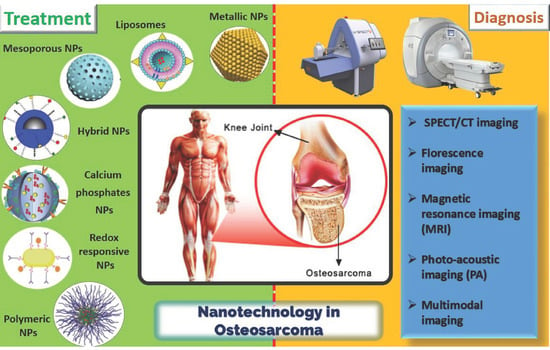
Recent Advances in Nanotechnology-Based Diagnosis and Treatments of Human Osteosarcoma
Abstract
:1. Introduction
2. Diagnosis of Human Osteosarcoma
2.1. Current Approaches for Diagnosis of OSA
2.2. Nanomaterials for Diagnosis of Human Osteosarcoma
2.2.1. Single-Photon Emission Computed Tomography (SPECT)/CT Imaging
2.2.2. Fluorescence Imaging
2.2.3. Magnetic Resonance Imaging (MRI)
2.2.4. Photo-Acoustic Imaging (PAI)
2.2.5. Multimodal Imaging
3. Nanomaterials for the Treatment of OSA
3.1. Polymeric Nanocarriers
3.2. Liposomes
3.3. Metallic Nanoparticles
3.4. Redox Responsive Nanocarriers
3.5. Hybrid Nanoparticles
3.6. Mesoporous Silica Nanocarriers
3.7. Calcium Phosphates Nanocarriers
3.8. Other NPs
4. Conclusions, Challenges, and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Apa | Apatinib |
| CaP | Calcium phosphate |
| CSCs | Cancer stem cells |
| CT | Commutated tomography |
| EphA2 | Ephrin alpha 2 receptors |
| FA | Folic acid |
| GSCT | Gemstone spectral commutated tomography |
| MLNs | Metastatic lymph nodes |
| MMP-2 | Matrix metalloproteinase-2 |
| MRI | Magnetic resonance imaging |
| MTX | Methotrexate |
| NPs | Nanoparticles |
| OSA | Osteosarcoma |
| PAI | Photoacoustic imaging |
| PEG | Poly ethylene glycol |
| PET | Positron emission tomography |
| PLGA | Poly (lactide-co-glycolide) |
| PMAN | Poly(2-(methylacryloyl)ethylnicotinate) |
| RGD | Arginine-glycine-aspartic acid |
| ROS | Reactive oxygen species |
| siRNA | Small interfering RNA |
| TAMs | Tumor-associated macrophages |
References
- Longhi, A.; Errani, C.; De Paolis, M.; Mercuri, M.; Bacci, G. Primary bone osteosarcoma in the pediatricage: State of the art. Cancer Treat. Rev. 2006, 32, 423–436. [Google Scholar] [CrossRef]
- Fletcher, C.D.; Unni, K.K.; Mertens, F. Pathology and Genetics of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2002; Volume 4. [Google Scholar]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef]
- Ozaki, T.; Flege, S.; Kevric, M.; Lindner, N.; Maas, R.; Delling, G.; Schwarz, R.; von Hochstetter, A.R.; Salzer-Kuntschik, M.; Berdel, W.E.; et al. Osteosarcoma of the pelvis: Experience of the Cooperative Osteosarcoma Study Group. J. Clin. Oncol. 2003, 21, 334–341. [Google Scholar] [CrossRef]
- Picci, P. Osteosarcoma (osteogenic sarcoma). Orphanet J. Rare Dis. 2007, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Mirabello, L.; Troisi, R.J.; Savage, A.S. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Wang, H.J.; Tsai, P.-C.; Langford, C.F.; Fosmire, S.P.; Jubala, C.M.; Getzy, D.M.; Cutter, G.R.; Modiano, J.F.; Breen, M. Influence of genetic background on tumor karyotypes: Evidence for breed-associated cytogenetic aberrations in canine appendicular osteosarcoma. Chromosome Res. 2009, 17, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Kempf-Bielack, B.; Bielack, S.S.; Jürgens, H.; Branscheid, D.; Berdel, W.E.; Göbel, G.U.E.; Helmke, K.; Jundt, G.; Kabisch, H.; Kevric, M.; et al. Osteosarcoma relapse after combined modality therapy:Ananalysis of unselected patients in the Cooperative Osteosarcoma Study Group(COSS). J. Clin. Oncol. 2005, 23, 559–568. [Google Scholar] [CrossRef]
- Miller, B.J.; Cram, P.; Lynch, C.F.; Buckwalter, J.A. Risk factors for metastatic disease at presentation with osteosarcoma: An analysis of the SEER database. J. Bone Jt. Surg. Am. Vol. 2013, 95. [Google Scholar] [CrossRef] [Green Version]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. 2010, 8, 705–718. [Google Scholar]
- Hamada, K.; Tomita, Y.; Inoue, A.; Fujimoto, T.; Hashimoto, N.; Myoui, A.; Yoshikawa, H.; Hatazawa, J. Evaluation of chemotherapy response in osteosarcoma with FDG-PET. Ann. Nucl. Med. 2009, 23, 89–95. [Google Scholar] [CrossRef]
- Huang, R.; Wang, M.; Zhu, Y.; Conti, P.S.; Chen, K. Development of PET probes for cancer imaging. Curr. Top. Med. Chem. 2015, 15, 795–819. [Google Scholar] [CrossRef] [PubMed]
- Schirra, C.O.; Brendel, B.; Anastasio, M.A.; Roessl, E. Spectral CT: A technology primer for contrast agent development. Contrast Media Mol. Imaging 2014, 9, 62–70. [Google Scholar] [CrossRef]
- Jin, Y.; Ni, D.; Gao, L.; Meng, X.; Lv, Y.; Han, F.; Zhang, H.; Liu, Y.; Yao, Z.; Feng, X.; et al. Harness the power of upconversion nanoparticles for spectral computed tomography diagnosis of osteosarcoma. Adv. Funct. Mater. 2018, 28, 1802656. [Google Scholar] [CrossRef]
- Chen, B.; Yang, J.-Z.; Wang, L.-F.; Zhang, Y.-J.; Lin, X.-J. Ifosfamide-loaded poly(lactic-co-glycolicacid) PLGA-dextran polymeric nanoparticles to improve the antitumor efficacy in Osteosarcoma. BMC Cancer 2015, 15, 752. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Miyake, K.; Oshiro, H.; Sugisawa, N.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Igarashi, K.; Chawla, S.P.; et al. Trabectedin and irinotecan combination regresses a cisplatinum-resistant osteosarcoma in a patient-derived orthotopic xenograft nude-mouse model. Biochem. Biophys. Res. Commun. 2019, 513, 326–331. [Google Scholar] [CrossRef]
- Schwartz, C.L.; Gorlick, R.; Teot, L.; Krailo, M.; Chen, Z.; Goorin, A.; Grier, H.E.; Bernstein, M.L.; Meyers, P.; Group, C.O. Multiple Drug Resistance in Osteogenic Sarcoma: INT0133 From the Children’s Oncology Group. J. Clin. Oncol. 2007, 25, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- PosthumaDeBoer, J.; van Royen, B.; Helder, M. Mechanisms of therapy resistance in osteosarcoma: A review. Oncol. Discov. 2013, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hou, H.; Zhang, P.; Zhang, Z. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Deliv. 2020, 27, 1044–1053. [Google Scholar] [CrossRef]
- Ferrari, S.; Serra, M. An update on chemotherapy for osteosarcoma. Expert Opin. Pharmacother. 2015, 16, 2727–2736. [Google Scholar]
- Wang, S.-Y.; Hu, H.-Z.; Qing, X.-C.; Zhang, Z.-C.; Shao, Z.-W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Ullah, M.W.; Siddique, R.; Liu, Y.; Ullah, I.; Xue, M.; Yang, G.; Hou, H. Catechins-modified selenium-doped hydroxyapatite nanomaterials for improved osteosarcoma therapy through generation of reactive oxygen species. Front. Oncol. 2019, 9, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Zhao, L.; Yang, Z.; Liu, Z.; Gu, J.; Bai, B.; Liu, J.; Xu, J.; Yang, H. Mechanisms of oxidative stress, apoptosis, and autophagy involved in graphene oxide nanomaterials anti-osteosarcoma effect. Int. J. Nanomed. 2018, 13, 2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Wu, W.; Yang, W.; Qing, X.; Shao, Z. Surface engineering of nanoparticles with ligands for targeted delivery to osteosarcoma. Colloids Surf. B Biointerfaces 2020, 190, 110891. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Lin, Z.; Li, M.; Hu, X.; Zhang, Y.; Peng, M.; Xia, H.; Han, G. Enhancing osteosarcoma killing and CT imaging using ultrahigh drug loading and NIR-responsive bismuth sulfide@mesoporous silica nanoparticles. Adv. Healthc. Mater. 2018, 7, 1800602. [Google Scholar] [CrossRef]
- Si, X.-Y.; Merlin, D.; Xiao, B. Recent advances in orally administered cell-specific nano therapeutics for inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7718. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandan, F.M.; Afzali, D.; Sargazi, G.; Gordan, M. Novel uranyl-curcumin-MOF photocatalysts with highly performance photocatalytic activity toward the degradation of phenol red from aqueous solution: Effective synthesis route, design and a controllable systematic study. J. Mater. Sci. Mater. Electron. 2018, 29, 18600–18613. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2019, 1–18. [Google Scholar] [CrossRef]
- Nematollahi, M.H.; Pardakhty, A.; Torkzadeh-Mahanai, M.; Mehrabanid, M.; Asadikaram, G. Changes in physical and chemical properties of niosome membrane induced by cholesterol: A promising approach for niosome bilayer intervention. RSC Adv. 2017, 7, 49463–49472. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, K.A.; Nematollahi, M.H.; Khanbabaei, H.; Nave, H.H.; Mirzaei, H.R.; Pourghadamyari, H.; Sahebkar, A. Targeted Delivery of CRISPR/Cas13asa Promising Therapeutic Approach to Treat. SARS-CoV-2. Curr. Pharmaceut. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, F.; Qi, X.; Dong, Y.; Zhang, Y.; Ge, Z.; Cai, G.; Zhang, X. Epidermal growth factor receptor aptamer-conjugated polymer-lipid hybrid nanoparticles enhance salinomycin delivery to osteosarcoma and cancer stem cells. Exp. Ther. Med. 2018, 15, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Lupusoru, R.V.; Pricop, D.A.; Uritu, C.M.; Arvinte, A.; Coroaba, A.; Esanu, I.; Zaltariov, M.F.; Silion, M.S.; Stefanescu, C.; Pinteala, M. Effect of TAT-DOX-PEG irradiated gold nanoparticles conjugates on human osteosarcoma cells. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Song, J.-X.; Zhang, M.-N.; Yuan, B.-S. A multiple drug loaded, functionalized pH-sensitive nanocarrier as therapeutic and epigenetic modulator for osteosarcoma. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raghubir, M.; Rahman, C.N.; Fang, J.; Matsui, H.; Mahajan, S.S. Osteosarcoma growth suppression by riluzole delivery via iron oxide nanocage in nude mice. Oncol. Rep. 2020, 43, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Sharma, S.; Javed, M.N.; Pottoo, F.H.; Barkat, M.A.; Alam, M.S.H.; Amir, M.; Sarafroz, M. Bioinspired nanocomposites: Applications in disease diagnosis and treatment. Pharm. Nanotechnol. 2019, 7, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Zhang, Q.; Sun, C.; Liu, G.-Y. Ifosfamide-loaded lipid-core-nanocapsules to increase the anticancer efficacy in MG63 osteosarcoma cells. Saudi J. Biol. Sci. 2018, 25, 1140–1145. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Nazmi, K.; De Boer, J.P.; Zandieh-Doulabi, B.; Forouzanfar, T.; Helder, M.N. EphA2 targeted doxorubicin-nanoliposomes for osteosarcoma treatment. Pharm. Res. 2017, 34, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, C.; Cheng, Y.; Li, D.; Gong, Y.; Liu, J.; Tian, H.; Chen, X. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 2014, 35, 8723–8734. [Google Scholar] [CrossRef]
- Bukchin, A.; Pascual-Pasto, G.; Cuadrado-Vilanova, M.; Castillo-Ecija, H.; Monterrubio, C.; Olaciregui, N.G.; Vila-Ubach, M.; Ordeix, L.; Mora, J.; Carcaboso, A.M.; et al. Glucosylated nanomicelles target glucose-avid pediatric patient-derived sarcomas. J. Control. Release 2018, 276, 59–71. [Google Scholar] [CrossRef]
- Fan, T.M.; Roberts, R.D.; Lizardo, M.M. Understanding and modeling metastasis biology to improve therapeutic strategies for combating osteosarcoma progression. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lv, L.; Ling, Z.; Wang, Y.; Liu, Y.; Li, L.; Liu, G.; Shen, L.; Yan, J.; Wang, Y. Long-circulating iodinated albumin–gadolinium nanoparticles as enhanced magnetic resonance and computed tomography imaging probes for osteosarcoma visualization. Anal. Chem. 2015, 87, 4299–4304. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Bilal, M.; Rahdar, A.; Arshad, R.; Kumar, A.; Hamishekar, H.; Kyzas, G.Z. Nanodiagnosis and nanotreatment of colorectal cancer: An overview. J. Nanopart. Res. 2021, 23, 1–25. [Google Scholar] [CrossRef]
- Barani, M.; Sabir, F.; Rahdar, A.; Arshad, R.; Kyzas, G.Z. Nanotreatment and Nanodiagnosis of Prostate Cancer: Recent Updates. Nanomaterials 2020, 10, 1696. [Google Scholar] [CrossRef]
- Bilal, M.; Barani, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanomaterials for the treatment and diagnosis of Alzheimer’s disease: An overview. NanoImpact 2020, 100251. [Google Scholar] [CrossRef]
- Nikazar, S.; Barani, M.; Rahdar, A.; Zoghi, M.; Kyzas, G.Z. Photo-and Magnetothermally Responsive Nanomaterials for Therapy, Controlled Drug Delivery and Imaging Applications. ChemistrySelect 2020, 5, 12590–12609. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Nasri, S.; Beyzaei, H.; Barani, M.; Trant, J.F. The synthesis of methotrexate-loaded F127 microemulsions and their in vivo toxicity in a rat model. J. Mol. Liq. 2020, 313, 113449. [Google Scholar] [CrossRef]
- Roberts, R.D.; Lizardo, M.M.; Reed, D.R.; Hingorani, P.; Glover, J.; Allen-Rhoades, W.; Fan, T.; Khanna, C.; Sweet-Cordero, E.A.; Cash, T. Provocative questions in osteosarcoma basic and translational biology: A report from the Children’s Oncology Group. Cancer 2019, 125, 3514–3525. [Google Scholar] [CrossRef] [PubMed]
- Sheen, H.; Kim, W.; Byun, B.H.; Kong, C.-B.; Song, W.S.; Cho, W.H.; Lim, I.; Lim, S.M.; Woo, S.-K. Metastasis risk prediction model in osteosarcoma using metabolic imaging phenotypes: A multivariable radiomics model. PLoS ONE 2019, 14, e0225242. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, J.; Zhang, X.; Wang, R.; Liang, Z.; He, Q.; Wang, Z.; Wang, K.; Wang, S. Label-free Raman imaging of live osteosarcoma cells with multivariate analysis. Appl. Microbiol. Biotechnol. 2019, 103, 6759–6769. [Google Scholar] [CrossRef] [PubMed]
- Yarmish, G.; Klein, M.J.; Landa, J.; Lefkowitz, R.A.; Hwang, S. Imaging characteristics of primary osteosarcoma: Nonconventional subtypes. Radiographics 2010, 30, 1653–1672. [Google Scholar] [CrossRef] [Green Version]
- Murphey, M.D.; Jaovisidha, S.W.; Temple, H.T.; Gannon, F.H.; Jelinek, J.S.; Malawer, M.M. Telangiectatic osteosarcoma: Radiologic-pathologic comparison. Radiology 2003, 229, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mc Auley, G.; Jagannathan, J.; O’Regan, K.; Krajewski, K.M.; Hornick, J.L.; Butrynski, J.; Ramaiya, N. Extraskeletal osteosarcoma: Spectrum of imaging findings. Am. J. Roentgenol. 2012, 198, W31–W37. [Google Scholar] [CrossRef]
- Kundu, Z.S. Classification, imaging, biopsy and staging of osteosarcoma. Indian J. Orthop. 2014, 48, 238–246. [Google Scholar] [CrossRef] [PubMed]
- O’Flanagan, S.J.; Stack, J.P.; McGee, H.M.; Dervan, P.; Hurson, B. Imaging of intramedullary tumour spread in osteosarcoma. A comparison of techniques. J. Bone Jt. Surg. Br. Vol. 1991, 73, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Saueressig, U.; van Buiren, M.V.; Kontny, U.; Niemeyer, C.; Köhler, G.; Ilyasov, K.; Langer, M. Osteosarcoma: Preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion-and perfusion-weighted magnetic resonance imaging. Investig. Radiol. 2006, 41, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Wittig, J.C.; Bickels, J.; Priebat, D.; Jelinek, J.; Kellar-Graney, K.; Shmookler, B.; Malawer, M.M. Osteosarcoma: A multidisciplinary approach to diagnosis and treatment. Am. Fam. Physician 2002, 65, 1123. [Google Scholar]
- Thoeni, R.F.; Mueller-Lisse, U.G.; Chan, R.; Do, N.K.; Shyn, P.B. Detection of small, functional islet cell tumors in the pancreas: Selection of MR imaging sequences for optimal sensitivity. Radiology 2000, 214, 483–490. [Google Scholar] [CrossRef]
- Hallscheidt, P.J.; Fink, C.; Haferkamp, A.; Bock, M.; Luburic, A.; Zuna, I.; Noeldge, G.; Kauffmann, G. Preoperative staging of renal cell carcinoma with inferior vena cava thrombus using multidetector CT and MRI: Prospective study with histopathological correlation. J. Comput. Assist. Tomogr. 2005, 29, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Pichler, B.J.; Judenhofer, M.S.; Pfannenberg, C. Multimodal Imaging Approaches: Pet./Ct and Pet/Mri, in Molecular Imaging I; Springer: Berlin/Heidelberg, Germany, 2008; pp. 109–132. [Google Scholar]
- Barani, M.; Torkzadeh-Mahani, M.; Mirzaei, M.; Nematollahi, M.H. Comprehensive evaluation of gene expression in negative and positive trigger-based targeting niosomes in HEK-293 cell line. Iran. J. Pharm. Res. 2020, 19, 166. [Google Scholar]
- Torkzadeh-Mahani, M.; Zaboli, M.; Barani, M.; Torkzadeh-Mahani, M. A combined theoretical and experimental study to improve the thermal stability of recombinant D-lactate dehydrogenase immobilized on a novel superparamagnetic Fe3O4NPs@ metal-organic framework. Appl. Organomet. Chem. 2020, 34, e5581. [Google Scholar] [CrossRef]
- Sonvico, F.; Dubernet, C.; Colombo, P.; Couvreur, P. Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Curr. Pharm. Des. 2005, 11, 2091–2105. [Google Scholar] [CrossRef]
- Jamali, Z.; Taheri-Anganeh, M.; Shabaninejad, Z.; Keshavarzi, A.; Taghizadeh, H.; Razavi, Z.S.; Mottaghi, R.; Abolhassan, M.; Movahedpour, A.; Mirzaei, H. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. IUBMB Life 2020, 72, 1306–1321. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, M.; Alvarez-Lorenzo, C.; Concheiro, A.; Santos, A.C.; Veiga, F.; Figueiras, A. Nanomedicine in osteosarcoma therapy: Micelleplexes for delivery of nucleic acids and drugs toward osteosarcoma-targeted therapies. Eur. J. Pharm. Biopharm. 2020, 148, 88–106. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Y.; Cheng, L.; Li, W.; Cao, X. SPECT/CT imaging of retroperitoneal extraskeletal osteosarcoma. Clin. Nucl. Med. 2014, 39, 200–202. [Google Scholar] [CrossRef]
- Gu, T.; Shi, H.; Xiu, Y.; Gu, Y. Primary pulmonary osteosarcoma: PET/CT and SPECT/CT findings. Clin. Nucl. Med. 2011, 36, e209–e212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, H.; Lu, W.; Shen, J.; Wang, Y.; Wang, Y. Bone-Seeking Albumin-Nanomedicine for In Vivo Imaging and Therapeutic Monitoring. ACS Biomater. Sci. Eng. 2020, 6, 647–653. [Google Scholar] [CrossRef]
- Bhushan, B.; Khanadeev, V.; Khlebtsov, B.; Khlebtsov, N.; Gopinath, P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv. Colloid Interface Sci. 2017, 246, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, L.; Motevalli, S.M.; Wu, X.; Yang, X.-H.; Li, X.; Han, L.; Magrini, A.; Guo, W.; Chang, J.; et al. Light-triggered retention and cascaded therapy of albumin-based theranostic nanomedicines to alleviate tumor adaptive treatment tolerance. Adv. Funct. Mater. 2018, 28, 1707291. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Sun, W.; Guo, Y.; Jia, H.-R.; Yu, X.-W.; Pan, G.-Y.; Wu, F.-G. Molecular Targeting-Mediated Mild—Temperature Photothermal Therapy with a Smart Albumin—Based Nanodrug. Small 2019, 15, 1900501. [Google Scholar] [CrossRef]
- Carpentier, A.; McNichols, R.J.; Stafford, R.J.; Guichard, J.-P.; Reizine, D.; Delaloge, S.; Vicaut, E.; Payen, D.; Gowda, A.; George, B. Laser thermal therapy: Real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg. Med. 2011, 43, 943–950. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, N.; Cornel, E.J.; Cai, H.; Xue, S.; Xi, H.; Fan, Z.; He, S.; Du, J. Bone-targeting polymer vesicles for simultaneous imaging and effective malignant bone tumor treatment. Biomaterials 2020, 269, 120345. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, H.; Zhang, M.; Wang, B.; Liu, Y.; Wang, Y.; Fang, X.; Zhang, J.; Yao, Z.; Bu, W. Negative CT Contrast Agents for the Diagnosis of Malignant Osteosarcoma. Adv. Sci. 2019, 6, 1901214. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.; He, W.; Ai, K.; Ren, X.; Liu, L.; Zhang, M.; Lu, L. Targeted imaging of damaged bone in vivo with gemstone spectral computed tomography. ACS Nano 2016, 10, 4164–4172. [Google Scholar] [CrossRef]
- Yin, X.-R.; Xia, W.; Yao, Z.-W.; He, H.-J.; Feng, X.-Y. The Initial Exploration of Adamkiewicz Artery Computed Tomographic Angiography With Monochromatic Reconstruction of Gemstone Spectral Imaging. J. Comput. Assist. Tomogr. 2016, 40, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Heilemann, M.; van de Linde, S.; Schüttpelz, M.; Kasper, R.; Seefeldt, B.; Mukherjee, A.; Tinnefeld, P.; Sauer, M. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. 2008, 47, 6172–6176. [Google Scholar] [CrossRef]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Schäferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed. 2012, 51, 3532–3554. [Google Scholar] [CrossRef]
- Kawada, K.; Taketo, M.M. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res. 2011, 71, 1214–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Sun, H.; Zhao, M.; Wang, A.; Qiu, S.; Gao, Y.; Ding, J.; Ji, S.-J.; Shi, H.; Gao, M. Rational design and synthesis of a metalloproteinase-activatable probe for dual-modality imaging of metastatic lymph nodes in vivo. J. Org. Chem. 2019, 84, 6126–6133. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, W.; Li, A.; Wang, B.; Ding, Q.; Xue, L.; Zeng, X.; Feng, Y.; Li, Q.; Wang, T.; et al. Specific Small-Molecule NIR-II Fluorescence Imaging of Osteosarcoma and Lung Metastasis. Adv. Healthc. Mater. 2020, 9, 1901224. [Google Scholar] [CrossRef]
- Schenck, J.F. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann. N. Y. Acad. Sci. 1992, 649, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; He, Z.Z.; Yang, Y.; Liu, J. MRI-based three-dimensional thermal physiological characterization of thyroid gland of human body. Med. Eng. Phys. 2014, 36, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Wiggett, A.J.; Downing, P.E. Functional MRI analysis of body and body part representations in the extrastriate and fusiform body areas. J. Neurophysiol. 2007, 98, 1626–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, S.; Yerneni, K.; Theruvath, J.L.; Graef, C.M.; Nejadnik, H.; Lenkov, O.; Pisani, L.; Rosenberg, J.; Mitra, S.; Sweet Cordero, A.S.; et al. Nanoparticle enhanced MRI can monitor macrophage response to CD47 mAb immunotherapy in osteosarcoma. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Pourtau, L.; Oliveira, H.; Thevenot, J.; Wan, Y.; Brisson, A.R.; Sandre, O.; Miraux, S.; Thiaudiere, E.; Lecommandoux, S. Antibody-functionalized magnetic polymersomes: In vivo targeting and imaging of bone metastases using high resolution MRI. Adv. Healthc. Mater. 2013, 2, 1420–1424. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Qin, H.; Chen, H.; Yang, H.; Xu, J.; Yang, S.; Hu, J.; Xing, D. Phage display-derived oligopeptide-functionalized probes for in vivo specific photoacoustic imaging of osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar] [CrossRef]
- Núñez, N.G.; Boari, J.T.; Ramos, R.N.; Richer, W.; Cagnard, N.; Anderfuhren, C.D.; Niborski, L.L.; Bigot, J.; Meseure, D.; Rochere, P.D.L.; et al. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat. Commun. 2020, 11, 1–15. [Google Scholar]
- Faghih, Z.; Rezaeifard, S.; Safaei, A.; Ghaderi, A.; Erfani, N. IL-17 and IL-4 producing CD8+ T cells in tumor draining lymph nodes of breast cancer patients: Positive association with tumor progression. Iran. J. Immunol. 2013, 10, 193–204. [Google Scholar]
- Xu, Z.; Wang, Y.; Han, J.; Xu, Q.; Ren, J.; Xu, J.; Wang, Y.; Chai, Z. Noninvasive multimodal imaging of osteosarcoma and lymph nodes using a 99mTc-labeled biomineralization nanoprobe. Anal. Chem. 2018, 90, 4529–4534. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wang, L.; Yu, J.; Lu, G.; Zhao, W.; Miao, C.; Zou, C.; Wu, J. Overcoming therapeutic failure in osteosarcoma via Apatinib-encapsulated hydrophobic poly (ester amide) nanoparticles. Biomater. Sci. 2020, 8, 5888–5899. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, Y.; Zhang, X. Poloxamer surface modified trimethyl chitosan nanoparticles for the effective delivery of methotrexate in osteosarcoma. Biomed. Pharmacother. 2017, 90, 872–879. [Google Scholar] [CrossRef]
- Irmak, G.; Öztürk, M.G.; Gümüşderelioğlu, M. Salinomycin Encapsulated Plga Nanoparticles Eliminate Osteosarcoma Cells Via Inducing/Inhibiting Multiple Signaling Pathways: Comparison With Free Salinomycin. J. Drug Deliv. Sci. Technol. 2020, 58, 101834. [Google Scholar] [CrossRef]
- Liénard, R.; Montesi, M.; Panseri, S.; Dozio, S.M.; Vento, F.; Mineo, P.G.; Piperno, A.; Winter, J.D.; Coulembier, O.; Scala, A. Design of naturally inspired jellyfish-shaped cyclopolylactides to manage osteosarcoma cancer stem cells fate. Mater. Sci. Eng. C 2020, 117, 111291. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Zhang, L.; Wang, Y.; Wang, S.; Zhang, Y.; Guo, H.; Ji, D.; Wang, Y. Alginate oligosaccharide DP5 exhibits antitumor effects in osteosarcoma patients following surgery. Front. Pharmacol. 2017, 8, 623. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, S.; Jana, S.K.; Pramanik, N. Biomedical Application of Doxorubicin Coated Hydroxyapatite—Poly (lactide-co-glycolide) Nanocomposite for Controlling Osteosarcoma Therapeutics. J. Nanosci. Nanotechnol. 2020, 20, 3994–4004. [Google Scholar] [CrossRef]
- Yuba, E.; Osaki, T.; Ono, M.; Park, S.; Harada, A.; Yamashita, M.; Azuma, K.; Tsuka, T.; Ito, N.; Imagawa, T.; et al. Bleomycin-loaded pH-sensitive polymer–lipid-incorporated liposomes for cancer chemotherapy. Polymers 2018, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- Gazzano, E.; Buondonno, I.; Marengo, A.; Rolando, B.; Chegaev, K.; Kopecka, J.; Saponara, S.; Sorge, M.; Hattinger, C.M.; Gasco, A. Hyaluronated liposomes containing H2S-releasing doxorubicin are effective against P-glycoprotein-positive/doxorubicin-resistant osteosarcoma cells and xenografts. Cancer Lett. 2019, 456, 29–39. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Xu, D.; Ke, X.; Ci, T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromol. 2020, 164, 2583–2597. [Google Scholar] [CrossRef]
- Giansanti, L.; Condello, M.; Altieri, B.; Galantini, L.; Meschini, S.; Mancini, G. Influence of lipid composition on the ability of liposome loaded voacamine to improve the reversion of doxorubicin resistant osteosarcoma cells. Chem. Phys. Lipids 2019, 223, 104781. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S. Advances in tumor targeted liposomes. Curr. Mol. Med. 2018, 18, 44–57. [Google Scholar] [CrossRef]
- Caliskan, Y.; Dalgic, A.D.; Gerekci, S.; Gulec, E.A.; Tezcaner, A.; Ozen, C.; Keskin, D. A new therapeutic combination for osteosarcoma: Gemcitabine and Clofazimine co-loaded liposomal formulation. Int. J. Pharm. 2019, 557, 97–104. [Google Scholar] [CrossRef]
- Gong, T.; Su, X.-T.; Xia, Q.; Wang, J.-G. Biodegradable combinatorial drug loaded pH-sensitive liposomes for enhanced osteosarcoma therapeutics. J. Biomater. Tissue Eng. 2017, 7, 952–961. [Google Scholar] [CrossRef]
- Evans, E.R.; Bugga, P.; Asthana, V.; Drezek, R. Metallic nanoparticles for cancer immunotherapy. Mater. Today 2018, 21, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, E.; Cui, Y.; Huang, Y. Nanotechnology-based combination therapy for overcoming multidrug-resistant cancer. Cancer Biol. Med. 2017, 14, 212. [Google Scholar] [CrossRef] [Green Version]
- Firdhouse, J.M.; Lalitha, P. Biosynthesis of silver nanoparticles and its applications. J. Nanotechnol. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, D.; Paul, A.; Chattopadhyay, A. Photocatalytic polypyrrole-TiO2-nanoparticles composite thin film generated at the air-water interface. Langmuir 2005, 21, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Nair, K.M.; Paul, N.; Koshy, E.P.; Mathew, B. Green synthesized metal nanoparticles as a selective inhibitor of human osteosarcoma and pathogenic microorganisms. Mater. Today Chem. 2019, 13, 128–138. [Google Scholar] [CrossRef]
- Wen, X.Z.; Wang, Q.; Dai, T.; Shao, J.; Wu, X.; Jiang, Z.; Jacob, J.A.; Jiang, C. Identification of possible reductants in the aqueous leaf extract of mangrove plant Rhizophora apiculata for the fabrication and cytotoxicity of silver nanoparticles against human osteosarcoma MG-63 cells. Mater. Sci. Eng. C 2020, 116, 111252. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Das, A.; Raha, S.; Barui, A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J. Photochem. Photobiol. B Biol. 2020, 203, 111778. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-K.; Kim, J.-H. Tangeretin-assisted platinum nanoparticles enhance the apoptotic properties of doxorubicin: Combination therapy for osteosarcoma treatment. Nanomaterials 2019, 9, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, J.-W.; Liu, B.; Liu, W.-D. Folic acid-tagged titanium dioxide nanoparticles for enhanced anticancer effect in osteosarcoma cells. Mater. Sci. Eng. C 2017, 76, 1181–1187. [Google Scholar] [CrossRef]
- Sisubalan, N.; Ramkumar, V.S.; Pugazhendhi, A.; Karthikeyan, C.; Indira, K.; Gopinath, K.; Hameed, A.S.H.; Basha, M.H.G. ROS-mediated cytotoxic activity of ZnO and CeO2 nanoparticles synthesized using the Rubia cordifolia L. leaf extract on MG-63 human osteosarcoma cell lines. Environ. Sci. Pollut. Res. 2018, 25, 10482–10492. [Google Scholar] [CrossRef]
- He, G.; Pan, X.; Liu, X.; Zhu, Y.; Ma, Y.; Du, C.; Liu, X.; Mao, C. HIF-1α-Mediated Mitophagy Determines ZnO Nanoparticle-Induced Human Osteosarcoma Cell Death both In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 48296–48309. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Chi, Y.; Yin, X.; Sun, K.; Feng, S.; Liu, J.; Chen, D.; Guo, C.; Wu, Z. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J. Control. Release 2017, 261, 113–125. [Google Scholar] [CrossRef]
- Feng, S.; Wu, Z.-X.; Zhao, Z.; Liu, J.; Sun, K.; Guo, C.; Wang, H.; Wu, Z. Engineering of bone-and CD44-dual-targeting redox-sensitive liposomes for the treatment of orthotopic osteosarcoma. ACS Appl. Mater. Interfaces 2019, 11, 7357–7368. [Google Scholar]
- Yin, X.; Feng, S.; Chi, Y.; Liu, J.; Sun, K.; Guo, C.; Wu, Z. Estrogen-functionalized liposomes grafted with glutathione-responsive sheddable chotooligosaccharides for the therapy of osteosarcoma. Drug Deliv. 2018, 25, 900–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Chi, Y.; Guo, C.; Feng, S.; Liu, J.; Sun, K.; Wu, Z. Chitooligosaccharides modified reduction-sensitive liposomes: Enhanced cytoplasmic drug delivery and osteosarcomas-tumor inhibition in animal models. Pharm. Res. 2017, 34, 2172–2184. [Google Scholar] [CrossRef]
- Gui, K.; Zhang, X.; Chen, F.; Ge, Z.; Zhang, S.; Qi, X.; Sun, J.; Yu, Z. Lipid-polymer nanoparticles with CD133 aptamers for targeted delivery of all-trans retinoic acid to osteosarcoma initiating cells. Biomed. Pharmacother. 2019, 111, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.-W.; Liao, W.; Ren, Z.-L. Enhanced anticancer effect of copper-loaded chitosan nanoparticles against osteosarcoma. RSC Adv. 2017, 7, 15971–15977. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Xiong, M.; Zhang, X.; Cai, G.; Chen, H.; Zeng, Q.; Yu, Z. Poly (lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. International journal of nanomedicine. Int. J. Nanomed. 2015, 10, 2537. [Google Scholar]
- Zhao, L.; Bi, D.; Qi, X.; Guo, Y.; Yue, F.; Wang, X.; Han, M. Polydopamine-based surface modification of paclitaxel nanoparticles for osteosarcoma targeted therapy. Nanotechnology 2019, 30, 255101. [Google Scholar] [CrossRef]
- Rudnick-Glick, S.; Corem-Salkmon, E.; Grinberg, I.; Margel, S. Targeted drug delivery of near IR fluorescent doxorubicin-conjugated poly(ethylene glycol) bisphosphonate nanoparticles for diagnosis and therapy of primary and metastatic bone cancer in a mouse model. J. Nanobiotechnol. 2016, 14, 80. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Tang, L.; Cai, K.; Tong, R.; Sternberg, R.; Yang, X.; Dobrucki, L.W.; Borst, L.B.; Kamstock, D.; Song, Z. Pamidronate functionalized nanoconjugates for targeted therapy of focal skeletal malignant osteolysis. Proc. Natl. Acad. Sci. USA 2016, 113, E4601–E4609. [Google Scholar] [CrossRef] [Green Version]
- Haghiralsadat, F.; Amoabediny, G.; Sheikhha, M.S.; Doulabi, B.Z.; Naderinezhad, S.; Helder, M.N.; Forouzanfar, T. New liposomal doxorubicin nanoformulation for osteosarcoma: Drug release kinetic study based on thermo and pH sensitivity. Chem. Biol. Drug Des. 2017, 90, 368–379. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Zandieh-Doulabi, B.; Forouzanfar, T.; Helder, M.N. Codelivery of doxorubicin and JIP1 siRNA with novel EphA2-targeted PEGylated cationic nanoliposomes to overcome osteosarcoma multidrug resistance. Int. J. Nanomed. 2018, 13, 3853. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-X.; Guo, C.-L.; Yao, W.-T.; Cai, Q.-Q.; Wang, Y.-S.; Wang, J.-Q. Vitamin E TPGS based liposomal delivery of doxorubicin in osteosarcoma cancer cells. Biomed. Res. 2017, 28. Available online: https://www.biomedres.info/biomedical-research/vitamin-e-tpgs-based-liposomal-delivery-of-doxorubicin-in-osteosarcoma-cancer-cells.html (accessed on 19 February 2021).
- Meyers, P.A. Muramyl Tripeptide-Phosphatidyl Ethanolamine Encapsulated in Liposomes (L-MTP-PE) in the Treatment of Osteosarcoma. In Current Advances in Osteosarcoma; Springer: Berlin/Heidelberg, Germany, 2020; pp. 133–139. [Google Scholar]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz-Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Stepniak, I.I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, H.; Zhou, M.; Li, B.; Liu, L.; Yang, X.; Wen, Y.; Yu, H.; Wang, H.; Chen, J.; Chen, L. Metal-Drug Nanoparticles-Mediated Osteolytic Microenvironment Regulation for Enhanced Radiotherapy of Orthotopic Osteosarcoma. Chem. Eng. J. 2020, 128103. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, B.; Gong, Y.; Chen, R.; Yang, W.; Lin, C.; Chen, M.; Qin, L.; Jia, Y.; Cai, K. Functionalization of Ti substrate with pH-responsive naringin-ZnO nanoparticles for the reconstruction of large bony after osteosarcoma resection. J. Biomed. Mater. Res. Part A 2020, 108, 2190–2205. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Panicker, S.; Abdallah, S.H.; Khan, A.A.; Han, C.; Chehimi, M.M.; Mohamed, A.A. Protein-Coated Aryl Modified Gold Nanoparticles for Cellular Uptake Study by Osteosarcoma Cancer Cells. Langmuir 2020, 36, 11765–11775. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, Q.; Cai, Y.; Zhu, X.; Zhu, C.; Li, Y.; Li, B. Poly (ethylene glycol)-sheddable reduction-sensitive polyurethane micelles for triggered intracellular drug delivery for osteosarcoma treatment. J. Orthop. Transl. 2020, 21, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Wang, Z.; Jiang, Y.; Zhang, T.; Wang, Z.; Hua, Y.; Song, Z.; Liu, J.; Xu, W.; Xu, J.; et al. Reduction-responsive polypeptide nanomedicines significantly inhibit progression of orthotopic osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102085. [Google Scholar] [CrossRef]
- Cheng, Q.; Blais, M.-O.; Harris, G.; Jabbarzadeh, E. PLGA-carbon nanotube conjugates for intercellular delivery of caspase-3 into osteosarcoma cells. PLoS ONE 2013, 8, e81947. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, W.; Shao, Z.; Yang, S.; Liu, X. Triggering of apoptosis in osteosarcoma cells by graphene/single-walled carbon nanotube hybrids via the ROS-mediated mitochondrial pathway. J. Biomed. Mater. Res. Part A 2017, 105, 443–453. [Google Scholar] [CrossRef]
- Gong, M.; Liu, H.; Sun, N.; Xie, Y.; Yan, F.; Cai, L. Polyethylenimine-dextran-coated magnetic nanoparticles loaded with miR-302b suppress osteosarcoma in vitro and in vivo. Nanomedicine 2020, 15, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Lenna, S.; Bellotti, C.; Duchi, S.; Martella, E.; Columbaro, M.; Dozza, B.; Ballestri, M.; Guerrini, A.; Sotgiu, G.; Frisoni, T.; et al. Mesenchymal stromal cells mediated delivery of photoactive nanoparticles inhibits osteosarcoma growth in vitro and in a murine in vivo ectopic model. J. Exp. Clin. Cancer Res. 2020, 39, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Shahabi, S.; Döscher, S.; Bollhorst, T.; Treccani, L.; Maas, M.; Dringen, R.; Rezwan, K. Enhancing cellular uptake and doxorubicin delivery of mesoporous silica nanoparticles via surface functionalization: Effects of serum. ACS Appl. Mater. Interfaces 2015, 7, 26880–26891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Miao, Y.; Ni, W.; Xiao, H.; Zhang, J. Cancer cell membrane coated silica nanoparticles loaded with ICG for tumour specific photothermal therapy of osteosarcoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2298–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.; Bi, J.; Tang, Y.; Qiao, S.-Z. Magnetic core–shell silica nanoparticles with large radial mesopores for siRNA delivery. Small 2016, 12, 4735–4742. [Google Scholar] [CrossRef] [PubMed]
- Son, D.K.; Kim, Y.-J. Anticancer activity of drug-loaded calcium phosphate nanocomposites against human osteosarcoma. Biomater. Res. 2017, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Wu, V.M.; Ghosh, S.; Uskoković, V. Gene delivery using calcium phosphate nanoparticles: Optimization of the transfection process and the effects of citrate and poly (L-lysine) as additives. J. Colloid Interface Sci. 2016, 471, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Hess, U.; Shahabi, S.; Treccani, L.; Streckbein, P.; Heiss, C.; Rezwan, K. Co-delivery of cisplatin and doxorubicin from calcium phosphate beads/matrix scaffolds for osteosarcoma therapy. Mater. Sci. Eng. C 2017, 77, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Boga, C.; Micheletti, G.; Cassani, M.C.; Fini, M.; Bigi, A. (9R)-9-Hydroxystearate-Functionalized Hydroxyapatite as Antiproliferative and Cytotoxic Agent toward Osteosarcoma Cells. Langmuir 2016, 32, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In vitro and in vivo mechanism of bone tumor inhibition by selenium-doped bone mineral nanoparticles. ACS Nano 2016, 10, 9927–9937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.M.; Mickens, J.; Uskoković, V. Bisphosphonate-functionalized hydroxyapatite nanoparticles for the delivery of the bromodomain inhibitor JQ1 in the treatment of osteosarcoma. ACS Appl. Mater. Interfaces 2017, 9, 25887–25904. [Google Scholar] [CrossRef]
- Han, X.G.; Yang, S.B.; Mo, H.M.; Wang, M.Q.; Zhou, F.; Li, H.J.; Qiao, H.; Mei, J.T.; Wang, Y.J.; Cheng, Y.W.; et al. Targeting of CXCR1 on Osteosarcoma Circulating Tumor Cells and Precise Treatment via Cisplatin Nanodelivery. Adv. Funct. Mater. 2019, 29, 1902246. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, T.; Yu, Y.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Shen, Y.; et al. Dual targeting curcumin loaded alendronate-hyaluronan-octadecanoic acid micelles for improving osteosarcoma therapy. Int. J. Nanomed. 2019, 14, 6425. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Ye, M.; Zhu, J.; Wang, Y.; Liang, C.; Tang, J.; Tao, H.; Shen, Y. Zinc phthalocyanine encapsulated in polymer micelles as a potent photosensitizer for the photodynamic therapy of osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Sun, Y.; Xiao, H.; Li, P.; Liu, M.; Ding, F.; Kan, W.; Miao, R. Targeted osteosarcoma chemotherapy using RGD peptide-installed doxorubicin-loaded biodegradable polymeric micelle. Biomed. Pharmacother. 2017, 85, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Low, S.A.; Yang, J.; Kopeček, J.I. Bone-targeted acid-sensitive doxorubicin conjugate micelles as potential osteosarcoma therapeutics. Bioconjugate Chem. 2014, 25, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Noy, J.-M.; Lu, H.; Hogg, P.J.; Yang, J.-L.; Stenzel, M. Direct Polymerization of the Arsenic Drug PENAO to Obtain Nanoparticles with High Thiol-Reactivity and Anti-Cancer Efficiency. Bioconjugate Chem. 2018, 29, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, C.; Song, Z.; Reyes-Vargas, E.; Clayton, F.; Huang, J.; Jensen, P.; Chen, X. Association of anti-HER2 antibody with graphene oxide for curative treatment of osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Saravanabhavan, S.S.; Rethinasabapathy, M.; Zsolt, S.; Kalambettu, A.B.; Elumalai, S.; Janakiraman, M.; Huh, Y.S.; Natesan, B. Graphene oxide functionalized with chitosan based nanoparticles as a carrier of siRNA in regulating Bcl-2 expression on Saos-2 & MG-63 cancer cells and its inflammatory response on bone marrow derived cells from mice. Mater. Sci. Eng. C 2019, 99, 1459–1468. [Google Scholar]
- Niu, G.; Yousefi, B.; Qujeq, D.; Marjani, A.; Asadi, J.; Wang, Z.; Mir, S.M. Melatonin and doxorubicin co-delivered via a functionalized graphene-dendrimeric system enhances apoptosis of osteosarcoma cells. Mater. Sci. Eng. C 2020, 119, 111554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, Q.; Tang, H.; Xie, Q.; Ma, M.; Tan, L.; Zhang, Y.; Yao, S. Characterization of and biomolecule immobilization on the biocompatible multi-walled carbon nanotubes generated by functionalization with polyamidoamine dendrimers. Colloids Surf. B Biointerfaces 2010, 80, 18–25. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Shao, J.; Hu, R.; Chen, H.; Xie, P.; Liu, C. Angiotensin II promotes the anticoagulant effects of rivaroxaban via angiotensin type 2 receptor signaling in mice. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, N.A.; Hartgerink, J.D. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Reithofer, M.R.; Chan, K.-H.; Lakshmanan, A.; Lam, D.H.; Mishra, A.; Gopalan, B.; Joshi, M.; Wang, S.; Hauser, C.A.E. Ligation of anti-cancer drugs to self-assembling ultrashort peptides by click chemistry for localized therapy. Chem. Sci. 2014, 5, 625–630. [Google Scholar] [CrossRef]





| Nanomaterials | Composition | Loaded Moiety | Outcomes Reported | References |
|---|---|---|---|---|
| Polymeric NPs | PLGA with surface CD133 aptamers | Salinomycin | Targeted CD133+ OSA cell and reduced the progression of osteosarcoma by enhanced infiltration in the cells | [127] |
| Polymeric NPs | Polydopamine, alendronate | Paclitaxel | Increased accumulation in the tumor cells as compared to other tissues | [128] |
| Polymeric NPs | PEG-bisphosphonate | Doxorubicin | Internalization by the cancer cells and suppression of tumor growth by cytotoxic effect | [129] |
| Polymeric NPs | Polylactide coated with pamidronate | Doxorubicin | Malignant bone targeted drug delivery with no cardiac and hematological toxicity, significant anti-tumor activity | [130] |
| Liposomes | DSPE-mPEG * | Doxorubicin | Thermo and pH sensitive release of drug in the OSA cells | [131] |
| Liposomes | DSPE-mPEG, cholestrol | Doxorubicin and SiRNA | Dual targeting of the OSA cells surface EphA2 receptors and intracellular JIP1 protein, increased nuclear localization of the liposomes | [132] |
| Liposomes | TPGS **, phosphatidylcholine, DSPE | Doxorubicin and vitamin E | Concentration dependent toxicity in the OSA cells and high apoptosis | [133] |
| Liposomes | phosphatidyl ethanolamine | Muramyl tripeptide | Stimulated macrophages to destroy the OSA tumor cells | [134] |
| Gold NPs | Tannic acid, HAuCl4 | --- | Increased expression of proapoptotic protein Bax in the OSA cells and decreased expression of anti-apoptotic protein Bcl-2 | [135] |
| Metallic NPs | Self-assembly of ferric ions with hyaluronic acid anchorage | Zoledronate | Inhibition of osteoclast activity, generated free radicals killed the OSA cells | [136] |
| Zinc oxide NPs | Titanium substrate and zinc acetate | Naringin | Reconstruction of large bony defects in OSA, leakage of bacterial RNA and DNA after the accumulation of ROS in the cells | [137] |
| Gold-aryl NPs | C6H4-4-COOH linkage in gold | Bovine serum albumin | Internalization in the OSA cells | [138] |
| Mesoporous silica NPs | Poly acrylic acid, lectin | Doxorubicin | 8-folds higher cytotoxicity than free drug | [139] |
| Micelles | PEG, polyurethane | Doxorubicin | Significant antitumor activity against Saos-2 cells | [140] |
| Micelles | Polypeptide (methoxy poly(ethylene glycol)-block-poly(S-tert-butylmercapto-L-cysteine) copolymers) | Doxorubicin | Decreased accumulation in the heart and increased accumulation in the OSA cells, inhibition of metastasis | [141] |
| Nanotube | PLGA | Caspase-3 | Suppress proliferation of OSA cells | [142] |
| Single walled carbon nanotube | graphene | -- | ROS mediated cell killing | [143] |
| Magnetic NPs | Polyethylenimine, dextran, iron oxide | miR-302b | Magnetic field delivered the NPs to the OSA cells and demonstrated cytotoxic effect | [144] |
| Photoactive mesenchymal stromal cells loaded with NPs | poly-methyl methacrylate | Human osteosarcoma MG-63 cells | Photodynamic therapy to kill OSA cells | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barani, M.; Mukhtar, M.; Rahdar, A.; Sargazi, S.; Pandey, S.; Kang, M. Recent Advances in Nanotechnology-Based Diagnosis and Treatments of Human Osteosarcoma. Biosensors 2021, 11, 55. https://doi.org/10.3390/bios11020055
Barani M, Mukhtar M, Rahdar A, Sargazi S, Pandey S, Kang M. Recent Advances in Nanotechnology-Based Diagnosis and Treatments of Human Osteosarcoma. Biosensors. 2021; 11(2):55. https://doi.org/10.3390/bios11020055
Chicago/Turabian StyleBarani, Mahmood, Mahwash Mukhtar, Abbas Rahdar, Saman Sargazi, Sadanand Pandey, and Misook Kang. 2021. "Recent Advances in Nanotechnology-Based Diagnosis and Treatments of Human Osteosarcoma" Biosensors 11, no. 2: 55. https://doi.org/10.3390/bios11020055
APA StyleBarani, M., Mukhtar, M., Rahdar, A., Sargazi, S., Pandey, S., & Kang, M. (2021). Recent Advances in Nanotechnology-Based Diagnosis and Treatments of Human Osteosarcoma. Biosensors, 11(2), 55. https://doi.org/10.3390/bios11020055