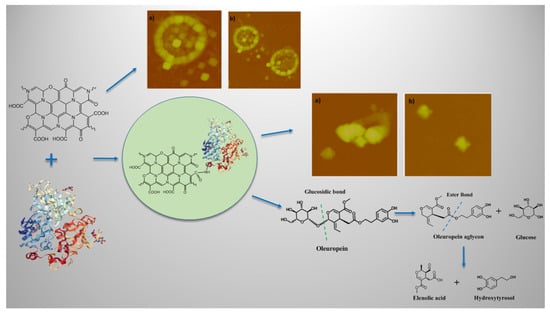
Enzymatic Conversion of Oleuropein to Hydroxytyrosol Using Immobilized β-Glucosidase on Porous Carbon Cuboids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Porous Carbon Cuboids (PCC) and Oxidized PPC (PCCox)
2.3. Covalent Immobilization of β-Glucosidase on PCC and PCCox
2.4. Non-Covalent Immobilization of β-Glucosidase on PCC and PCCox
2.5. Determination of the Immobilization Yield
2.6. Determination of the Hydrolyitc Activity of Immobilized β-Glucosidase
2.7. Thermal Stability of Free and Immobilized β-Glucosidases
2.8. Hydrolysis of Oleuropein to Hydroxytyrosol
2.9. High Performance Liquid Chromatography (HPLC) Analysis
2.10. Reusability Studies of Immobilized β-Glucosidases
2.11. Fourier-Transform Infrared Spectroscopy (FTIR)
2.12. Fluorescence Spectroscopy
2.13. X-ray Photoelectron Spectroscopy
2.14. Atomic Force Microscopy
2.15. Raman Spectroscopy
3. Results and Discussion
3.1. Characterization of PCC and PCCox
3.2. Immobilization Efficiency and Activity of Immobilized β-Glucosidase
3.3. Characterization of Bio-Nanoconjugates
3.4. Thermal Stability of Free and Immobilized β-Glucosidase
3.5. Use of Immobilized β-Glucosidase for the Conversion of Oleuropein to Hydroxytyrosol
3.6. Reusability of Immobilized β-Glucosidase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bolivar, J.M.; Eisl, I.; Nidetzky, B. Advanced characterization of immobilized enzymes as heterogeneous biocatalysts. Catal. Today 2015, 259, 66–80. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Hong, T.; Liu, W.; Li, M.; Chen, C. Recent advances in the fabrication and application of nanomaterial-based enzymatic microsystems in chemical and biological sciences. Anal. Chim. Acta 2019, 1067, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, I.V.; Vorhaben, T.; Tsoufis, T.; Rudolf, P.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Development of effective nanobiocatalytic systems through the immobilization of hydrolases on functionalized carbon-based nanomaterials. Bioresour. Technol. 2012, 115, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Bilal, M.; Rasheed, T.; Sharma, A.; Iqbal, H.M.N. Graphene and graphene oxide: Functionalization and nano-bio-catalytic system for enzyme immobilization and biotechnological perspective. Int. J. Biol. Macromol. 2018, 120, 1430–1440. [Google Scholar] [CrossRef]
- Li, Y.; Gao, F.; Wei, W.; Qu, J.; Ma, G.; Zhou, W. Enzymatic pore size of macroporous polystyrene microspheres affects lipase immobilization. J. Mol. Catal. B Enzym. 2010, 66, 182–189. [Google Scholar] [CrossRef]
- Bayne, L.; Ulijn, R.V.; Halling, P.J. Effect of pore size on the performance of immobilised enzymes. Chem. Soc. Rev. 2013, 42, 9000–9010. [Google Scholar] [CrossRef]
- Wan, D.; Tian, L.; Li, X.; Li, B.; Zhang, Q. A versatile strategy for enzyme immobilization: Fabricating lipase/inorganic hybrid nanostructures on macroporous resins with enhanced catalytic properties. Biochem. Eng. J. 2018, 139, 101–108. [Google Scholar] [CrossRef]
- Luangon, B.; Siyasukh, A.; Winayanuwattikun, P.; Tanthapanichakoon, W. Enzymatic flow-through immobilization of Candida rugosa lipase on hierarchical micro-/macroporous carbon monolith. J. Mol. Catal. B Enzym. 2012, 75, 80–85. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Yan, Y.; Hu, Y.; Zhang, Y.; Tang, Y. Protein adsorption onto nanozeolite: Effect of micropore openings. J. Colloid Interface Sci. 2013, 406, 130–138. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Tsoufis, T.; Enotiadis, A.; Gournis, D.; Stamatis, H. Functionalized multi-wall carbon nanotubes for lipase immobilization. Adv. Eng. Mater. 2010, 12, 179–183. [Google Scholar] [CrossRef]
- Patila, M.; Pavlidis, I.V.; Diamanti, E.K.; Katapodis, P.; Gournis, D.; Stamatis, H. Enhancement of cytochrome c catalytic behaviour by affecting the heme environment using functionalized carbon-based nanomaterials. Process Biochem. 2013, 48, 1010–1017. [Google Scholar] [CrossRef]
- Jin, L.; Yang, K.; Yao, K.; Zhang, S.; Tao, H.; Lee, S.; Liu, Z.; Peng, R. Functionalized graphene oxide in enzyme engineering: A selective modulator for enzyme activity and thermostability. ACS Nano 2012, 6, 4864–4875. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Patila, M.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Graphene-based nanobiocatalytic systems: Recent advances and future prospects. Trends Biotechnol. 2014, 32, 312–320. [Google Scholar] [CrossRef]
- Hao, G.P.; Mondin, G.; Zheng, Z.; Biemelt, T.; Klosz, S.; Schubel, R.; Eychm ller, A.; Kaskel, S. Unusual ultra-hydrophilic, porous carbon cuboids for atmospheric-water capture. Angew. Chem. Int. Ed. 2015, 54, 1941–1945. [Google Scholar] [CrossRef]
- Karageorgou, D.; Thomou, E.; Vourvou, N.T.; Lyra, K.M.; Chalmpes, N.; Enotiadis, A.; Spyrou, K.; Katapodis, P.; Gournis, D.; Stamatis, H. Antibacterial and algicidal effects of porous carbon cuboid nanoparticles. ACS Omega 2019, 4, 4991–5001. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Eur. J. Inorg. Chem. 1898, 32, 1481–1487. [Google Scholar] [CrossRef]
- Stergiou, D.V.; Diamanti, E.K.; Gournis, D.; Prodromidis, M.I. Comparative study of different types of graphenes as electrocatalysts for ascorbic acid. Electrochem. Commun. 2010, 12, 1307–1309. [Google Scholar] [CrossRef]
- Gengler, R.Y.N.; Veligura, A.; Enotiadis, A.; Diamanti, E.K.; Gournis, D.; Józsa, C.; Van Wees, B.J.; Rudolf, P. Large-yield preparation of high-electronic-quality graphene by a langmuir-schaefer approach. Small 2010, 6, 35–39. [Google Scholar] [CrossRef]
- Liaros, N.; Tucek, J.; Dimos, K.; Bakandritsos, A.; Andrikopoulos, K.S.; Gournis, D.; Zboril, R.; Couris, S. The effect of the degree of oxidation on broadband nonlinear absorption and ferromagnetic ordering in graphene oxide. Nanoscale 2016, 8, 2908–2917. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Ahmed, A.; Nasim, F.; Batool, K.; Bibi, A. Microbial β-glucosidase: Sources, production and applications. J. Appl. Environ. Microbiol. 2017, 5, 31–46. [Google Scholar] [CrossRef]
- Achmon, Y.; Fishman, A. The antioxidant hydroxytyrosol: Biotechnological production challenges and opportunities. Appl. Microbiol. Biotechnol. 2014, 99, 1119–1130. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 4728. [Google Scholar]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar]
- Zhang, Z.L.; Chen, J.; Xu, Q.; Rao, C.; Qiao, C. Efficient synthesis of hydroxytyrosol from 3,4-dihydroxybenzaldehyde. Synth. Commun. 2012, 42, 794–798. [Google Scholar] [CrossRef]
- Liu, M.; Yong, Q. Efficient bioconversion of oleuropein from olive leaf extract to antioxidant hydroxytyrosol by enzymatic hydrolysis and high-temperature degradation. Biotechnol. Appl. Biochem. 2018, 5, 680–689. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, C.; Ye, J.; Tao, R.; Zhang, Y. Enzymatic hydrolysis of oleuropein from Olea europea (Olive) leaf extract and antioxidant activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef]
- Khoufi, S.; Hamza, M.; Sayadi, S. Enzymatic hydrolysis of olive wastewater for hydroxytyrosol enrichment. Bioresour. Technol. 2011, 102, 9050–9058. [Google Scholar] [CrossRef]
- Mazzei, R.; Drioli, E.; Giorno, L. Enzyme membrane reactor with heterogenized β-glucosidase to obtain phytotherapic compound: Optimization study. J. Memb. Sci. 2012, 390–391, 121–129. [Google Scholar] [CrossRef]
- Nikolaivits, E.; Termentzi, A.; Skaltsounis, A.L.; Fokialakis, N.; Topakas, E. Enzymatic tailoring of oleuropein from Olea europaea leaves and product identification by HRMS/MS spectrometry. J. Biotechnol. 2017, 253, 48–54. [Google Scholar] [CrossRef]
- Sehgal, D.; Vijay, I.K. A method for the high efficiency of water-soluble carbodiimide-mediated amidation. Anal. Biochem. 1994, 218, 87–91. [Google Scholar] [CrossRef]
- Lau, S.C.; Lim, H.N.; Basri, M.; Reza, H.; Masoumi, F.; Ahmad, A. Enhanced biocatalytic esterification with lipase-immobilized chitosan/graphene oxide beads. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Orfanakis, G.; Patila, M.; Catzikonstantinou, A.V.; Lyra, K.; Kouloumpis, A.; Spyrou, K.; Katapodis, P. Hybrid Nanomaterials of magnetic iron nanoparticles and graphene oxide as matrices for the immobilization of β-glucosidase: Synthesis, characterization and biocatalytic properties. Front. Mater. 2018, 5, 11. [Google Scholar] [CrossRef]
- Prestrelski, S.J.; Tedeschi, N.; Arakawa, T.; Carpenter, J.F. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys. J. 1993, 65, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Secundo, F.; Carrea, G.; Molecolare, R.; Bianco, M. Mono- and disaccharides enhance the activity and enantioselectivity of Burkholderia cepacia lipase in organic solvent but do not significantly affect its conformation. Biotechnol. Bioeng. 2005, 92, 438–446. [Google Scholar] [CrossRef]
- Secundo, F.; Barletta, G.L.; Dumitriu, E.; Carrea, G.; Molecolare, R.; Bianco, M. Can an inactivating agent increase enzyme activity in organic solvent? Effects of 18-crown-6 on lipase activity, enantioselectivity and conformation. Biotechnol. Appl. Biochem. 2007, 97, 12–18. [Google Scholar] [CrossRef]
- Tzialla, A.A.; Pavlidis, I.V.; Felicissimo, M.P.; Rudolf, P.; Gournis, D.; Stamatis, H. Lipase immobilization on smectite nanoclays: Characterization and application to the epoxidation of α-pinene. Bioresour. Technol. 2010, 101, 1587–1594. [Google Scholar] [CrossRef]
- Kouloumpis, A.; Thomou, E.; Chalmpes, N.; Dimos, K.; Spyrou, K.; Bourlinos, A.B.; Koutselas, I.; Gournis, D.; Rudolf, P. Graphene/carbon dot hybrid thin films prepared by a modified Langmuir-Schaefer method. ACS Omega 2017, 2, 2090–2099. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabo, T.; Szeri, A.; Dekany, I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Spyrou, K.; Potsi, G.; Diamanti, E.K.; Ke, X.; Serestatidou, E.; Verginadis, I.I.; Velalopoulou, A.P.; Evangelou, A.M.; Deligiannakis, Y.; Van Tendeloo, G.; et al. Towards novel multifunctional pillared nanostructures: Effective intercalation of adamantylamine in graphene oxide and smectite clays. Adv. Funct. Mater. 2014, 24, 5841–5850. [Google Scholar] [CrossRef]
- Simons, W.W. Sadtler Research Laboratories The Sadtler Handbook of Infrared Spectra; Sadtler Research Laboratories: Philadelphia, PA, USA, 1978; ISBN 9780845600344. [Google Scholar]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules: Volume Two Advances in Infrared Group Frequencies; Springer: Amsterdam, The Netherlands, 1980; ISBN 9789401165228. [Google Scholar]
- Enotiadis, A.; Angjeli, K.; Baldino, N.; Nicotera, I.; Gournis, D. Graphene-based nafion nanocomposite membranes: Enhanced proton transport and water retention by novel organo-functionalized graphene oxide nanosheets. Small 2012, 8, 3338–3349. [Google Scholar] [CrossRef]
- Zygouri, P.; Tsoufis, T.; Kouloumpis, A.; Patila, M.; Potsi, G.; Sevastos, A.A.; Sideratou, Z.; Katsaros, F.; Charalambopoulou, G.; Stamatis, H.; et al. Synthesis, characterization and assessment of hydrophilic oxidized carbon nanodiscs in bio-related applications. RSC Adv. 2018, 8, 122–131. [Google Scholar] [CrossRef]
- Patila, M.; Pavlidis, I.V.; Kouloumpis, A.; Dimos, K.; Spyrou, K.; Katapodis, P.; Gournis, D.; Stamatis, H. Graphene oxide derivatives with variable alkyl chain length and terminal functional groups as supports for stabilization of cytochrome c. Int. J. Biol. Macromol. 2016, 84, 227–235. [Google Scholar] [CrossRef]
- Gao, Y.; Kyratzis, I. Covalent immobilization of proteins on carbon nanotubes using the cross-linker 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide—A critical assessment. Bioconjug. Chem. 2008, 19, 1945–1950. [Google Scholar] [CrossRef]
- Xue, Y.M.; Xu, C.Y.; Hou, J.J.; Li, X.Q.; Cao, Z.G. Enhanced soluble expression of a thermostble β glucosidase from Thermotoga maritima in Escherichia coli and its applicaton in immobilization 1. Appl. Biochem. Microbiol. 2015, 51, 306–315. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, O.; Yoon, Y.; Ryu, K. Immobilization of horseradish peroxidase on multi-wall carbon nanotubes and its electrochemical properties. Biotechnol. Lett. 2006, 28, 39–43. [Google Scholar] [CrossRef]
- Gómez, J.M.; Romero, M.D.; Fernández, T.M. Immobilization of β-glucosidase on carbon nanotubes. Catal. Lett. 2005, 101, 275–278. [Google Scholar]
- Çelik, A.; Dinçer, A.; Aydemir, T. Characterization of β-glucosidase immobilized on chitosan-multiwalled carbon nanotubes (MWCNTS) and their application on tea extracts for aroma enhancement. Int. J. Biol. Macromol. 2016, 89, 406–414. [Google Scholar] [CrossRef]
- Patila, M.; Kouloumpis, A.; Gournis, D.; Rudolf, P.; Stamatis, H. Laccase-functionalized graphene oxide assemblies as efficient nanobiocatalysts for oxidation reactions. Sensors (Switzerland) 2016, 16, 287. [Google Scholar] [CrossRef]
- Patila, M.; Diamanti, E.K.; Bergouni, D.; Polydera, A.C.; Gournis, D.; Stamatis, H. Preparation and biochemical characterisation of nanoconjugates of functionalized carbon nanotubes and cytochrome c. Nanomed. Res. J. 2018, 3, 10–18. [Google Scholar]
- Zhou, J.; Zhang, J.; David, A.E.; Yang, V.C. Magnetic tumor targeting of β -glucosidase immobilized iron oxide nanoparticles. Nanotechnology 2013, 24, 1–12. [Google Scholar] [CrossRef]
- Chiaradia, V.; Soares, N.S.; Valério, A.; de Oliveira, D.; Araújo, P.H.H.; Sayer, C. Immobilization of Candida antarctica Lipase B on magnetic poly(urea-urethane) nanoparticles. Appl. Biochem. Biotechnol. 2016, 180, 558–575. [Google Scholar] [CrossRef]
- Ayoub, F.D.P.; Caseli, L. Biointerfaces controlling the molecular architecture of lactase immobilized in Langmuir-Blodgett films of phospholipids to modulate the enzyme activity. Colloids Surf. B Biointerfaces 2017, 150, 8–14. [Google Scholar] [CrossRef]
- Junior, R.; Caseli, L. Adsorption and enzyme activity of asparaginase at lipid Langmuir and Langmuir-Blodgett films. Mater. Sci. Eng. C 2017, 73, 579–584. [Google Scholar] [CrossRef]
- Serefoglou, E.; Litina, K.; Gournis, D.; Kalogeris, E.; Tzialla, A.A.; Pavlidis, I.V.; Stamatis, H.; Maccallini, E.; Lubomska, M.; Rudolf, P. Smectite clays as solid supports for immobilization of β-glucosidase: Synthesis, characterization and biochemical properties. Chem. Mater. 2008, 20, 4106–4115. [Google Scholar] [CrossRef]
- Spyrou, K.; Calvaresi, M.; Diamanti, E.K.; Tsoufis, T.; Gournis, D.; Rudolf, P.; Zerbetto, F. Graphite oxide and aromatic amines: Size matters. Adv. Funct. Mater. 2015, 25, 263–269. [Google Scholar] [CrossRef]
- Verma, M.L.; Chaudhary, R.; Tsuzuki, T.; Barrow, C.J.; Puri, M. Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: Application in cellobiose hydrolysis. Bioresour. Technol. 2013, 135, 2–6. [Google Scholar] [CrossRef]
- Chen, T.; Yang, W.; Guo, Y.; Yuan, R.; Xu, L.; Yan, Y. Enhancing catalytic performance of β-glucosidase via immobilization on metal ions chelated magnetic nanoparticles. Enzym. Microb. Technol. 2014, 63, 50–57. [Google Scholar] [CrossRef]
- Briante, R.; Cara, F.L.; Tonziello, M.P.; Febbraio, F.; Nucci, R. Antioxidant activity of the main bioactive derivatives from oleuropein hydrolysis by hyperthermophilic β-glycosidase. J. Agric. Food Chem. 2001, 49, 3198–3203. [Google Scholar] [CrossRef]
- Hamza, M.; Sayadi, S. Original article high production of Aspergillus niger β-glucosidase at pilot-scale and application for hydroxytyrosol release from olive by-product. Food Sci. Technol. 2015, 50, 1882–1890. [Google Scholar]
- Chang, J.; Lee, Y.; Fang, S.; Park, D.; Choi, Y. Hydrolysis of isoflavone glycoside by immobilization of β-glucosidase on a chitosan-carbon in two-phase system. Int. J. Biol. Macromol. 2013, 61, 465–470. [Google Scholar] [CrossRef]









| Immobilization Yield %–(Activity U mg−1 Enzyme) | ||||
|---|---|---|---|---|
| Albgl | Tmbgl | |||
| Covalent | Non-covalent | Covalent | Non-covalent | |
| PCC | 72–(0.8) | 80–(0.5) | 95–(24) | 94–(18) |
| PCCox | 90–(5) | 62–(9) | 93–(35) | 94–(37) |
| free | 9.5 | 45 | ||
| Sample | Atomic Percentage % | ||
|---|---|---|---|
| C | O | N | |
| PCC-Tmbgl-cov | 75.1 ± 3.0 | 14.1 ± 1.1 | 10.8 ± 0.9 |
| PCC-Tmbgl-nc | 75.1 ± 3.0 | 13.5 ± 1.1 | 11.4 ± 0.9 |
| PCCox-Tmbgl-cov | 55.5 ± 2.2 | 40.7 ± 2.4 | 3.8 ± 0.3 |
| PCCox-Tmbgl-nc | 72 ± 2.9 | 23 ± 1.4 | 5 ± 0.4 |
| Sample | C/N Ratio |
|---|---|
| PCC-Tmbgl-cov | 6.9 |
| PCC-Tmbgl-nc | 6.6 |
| PCCox-Tmbgl-cov | 14.6 |
| PCCox-Tmbgl-nc | 14.4 |
| Sample | Half-Life Time (Hours) |
|---|---|
| Albgl | 0.6 ± 0.04 |
| PCC-Albgl-cov | 0.9 ± 0.10 |
| PCC-Albgl-nc | 0.7 ± 0.07 |
| PCCox-Albgl-cov | 0.9 ± 0.06 |
| PCCox-Albgl-nc | 0.8 ± 0.05 |
| Sample | Initial Reaction Rate mM h−1 g−1 of Biocatalyst | % Conversion Yield of OLE |
|---|---|---|
| Free Albgl | 0.05 | 50 |
| PCCox-Albgl-cov | 0.18 | 92 |
| PCCox-Albgl-nc | 0.16 | 90 |
| Free Tmbgl | 0.14 | 78 |
| PCCox-Tmbgl-cov | 0.20 | 98 |
| PCCox-Tmbgl-nc | 0.19 | 95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzikonstantinou, A.V.; Gkantzou, E.; Thomou, E.; Chalmpes, N.; Lyra, K.-M.; Kontogianni, V.G.; Spyrou, K.; Patila, M.; Gournis, D.; Stamatis, H. Enzymatic Conversion of Oleuropein to Hydroxytyrosol Using Immobilized β-Glucosidase on Porous Carbon Cuboids. Nanomaterials 2019, 9, 1166. https://doi.org/10.3390/nano9081166
Chatzikonstantinou AV, Gkantzou E, Thomou E, Chalmpes N, Lyra K-M, Kontogianni VG, Spyrou K, Patila M, Gournis D, Stamatis H. Enzymatic Conversion of Oleuropein to Hydroxytyrosol Using Immobilized β-Glucosidase on Porous Carbon Cuboids. Nanomaterials. 2019; 9(8):1166. https://doi.org/10.3390/nano9081166
Chicago/Turabian StyleChatzikonstantinou, Alexandra V., Elena Gkantzou, Eleni Thomou, Nikolaos Chalmpes, Kyriaki-Marina Lyra, Vasiliki G. Kontogianni, Konstantinos Spyrou, Michaela Patila, Dimitrios Gournis, and Haralambos Stamatis. 2019. "Enzymatic Conversion of Oleuropein to Hydroxytyrosol Using Immobilized β-Glucosidase on Porous Carbon Cuboids" Nanomaterials 9, no. 8: 1166. https://doi.org/10.3390/nano9081166
APA StyleChatzikonstantinou, A. V., Gkantzou, E., Thomou, E., Chalmpes, N., Lyra, K. -M., Kontogianni, V. G., Spyrou, K., Patila, M., Gournis, D., & Stamatis, H. (2019). Enzymatic Conversion of Oleuropein to Hydroxytyrosol Using Immobilized β-Glucosidase on Porous Carbon Cuboids. Nanomaterials, 9(8), 1166. https://doi.org/10.3390/nano9081166






_Stamatis.png)