Phy. 2016, 18, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Tao, S.; Song, X.; Zhang, P.; Gao, L. A silicon/double-shelled carbon yolk-like nanostructure as high-performance anode materials for lithium-ion battery. J. Electrochem. Soc. 2015, 162, A1530–A1536. [Google Scholar] [CrossRef]
- Sun, Z.; Song, X.; Zhang, P.; Gao, L. Controlled synthesis of yolk-mesoporous shell Si@SiO2 nanohybrid designed for high performance Li ion battery. RSC Adv. 2014, 4, 20814–20820. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Liang, J.; Hu, X.; Yu, K. Preparation of disordered carbon from rice husks for lithium-ion batteries. New J. Chem. 2016, 40, 325–329. [Google Scholar] [CrossRef]
- Kaskhedikar, N.A.; Maier, J. Lithium storage in carbon nanostructures. Adv. Mater. 2009, 21, 2664–2680. [Google Scholar] [CrossRef]
- Xiang, J.; Lv, W.; Mu, C.; Zhao, J.; Wang, B. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloy. Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Jin, M.; Liang, M.; Dayeh, S.A.; Picraux, S.T.; Zhi, L. Adaptable silicon–carbon nanocables sandwiched between reduced graphene oxide sheets as lithium ion battery anodes. ACS Nano 2013, 7, 1437–1445. [Google Scholar] [CrossRef]
- Yun, Q.; Qin, X.; Lv, W.; He, Y.-B.; Li, B.; Kang, F.; Yang, Q.-H. “Concrete” inspired construction of a silicon/carbon hybrid electrode for high performance lithium ion battery. Carbon 2015, 93, 59–67. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Liu, Y.; Zhang, K.; Emani, S.; Sawicki, M.S.; Shamie, J.S.; Shaw, L.L. Hollow silicon nanospheres encapsulated with a thin carbon shell: An electrochemical study. Electrochim. Acta 2016, 215, 126–141. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.-M.; Wang, Z.-H.; Shao, Q.-G.; Li, X.; Yuan, L.-X.; Hu, X.-L.; Zhang, W.-X.; Huang, Y.-H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Zai, J.; Dai, F.; Gordin, M.L.; Wang, D. Dual conductive network-enabled graphene/Si–C composite anode with high areal capacity for lithium-ion batteries. Nano Energy 2014, 6, 211–218. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Qi, K.; Ge, X.; Zhang, Q.; Zhang, B. One-step synthesis of 3D sulfur/nitrogen dual-doped graphene supported nano silicon as anode for Li-ion batteries. Appl. Surf. Sci. 2018, 433, 367–373. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Kalubarme, R.S.; Kim, J.; Jo, Y.; Woo, H.; Cho, S.; Pawar, S.M.; Park, C.-J.; Lee, Y.-W.; Sohn, J.I.; et al. Nickel titanate lithium-ion battery anodes with high reversible capacity and high-rate long-cycle life performance. J. Mater. Chem. A 2016, 4, 4691–4699. [Google Scholar] [CrossRef]
- Jia, D.; Huang, J. A bio-inspired nanofibrous silicon/carbon composite as an anode material for lithium-ion batteries. New J. Chem. 2017, 41, 4887–4900. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Z.; Ci, L.; Zhai, W.; Ai, Q.; Xiong, S. Chemical dealloying synthesis of porous silicon anchored by in situ generated graphene sheets as anode material for lithium-ion batteries. J. Power Sources 2015, 287, 177–183. [Google Scholar] [CrossRef]








| Samples | BET Analysis | BJH Analysis | ||
|---|---|---|---|---|
| Specific Surface Area (m2/g) | Pore Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | |
| nc-Si | 232.8 | 153.5 | 0.2936 | 5.83 |
| AC | 1068.5 | 755.8 | 0.4932 | 2.41 |
| AC<nc-Si>AC | 498.5 | 394.9 | 0.386 | 2.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Aqueel Ahmed, A.T.; Inamdar, A.I.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Activated Carbon-Decorated Spherical Silicon Nanocrystal Composites Synchronously-Derived from Rice Husks for Anodic Source of Lithium-Ion Battery. Nanomaterials 2019, 9, 1055. https://doi.org/10.3390/nano9071055
Sekar S, Aqueel Ahmed AT, Inamdar AI, Lee Y, Im H, Kim DY, Lee S. Activated Carbon-Decorated Spherical Silicon Nanocrystal Composites Synchronously-Derived from Rice Husks for Anodic Source of Lithium-Ion Battery. Nanomaterials. 2019; 9(7):1055. https://doi.org/10.3390/nano9071055
Chicago/Turabian StyleSekar, Sankar, Abu Talha Aqueel Ahmed, Akbar I. Inamdar, Youngmin Lee, Hyunsik Im, Deuk Young Kim, and Sejoon Lee. 2019. "Activated Carbon-Decorated Spherical Silicon Nanocrystal Composites Synchronously-Derived from Rice Husks for Anodic Source of Lithium-Ion Battery" Nanomaterials 9, no. 7: 1055. https://doi.org/10.3390/nano9071055
APA StyleSekar, S., Aqueel Ahmed, A. T., Inamdar, A. I., Lee, Y., Im, H., Kim, D. Y., & Lee, S. (2019). Activated Carbon-Decorated Spherical Silicon Nanocrystal Composites Synchronously-Derived from Rice Husks for Anodic Source of Lithium-Ion Battery. Nanomaterials, 9(7), 1055. https://doi.org/10.3390/nano9071055