Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat
Abstract
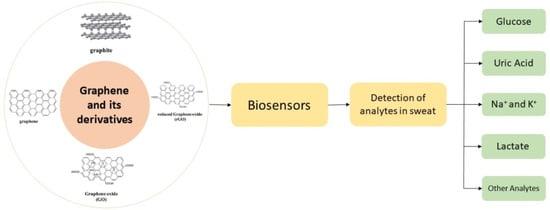
:1. Introduction
2. Techniques for the Production of Graphene
2.1. Top-Down Methods
2.1.1. Oxidative Exfoliation Reduction
2.1.2. Arc Discharge Method
2.1.3. Liquid Exfoliation
2.1.4. Un-Zipping CNTs
2.1.5. Mechanical Exfoliation
2.2. Bottom-Up Methods
2.2.1. Chemical Vapour Deposition
2.2.2. Epitaxial Growth on SiC

2.2.3. Pyrolysis
2.2.4. Substrate-Free Gas-Phase (SFGP)
2.2.5. Total Organic Synthesis
2.2.6. Template Route
3. Graphene Derivatives (GPDs)
3.1. Graphene Oxide
3.2. Reduced Graphene Oxide
3.3. Graphene Nanoribbons
3.4. Graphene Nano-Walls
3.5. Graphene Quantum Dots (GQDS)
4. Defects in Graphene
4.1. Intrinsic Defects
4.1.1. Single Vacancy Defects
4.1.2. Multiple Vacancy Defects
4.1.3. Line Defects
4.1.4. Out-of-Plane Carbon Adatoms
4.2. Extrinsic Defects
4.2.1. Foreign Adatoms
4.2.2. Substitutional Impurities
4.3. Double Graphene Structure Defects
5. Properties of Graphene Materials
5.1. Mechanical Properties
5.2. Electrical Properties
5.3. Thermal Properties
5.4. Non-Toxic Nature
6. Biosensors for Sweat Analysis
6.1. Sweat Chemistry
6.2. Features of a Good Biosensor
6.2.1. Substitutional Impurities
6.2.2. Sensitivity
6.2.3. Flexibility and Mechanical Strength
6.2.4. Self-Healing Ability
6.2.5. Self-Cleaning Ability
6.2.6. Optical Transparency
6.2.7. Ability to Power Itself
6.2.8. Interfacing and Biocompatibility with Skin
6.3. Graphene and Its Derivatives for Biosensors
- (1)
- Splendidly large surface area: For single-layer graphene, the predicted surface area is 2630 m2/g, leading to a high density of bound recognition components or analyte molecules. This aids in the downsizing and high detection sensitivity of the apparatus.
- (2)
- Exceptional electrical characteristics and electron transport abilities: Graphene’s sp2-hybridized carbon atoms create a massive free-flowing electron conjugate system. Graphene is an appealing material for electrochemical sensing because of its unique characteristics.
- (3)
- Extreme mechanical toughness and malleability: Graphene, a two-dimensional material with a thickness of only 0.335 nm, has a hardness greater than diamond because of its strong C=C bonding in the atomic plane. However, unlike diamond, the interlayer bonding of graphene is weak owing to the action of Van der Waals forces. As a result, this is a huge step forward for the evolution of portable sensors that may be worn on the body.
6.4. Wearable Biosensors
Wearable Biochemical Sensors for Sweat Analysis
7. Wearable Sweat Sensor Technology
7.1. Colorimetric Sweat Sensors
7.2. Electrochemical Sweat Sensors
7.3. Hybrid Sweat Sensors
8. Detection of Analytes from Sweat Using Graphene
8.1. Glucose
Glucose-Sensing Mechanisms
- The mutarotation of glucose
- Chemisorption model
- IHOAM model
- Graphene-based electrochemical sensing of glucose
8.2. Lactate
8.3. Uric Acid
8.4. Detection of K+ and Na+
8.5. Other Analytes
9. Challenges
10. Future Outlook
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaupel, J.W. Biodemography of human ageing. Nature 2010, 464, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Takei, K.; Honda, W.; Harada, S.; Arie, T.; Akita, S. Toward flexible and wearable human-interactive health-monitoring devices. Adv. Healthc. Mater. 2015, 4, 487–500. [Google Scholar] [CrossRef]
- Pantelopoulos, A.; Bourbakis, N.G. Prognosis—A wearable health-monitoring system for people at risk: Methodology and modeling. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Murray, S.S.; Levy, S.; Venter, J.C. An agenda for personalized medicine. Nature 2009, 461, 724–726. [Google Scholar] [CrossRef]
- Narayan, R.J.; Verma, N. Nanomaterials as implantable sensors bt-materials for chemical sensing. In Materials for Chemical Sensing; Paixao, T.R.L.C., Reddy, S.M., Eds.; Springer International Publishing: Midtown Manhattan, NY, USA, 2016; pp. 123–139. [Google Scholar]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and miniaturized sensor technologies for personalized and preventive medicine. Adv. Funct. Mater. 2017, 27, 160527. [Google Scholar] [CrossRef]
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-enabled wearable sensors for healthcare. Adv. Healthc. Mater. 2017, 7, 1700889. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhang, T. Flexible sensing electronics for wearable/attachable health monitoring. Adv. Sci. 2017, 13, 1602790. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Peng, R.; Fan, Z.; Lin, Y. Self-powered and wearable biosensors for healthcare. Mater. Today Energy 2022, 23, 100900. [Google Scholar] [CrossRef]
- Murdoch, T.B.; Detsky, A.S. The inevitable application of big data to health care. JAMA 2013, 309, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.W.; Saria, S.; Ohno-Machado, L.; Shah, A.; Escobar, G. Big data in health care: Using analytics to identify and manage high-risk and high-cost patients. Health Aff. 2014, 33, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, W.; Raghupathi, V. Big data analytics in healthcare: Promise and potential. Health Inf. Sci. Syst. 2014, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Ravì, D.; Wong, C.; Deligianni, F.; Berthelot, M.; Andreu-Perez, J.; Lo, B.; Yang, G.Z. Deep learning for health informatics. IEEE J. Biomed. Health Inform. 2017, 21, 4–21. [Google Scholar]
- Banaee, H.; Ahmed, M.; Loutfi, A. Data mining for wearable sensors in health monitoring systems: A review of recent trends and challenges. Sensors 2013, 13, 17472–17500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Weiss, N.O.; Zhou, H.; Liao, L.; Liu, Y.; Jiang, S.; Huang, Y.; Duan, X. Graphene: An emerging electronic material. Adv. Mater. 2012, 24, 5782–5825. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Yang, H.; Xue, T.; Li, F.; Liu, W.; Song, Y. Graphene: Diversified flexible 2D material for wearable vital signs monitoring. Adv. Mater. Technol. 2018, 4, 1800574. [Google Scholar] [CrossRef]
- Wang, T.H.; Li, Z.; Liang, B.; Cai, Y.; Wang, Z.; Yang, C.; Luo, Y.; Sun, J.; Ye, X.; Chen, Y.; et al. A Power-Harvesting CGM Chiplet Featuring Silicon-Based Enzymatic Glucose Sensor. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 4626–4630. [Google Scholar]
- Ameri, S.K.; Singh, P.K.; D’Angelo, R.; Stoppel, W.; Black, L.; Sonkusale, S.R. Three dimensional graphene scaffold for cardiac tissue engineering and in-situ electrical recording. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4201–4203. [Google Scholar]
- Park, M.V.D.Z.; Bleeker, E.A.J.; Brand, W.; Cassee, F.R.; van Elk, M.; Gosens, I.; de Jong, W.H.; Meesters, J.A.J.; Peijnenburg, W.J.G.M.; Quik, J.T.K.; et al. Considerations for safe innovation: The case of graphene. ACS Nano 2017, 11, 9574–9593. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kim, H.K.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, Y.; Tao, T.H. Recent Progress in Bio-Integrated Intelligent Sensing System. Adv. Intell. Syst. 2022, 2100280. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Song, C.; Cho, H.R.; Ghaffari, R.; Choi, T.K.; Kim, K.H.; Lee, Y.B.; Ling, D.; Lee, H.; et al. An endoscope with integrated transparent bioelectronics and theranostic nanoparticles for colon cancer treatment. Nat. Commun. 2015, 6, 10059. [Google Scholar] [CrossRef] [Green Version]
- Lo, L.W.; Zhao, J.; Aono, K.; Li, W.; Wen, Z.; Pizzella, S.; Wang, Y.; Chakrabartty, S.; Wang, C. Stretchable Sponge Electrodes for Long-Term and Motion-Artifact-Tolerant Recording of High-Quality Electrophysiologic Signals. ACS Nano 2022, 16, 11792–11801. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Ahsan, W.; Mangla, B.; Javed, S.; Hassan, M.Z.; Asmari, M.; Al Bratty, M.; Najmi, A. Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements. Nanotechnol. Rev. 2022, 11, 96–116. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon N. Y. 2010, 48, 2127–2150. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Pascal, T.A.; Kim, H.; Goddard, W.A., III; Greer, J.R. Electronic-mechanical coupling in graphene from in situ nanoindentation experiments and multiscale atomistic simulations. Nano Lett. 2011, 11, 1241–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Rudrapati, R. Graphene: Fabrication Methods, Properties, and Applications in Modern Industries. In Graphene Production and Application; Ameen, S., Akhtar, M.S., Shin, H., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Nair, A.S.; Nallusamy, V.; Jayasankar, K.; Ss, S. Scalable preparation of graphene from graphite ore via mechano-chemical ball milling. Mater. Manuf. Processes 2022, 37, 113–122. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene ox-ide based on improved hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilisik, K.; Akter, M. Graphene nanoplatelets/epoxy nanocomposites: A review on functionalization, characterization techniques, properties, and applications. J. Reinf. Plast. Compos. 2022, 41, 99–129. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based mate-rials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cruz, A.; Ruiz-Hernández, A.R.; Vega-Clemente, J.F.; Luna-Gazcón, D.G.; Campos-Delgado, J. A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J. Mater. Sci. 2022, 1–36. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Jankovsky, O.; Hrdlickova Kuckova, S.; Pumera, M.; Simek, P.; Sedmidubsky, D.; Sofer, Z. Carbon fragments are ripped offfrom graphite oxide sheets during their thermal reduction. New J. Chem. 2014, 38, 5700–5705. [Google Scholar] [CrossRef]
- Somanathan, T.; Prasad, K.; Ostrikov, K.K.; Saravanan, A.; Krishna, V.M. Graphene oxide synthesis from agro waste. Nanomater. 2015, 5, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Tian, Z.; Simon, G.P.; Li, D. Scalable production of graphene via wet chemistry: Progress and challenges. Mater. Today 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Yoon, G.; Seo, D.-H.; Ku, K.; Kim, J.; Jeon, S.; Kang, K. Factors affecting the exfolia-tion of graphite intercalation compounds for graphene synthesis. Chem. Mater. 2015, 27, 2067–2073. [Google Scholar] [CrossRef]
- Khan, M.; Shakoor, A.; Tiaz Khan, G.; Sultana, S.; Zia, A. A study of stable graphene oxide dispersions in various solvents. J. Chem. Soc. Pak. 2015, 37, 62–67. [Google Scholar]
- Guo, S.; Garaj, S.; Bianco, A.; Ménard-Moyon, C. Controlling covalent chemistry on graphene oxide. Nat. Rev. Phys. 2022, 4, 247–262. [Google Scholar] [CrossRef]
- Chen, W.T.; Muruganantham, R.; Liu, W.R. Construction of 3D porous graphene aerogel wrapped silicon composite as anode materials for high-efficient lithium-ion storage. Surf. Coat. Technol. 2022, 434, 128147. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Li, H.; Lu, J.; Fu, Q.; Chu, Y. Graphene nanosheets synthesis via chemical reduction of graphene oxide using sodium acetate trihydrate solu-tion. Synth. Metal. 2014, 193, 132–138. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Strom, V.; Far-Ris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene ox-ide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [Green Version]
- Bo, Z.; Shuai, X.; Mao, S.; Yang, H.; Qian, J.; Chen, J.; Yan, J.; Cen, K. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 2014, 4, 4684. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yan, L.; Bangal, P.R. Chemical reduction of graphene oxide to graphene by sulfur-containing compounds. J. Phys. Chem. C 2010, 114, 19885–19890. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Murali, S.; Zhu, Y.; Ruoff, R.S.; Bielawski, C.W. Reduction of graphite oxide using alcohols. J. Mater. Chem. 2011, 21, 3443–3447. [Google Scholar] [CrossRef]
- Mei, X.; Ouyang, J. Ultrasonication-assisted ultrafast reduction of graphene ox-ide by zinc powder at room temperature. Carbon 2011, 49, 5389–5397. [Google Scholar] [CrossRef]
- Khanra, P.; Kuila, T.; Kim, N.H.; Bae, S.H.; Yu, D.-S.; Lee, J.H. Simultaneous bio-func-tionalization and reduction of graphene oxide by baker’s yeast. Chem. Eng. J. 2012, 183, 526–533. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bardhan, N.M.; Chen, G.-Y.; Li, Z.; Belcher, A.M.; Grossman, J.C. New in-sights into the thermal reduction of graphene oxide: Impact of oxygen clus-tering. Carbon 2016, 100, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Peng, Y.; Yang, Y.; Chen, J.; Tian, H.; Cui, X.; Zheng, W. Hydrothermal re-duction of graphene oxide; effect on surface-enhanced Raman scattering. J. Raman Spectrosc. 2016, 48, 97–103. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L. Preparation of graphene by a low-temperature thermal reduc-tion at atmosphere pressure. Nanoscale 2010, 2, 559–563. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Moussa, S.; Hassan, H.M.; Aluri, H.S.; Collinson, M.M.; El-Shall, M.S. Photothermal deoxygenation of graphite oxide with laser excitation in so-lution and graphene-aided increase in water temperature. J. Phys. Chem. Lett. 2010, 1, 2804–2809. [Google Scholar]
- Yun, Y.S.; Yoon, G.; Park, M.; Cho, S.Y.; Lim, H.-D.; Kim, H.; Park, Y.W.; Kim, B.H.; Kang, K. Restoration of thermally reduced graphene oxide by atomic-level selenium doping. NPG Asia Mater. 2016, 8, e338. [Google Scholar] [CrossRef] [Green Version]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.H.D.R.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- El-khatib, A.M.; Bondouk, I.I.; Omar, K.M.; Hamdy, A.; El-khatib, M. Impact of changing electrodes dimensions and different ACs on the characteristics of nano composites NZnO/MWCNTs prepared by the arc discharge method. Surf. Interfaces 2022, 29, 101736. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Shi, Z.; Gu, Z. Synthesis, isolation and spectroscopic characterization of Yb-containing high metallofullerenes. Chem. Phys. Lett. 2005, 409, 192–196. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Large scale synthesis of N-doped multi-layered graphene sheets by simple arc-discharge method. Carbon 2010, 48, 255–259. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Subrahmanyam, K.S.; Matte, H.R.; Abdulhakeem, B.; Govindaraj, A.; Das, B.; Kumar, P.; Ghosh, A.; Late, D.J. A study of the synthetic methods and properties of graphenes. Sci. Technol. Adv. Mater. 2010, 11, 054502. [Google Scholar] [CrossRef] [PubMed]
- Gacem, A.; Modi, S.; Yadav, V.K.; Islam, S.; Patel, A.; Dawane, V.; Jameel, M.; Inwati, G.K.; Piplode, S.; Solanki, V.S.; et al. Recent Advances in Methods for Synthesis of Carbon Nanotubes and Carbon Nanocomposite and their Emerging Applications: A Descriptive Review. J. Nanomater. 2022, 2022, 7238602. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Synthesis of single-wall carbon nanohorns by arc-discharge in air and their formation mechanism. Carbon 2010, 48, 1580–1585. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Sheng, L.; Yu, L.; An, K.; Xu, J.; Ando, Y.; Zhao, X. Mass-production of highly-crystalline few-layer graphene sheets by arc discharge in various H2-inert gas mixtures. Chem. Phys. Lett. 2012, 538, 72–76. [Google Scholar] [CrossRef]
- Mustafa, L.; Yenny, H.; Paul, J.K.; Ronan, J.S.; Valeria, N.; Lisa, S.K.; Fiona, M.B.; Sukanta, D.; Zhiming, W.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K. Liquid-phase exfoliation of graphite towards solubilized graphenes. Small 2009, 5, 1841–1845. [Google Scholar] [CrossRef]
- Keeley, G.P.; O’Neill, A.; McEvoy, N.; Peltekis, N.; Coleman, J.N.; Duesberg, G.S. Electrochemical ascorbic acid sensor based on DMF-exfoliated graphene. J. Mater. Chem. 2010, 20, 7864–7869. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.N. Liquid Exfoliation of Defect-Free Graphene. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, W.; Fang, D.; Wang, T.; Huang, J.; Li, Q.; Jiang, M.; Liu, L.; Li, Q.; Dong, L.; et al. A binary solvent system for improved liquid phase exfoliation of pristine graphene materials. Carbon 2015, 94, 405–411. [Google Scholar] [CrossRef]
- Cano-Marquez, A.G.; Rodriguez-Macias, F.J.; Campos-Delgado, J.; Espinosa-González, C.G.; Tristán-López, F.; Ramírez-González, D.; Cullen, D.A.; Smith, D.J.; Terrones, M.; Vega-Cantú, Y.I. Ex-MWNTs: Graphene sheets and ribbons produced by lithium intercalation and exfoliation of carbon nanotubes. Nano Lett. 2009, 9, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, M.; Alam, S.; Uddin, M.; Islam, M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Small, J.P.; Pontius, W.V.; Kim, P. Fabrication and electric-field-dependent transport measurements of mesoscopic graphite devices. Appl. Phys. Lett. 2005, 86, 073104. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.K.; Chakraborty, B. Understanding Graphene via Raman Scattering; Rao, C.N.R., Sood, A.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hiura, H.; Ebbesen, T.W.; Fujita, J.; Tanigaki, K.; Takada, T. Role of sp3 defect structures in graphite and carbon nanotubes. Nature 1994, 367, 148–151. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Huang, H.; Ruoff, R.S. Tailoring graphite with the goal of achieving single sheets. Nanotechnology 1999, 10, 269. [Google Scholar] [CrossRef]
- Bernhardt, T.M.; Kaiser, B.; Rademann, K. Formation of superperiodic patterns on highly oriented pyrolytic graphite by manipulation of nanosized graphite sheets with the STM tip. Surf. Sci. 1998, 408, 86–94. [Google Scholar] [CrossRef]
- Liang, X.; Chang, A.S.; Zhang, Y.; Harteneck, B.D.; Choo, H.; Olynick, D.L.; Cabrini, S. Electrostatic force assisted exfoliation of prepatterned few-layer graphenes into device sites. Nano Lett. 2009, 9, 467–472. [Google Scholar] [CrossRef]
- Ci, L.; Song, L.; Jariwala, D.; Elias, A.L.; Gao, W.; Terrones, M.; Ajayan, P.M. Graphene shape control by multistage cutting and transfer. Adv. Mater. 2009, 21, 4487–4491. [Google Scholar] [CrossRef]
- Chen, J.H.; Ishigami, M.; Jang, C.; Hines, D.R.; Fuhrer, M.S.; Williams, E.D. Printed graphene circuits. Adv. Mater. 2007, 19, 3623–3627. [Google Scholar] [CrossRef] [Green Version]
- Dathbun, A.; Chaisitsak, S. Effects of three parameters on graphene synthesis by chemical vapor deposition. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1018–1021. [Google Scholar]
- Reinke, M.; Kuzminykh, Y.; Hoffmann, P. Low temperature chemical vapor deposition using atomic layer deposition chemistry. Chem. Mater. 2015, 27, 1604–1611. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Chae, S.J.; Günes, F.; Kim, K.K.; Kim, E.S.; Han, G.H.; Kim, S.M.; Shin, H.J.; Yoon, S.M.; Choi, J.Y.; Park, M.H.; et al. August. Synthesis of large-area graphene layers on nickel film by chemical vapor deposition: Wrinkle formation. In Carbon Nanotubes, Graphene, and Associated Devices II; SPIE: Washington, DC, USA, 2009; Volume 7399, pp. 95–105. [Google Scholar]
- Lin, M.Y.; Guo, W.C.; Wu, M.H.; Wang, P.Y.; Liu, T.H.; Pao, C.W.; Chang, C.C.; Lee, S.C.; Lin, S.Y. Low-temperature grown graphene films by using molecular beam epitaxy. Appl. Phys. Lett. 2012, 101, 221911. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Z.; Li, X.; Zhu, H. Synthesis of two dimensional materials on extremely clean surfaces. Nano Today 2018, 22, 7–9. [Google Scholar] [CrossRef]
- Li, Z.; Wu, P.; Wang, C.; Fan, X.; Zhang, W.; Zhai, X.; Zeng, C.; Li, Z.; Yang, J.; Hou, J. Low-temperature growth of graphene by chemical vapor deposition using solid and liquid carbon sources. ACS Nano 2011, 5, 3385–3390. [Google Scholar] [CrossRef]
- Giorgi, R.; Dikonimos, T.; Falconieri, M.; Gagliardi, S.; Lisi, N.; Morales, P.; Pilloni, L.; Salernitano, E. Synthesis of graphene films on copper substrates by CVD of different precursors. In GraphITA 2011; Springer: Berlin/Heidelberg, Germany, 2012; pp. 109–118. [Google Scholar]
- Rümmeli, M.H.; Bachmatiuk, A.; Dianat, A.; Scott, A.; Börrnert, F.; Ibrahim, I.; Zhang, S.; Borowiak-Palen, E.; Cuniberti, G.; Büchner, B. Low temperature CVD growth of graphene nano-flakes directly on high K dielectrics. MRS Online Proc. Libr. (OPL) 2011, 1284, 19–24. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, W.W.; Chai, S.P.; Mohamed, A.R.; Aziz, A.; Khe, C.S.; Hidayah, N.M.; Hashim, U. Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv. 2017, 7, 15644–15693. [Google Scholar] [CrossRef]
- Volotskova, O.; Levchenko, I.; Shashurin, A.; Raitses, Y.; Ostrikov, K.; Keidar, M. Single-step synthesis and magnetic separation of graphene and carbon nanotubes in arc discharge plasmas. Nanoscale 2010, 2, 2281–2285. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.H.; Chen, S.H.; Lin, W.T.; Li, M.C.; Lin, Y.C.; Kuo, C.C. Low-temperature synthesis of graphene on Cu using plasma-assisted thermal chemical vapor deposition. Nanoscale Res. Lett. 2013, 8, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudaya, C.; Ahn, M.; Oh, S.H.; Jeon, B.J.; Sung, Y.E.; Lee, J.K. Simultaneous etching and transfer—Free multilayer graphene sheets derived from C60 thin films. J. Ind. Eng. Chem. 2018, 64, 70–75. [Google Scholar] [CrossRef]
- Agudosi, E.S.; Abdullah, E.C.; Numan, A.; Mubarak, N.M.; Khalid, M.; Omar, N. A review of the graphene synthesis routes and its applications in electrochemical energy storage. Crit. Rev. Solid State Mater. Sci. 2020, 45, 339–377. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Addou, R.; Dahal, A.; Sutter, P.; Batzill, M. Monolayer graphene growth on Ni (111) by low temperature chemical vapor deposition. Appl. Phys. Lett. 2012, 100, 021601. [Google Scholar] [CrossRef]
- Gallo, E.M.; Willner, B.I.; Hwang, J.; Sun, S.; Spencer, M.; Salagaj, T.; Mitchel, W.C.; Sbrockey, N.; Tompa, G.S. Chemical vapor deposition of graphene on copper at reduced temperatures. In Carbon Nanotubes, Graphene, and Associated Devices V; SPIE: Washington, DC, USA, 2012; Volume 8462, pp. 9–15. [Google Scholar]
- Lee, E.; Lee, H.C.; Jo, S.B.; Lee, H.; Lee, N.S.; Park, C.G.; Lee, S.K.; Kim, H.H.; Bong, H.; Cho, K. Heterogeneous Solid Carbon Source-Assisted Growth of High-Quality Graphene via CVD at Low Temperatures. Adv. Funct. Mater. 2016, 26, 562–568. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Guermoune, A.; Chari, T.; Popescu, F.; Sabri, S.S.; Guillemette, J.; Skulason, H.S.; Szkopek, T.; Siaj, M. Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 2011, 49, 4204–4210. [Google Scholar] [CrossRef]
- Unarunotai, S.; Murata, Y.; Chialvo, C.E.; Kim, H.S.; MacLaren, S.; Mason, N.; Petrov, I.; Rogers, J.A. Transfer of graphene layers grown on SiC wafers to other substrates and their integration into field effect transistors. Appl. Phys. Lett. 2009, 95, 202101. [Google Scholar] [CrossRef] [Green Version]
- Forbeaux, I.; Themlin, J.M.; Debever, J.M. Heteroepitaxial graphite on 6 H-SiC (0001): Interface formation through conduction-band electronic structure. Phys. Rev. B 1998, 58, 16396–16406. [Google Scholar] [CrossRef]
- Mañes, J.L.; Guinea, F.; Vozmediano, M.A.H. Existence and topological stability of Fermi points in multilayered graphene. Phys. Rev. B 2007, 75, 155424. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Ihm, J.; Choi, H.J.; Son, Y.-W. Origin of Anomalous Electronic Structures of Epitaxial Graphene on Silicon Carbide. Phys. Rev. Lett. 2008, 100, 176802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.X.; Hoh, H.Y.; Ang, P.K.; Loh, K.P. Direct Voltammetric Detection of DNA and pH Sensing on Epitaxial Graphene: An Insight into the Role of Oxygenated Defects. Anal. Chem. 2010, 82, 7387–7393. [Google Scholar] [CrossRef] [PubMed]
- Kruskopf, M.; Pakdehi, D.M.; Pierz, K.; Wundrack, S.; Stosch, R.; Dziomba, T.; Götz, M.; Baringhaus, J.; Aprojanz, J.; Tegenkamp, C.; et al. Comeback of epitaxial graphene for electronics: Large-area growth of bilayer-free graphene on SiC. 2D Mater. 2016, 3, 041002. [Google Scholar] [CrossRef]
- Momeni Pakdehi, D.; Aprojanz, J.; Sinterhauf, A.; Pierz, K.; Kruskopf, M.; Willke, P.; Baringhaus, J.; Stöckmann, J.P.; Traeger, G.A.; Hohls, F.; et al. Minimum resistance anisotropy of epitaxial graphene on SiC. ACS Appl. Mater. Interfaces 2018, 10, 6039–6045. [Google Scholar] [CrossRef] [PubMed]
- Norimatsu, W.; Kusunoki, M. Epitaxial graphene on SiC {0001}: Advances and perspectives. Phys. Chem. Chem. Phys. 2014, 16, 3501–3511. [Google Scholar] [CrossRef]
- Dean, C.R.; Young, A.F.; Meric, I.; Lee, C.; Wang, L.; Sorgenfrei, S.; Watanabe, K.; Taniguchi, T.; Kim, P.; Shepard, K.L.; et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 2010, 5, 722–726. [Google Scholar] [CrossRef]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.Z.; Hwang, C.G.; Jozwiak, C.M.; Köhl, A.; Schmid, A.K.; Lanzara, A. New synthesis method for the growth of epitaxial graphene. J. Electron Spectrosc. Relat. Phenom. 2011, 184, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Amirov, R.K.; Atamanuk, I.N.; Vorobieva, N.A.; Isakaev, E.H.; Shavelkina, M.B.; Shkolnikov, E.I. Synthesis of graphene-like materials by pyrolysis of hydrocarbons in thermal plasma and their properties. J. Phys. Conf. Ser. 2015, 653, 012161. [Google Scholar]
- Choucair, M.; Thordarson, P.; Stride, J.A. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 2009, 4, 30–33. [Google Scholar] [CrossRef]
- Dato, A.; Radmilovic, V.; Lee, Z.; Phillips, J.; Frenklach, M. Substrate-free gas-phase synthesis of graphene sheets. Nano Lett. 2008, 8, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple method of preparing graphene flakes by an arc-discharge method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Dato, A.; Frenklach, M. Substrate-free microwave synthesis of graphene: Experimental conditions and hydrocarbon precursors. New J. Phys. 2010, 12, 125013. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J. Graphene: Synthesis and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zheng, Q.; Kim, J.-K. Synthesis, Structure, and Properties of Graphene and Graphene Oxide. In Graphene for Transparent Conductors: Synthesis, Properties and Applications; Springer: New York, NY, USA, 2015; pp. 29–94. [Google Scholar]
- Yang, X.; Dou, X.; Rouhanipour, A.; Zhi, L.; Räder, H.J.; Müllen, K. Two-dimensional graphene nanoribbons. J. Am. Chem. Soc. 2008, 130, 4216–4217. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wen, W.; Ou, J.Z. Construction of adsorbents with graphene and its derivatives for wastewater treatment: A review. Environ. Sci. Nano 2022, 9, 3226–3276. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, L.-S. Synthesis of large, stable colloidal graphene quantum dots with tunable size. J. Am. Chem. Soc. 2010, 132, 5944–5945. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Zhang, H.; Huang, L.; Wu, B.; Chen, J.; Yu, G. Scalable synthesis of few-layer graphene ribbons with controlled morphologies by a template method and their applications in nanoelectromechanical switches. J. Am. Chem. Soc. 2009, 131, 11147–11154. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Wu, J.; Jiang, X.; Cao, P.; Hu, X.; Pan, T.; Qiu, C.; Yang, J.; Song, Y.; et al. Bottom-up fabrication of graphene on silicon/silica substrate via a facile soft-hard template approach. Sci. Rep. 2015, 5, 13480. [Google Scholar] [CrossRef] [Green Version]
- Deka, M.J.; Baruah, U.; Chowdhury, D. Insight into electrical conductivity of graphene and functionalized graphene: Role of lateral dimension of graphene sheet. Mater. Chem. Phys. 2015, 163, 236–244. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Delibozov, N. Analysis of graphene nanoribbons passivated with gold, copper and indium. Int. J. Theor. Appl. Nanotechnol. 2013, 1, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Santhosh, N.M.; Filipic, G.; Kovacevic, E.; Jagodar, A.; Berndt, J.; Strunskus, T.; Kondo, H.; Hori, M.; Tatarova, E.; Cvelbar, U. N-graphene nanowalls via plasma nitrogen incorporation and substitution: The experimental evidence. Nano-Micro Lett. 2020, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, J.; Chen, D. Graphene-Based Materials in Electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Ceng, H.-M.; Zhao, J.; Pei, S.; Ren, W.; Gao, L. Efficient Preparation of Large-Area Graphene Oxide Sheets for Transparent Conductive Films. ACS Nano 2010, 4, 5245–5252. [Google Scholar]
- Santoro, C.; Bollella, P.; Erable, B.; Atanassov, P.; Pant, D. Oxygen reduction reaction electrocatalysis in neutral media for bioelectrochemical systems. Nat. Catal. 2022, 5, 473–484. [Google Scholar] [CrossRef]
- L€utfi, M.; Eren, T.; Atar, N. A Novel and Sensitive Electrochemical DNA Biosensor Based on Fe @ Au Nanoparticles Decorated Graphene Oxide. Electrochim. Acta 2014, 125, 38–47. [Google Scholar]
- Pei, S.; Cheng, H. The Reduction of Graphene Oxide. Carbon 2011, 50, 3210–3228. [Google Scholar] [CrossRef]
- Fan, B.X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G. Deoxygenation of Exfoliated Graphite Oxide Under Alkaline Conditions: A Green Route to Graphene Preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, T.; Ruoff, R.S. Stable Aqueous Dispersions of Graphitic Nanoplatelets via the Reduction of Exfoliated Graphite Oxide in the Presence of Poly (Sodium 4-Styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Purwaningsih, H.; Suari, N.M.I.P.; Widiyastuti, W.; Setyawan, H. Preparation of rGO/MnO2 Composites through Simultaneous Graphene Oxide Reduction by Electrophoretic Deposition. ACS Omega 2022, 7, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; Mcwilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution Properties of Graphite and Graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Chia, P.; Chua, L.; Zhao, L.; Png, R.; Sivaramakrishnan, S.; Zhou, M.; Goh, R.G.; Friend, R.H.; Wee, A.T.; et al. Band-Like Transport in Surface-Functionalized Highly Solution-Processable Graphene Nanosheets. Adv. Mater. 2008, 20, 3440–3446. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-Throughput Solution Processing of Large-Scale Graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Wang, Y.; Hou, C.; Zhang, Y.; He, F. Preparation of Graphene Nano-Sheets Bonded PDA/MOFs Microcapsules Immobilized Glucose Oxidase as Mimetic Multi-Enzyme System for Electrochemical Sensing of Glucose. Mater. Chem. B 2016, 4, 3695–3702. [Google Scholar]
- Niu, X.; Zheng, W.; Yin, C.; Weng, W.; Li, G.; Sun, W. Electrochemical DNA Biosensor Based on Gold Nanoparticles and Partially Reduced Graphene Oxide Modified Electrode for the Detection of Listeria Monocytogenes Hly Gene Sequence. J. Electroanal. Chem. 2017, 806, 116–122. [Google Scholar] [CrossRef]
- Krittayavathananon, A.; Sawangphruk, M. Impedimetric Sensor of ss-HSDNA/Reduced Graphene Oxide Aerogel Electrode toward Aflatoxin B1 Detection: Effects of Redox Mediator Charges and Hydrodynamic Diffusion. Anal. Chem. 2017, 89, 13283–13289. [Google Scholar] [CrossRef] [PubMed]
- Hod, O.; Scuseria, G.E. Electronic Structure and Stability of Semiconducting Graphene Nanoribbons. Nano Lett. 2006, 6, 2748–2754. [Google Scholar]
- Ezawa, M. Peculiar Width Dependence of the Electronic Properties of Carbon Nanoribbons. Phys. Rev. B 2006, 73, 045432. [Google Scholar] [CrossRef] [Green Version]
- Asadian, E.; Shahrokhian, S.; Zad, A.I. Highly Sensitive Nonenzymetic Glucose Sensing Platform Based on MOF-Derived NiCo LDH Nanosheets/Graphene Nanoribbons Composite. J. Electroanal. Chem. 2018, 808, 114–123. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Rahighi, R. Toward Single-DNA Electrochemical. ACS Nano 2012, 6, 2904–2916. [Google Scholar] [CrossRef]
- Kumar, P.; Ganguly, A.; Yang, W.; Wu, C.; Hwang, J.; Tai, Y.; Chen, K.; Chen, L.; Chattopadhyay, S. Edge Promoted Ultrasensitive Electrochemical Detection of Organic Bio-Molecules on Epitaxial Graphene Nanowalls. Biosens. Bioelectron. 2015, 70, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Razmi, H.; Mohammad-Rezaei, R. Biosensors and Bioelectronics Graphene Quantum Dots as a New Substrate for Immobilization and Direct Electrochemistry of Glucose Oxidase: Application to Sensitive Glucose Determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Bing, X.; Wang, G. Label Free C-reactive Protein Detection Based on An Electrochemical Sensor for Clinical Application. Int. J. Electrochem. Sci. 2017, 12, 6304–6314. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. In Situ Growth of Surfactant-Free Gold Nanoparticles on Nitrogen-Doped Graphene Quantum Dots for Electrochemical Detection of Hydrogen Peroxide in Biological Environments. Anal. Chem. 2014, 87, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Zou, X.; Wu, S.; Wan, D.; Cao, A. Ultrafine Graphene Nanomesh With Large On/Off Ratio for High-Performance Flexible Biosensors. Adv. Funct. Mater. 2017, 27, 1604096. [Google Scholar] [CrossRef]
- Ruiyi, L.; Juanjuan, Z.; Zhouping, W.; Zaijun, L.; Junkang, L.; Zhiguo, G. Novel Graphene-Gold Nanohybrid With Excellent Electrocatalytic Performance for the Electrochemical Detection of Glucose. Sens. Actuators B Chem. 2015, 208, 421–428. [Google Scholar] [CrossRef]
- Krasheninnikov, A.V.; Lehtinen, P.O.; Foster, A.S.; Nieminen, R.M. Bending the rules: Contrasting vacancy energetics and migration in graphite and carbon nanotubes. Chem. Phys. Lett. 2006, 418, 132–136. [Google Scholar] [CrossRef]
- Lu, A.J.; Pan, B.C. Nature of Single Vacancy in Achiral Carbon Nanotubes. Phys. Rev. Lett. 2004, 92, 105504. [Google Scholar] [CrossRef] [PubMed]
- El-Barbary, A.A.; Telling, R.H.; Ewels, C.P.; Heggie, M.I.; Briddon, P.R. Structure and energetics of the vacancy in graphite. Phys. Rev. B 2003, 68, 144107. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, A.; Uenaga, K.; Gloter, A.; Urita, K.; Lijima, S. Direct evidence for atomic defects in graphene layers. Nature 2004, 430, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Tapasztó, L.; Dobrik, G.; Nemes-Incze, P.; Vertesy, G.; Lambin, P.; Biró, L.P. Tuning the electronic structure of graphene by ion irradiation. Phys. Rev. B 2008, 78, 233407. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.N.; Wang, R.; Wang, X.W.; Zhang, D.D.; Qiu, X.H. Nanoscale charge distribution and energy band modification in defect-patterned graphene. Nanoscale 2012, 4, 2651–2657. [Google Scholar] [CrossRef]
- Henderson, M.A. Surface perspective on self-diffusion in rutile TiO2. Surf. Sci. 1999, 419, 174–187. [Google Scholar] [CrossRef]
- Tolchard, J.R.; Islam, M.S.; Slater, P.R. Defect chemistry and oxygen ion migration in the apatite-type materials La9.33Si6O26 and La8Sr2Si6O26. J. Mater. Chem. 2003, 13, 1956–1961. [Google Scholar] [CrossRef] [Green Version]
- Banhart, F.; Li, J.X.; Krasheninnikov, A.V. Carbon nanotubes under electron irradiation: Stability of the tubes and their action as pipes for atom transport. Phys. Rev. B 2005, 71, 241408. [Google Scholar] [CrossRef] [Green Version]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Li, L.; Reich, S.; Robertson, J. Defect energies of graphite: Density-functional calculations. Phys. Rev. B 2005, 72, 184109. [Google Scholar] [CrossRef] [Green Version]
- Krasheninnikov, A.V.; Banhart, F.; Li, J.X.; Foster, A.S.; Nieminen, R.M. Stability of carbon nanotubes under electron irradiation: Role of tube diameter and chirality. Phys. Rev. B 2005, 72, 125428. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Alfè, D.; Michaelides, A.; Wang, E. Stone-Wales defects in graphene and other planar sp2-bonded materials. Phys. Rev. B 2009, 80, 033407. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.C.; Kisielowski, C.; Erni, R.; Rossell, M.D.; Crommie, M.F.; Zettl, A. Direct Imaging of Lattice Atoms and Topological Defects in Graphene Membranes. Nano Lett. 2008, 8, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintterlin, J.; Bocquet, M.L. Graphene on metal surfaces. Surf. Sci. 2009, 603, 1841–1852. [Google Scholar] [CrossRef]
- Huang, P.Y.; Ruiz-Vargas, C.S.; van der Zande, A.M.; Whitney, W.S.; Levendorf, M.P.; Kevek, J.W.; Garg, S.; Alden, J.S.; Hustedt, C.J.; Zhu, Y.; et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature 2011, 469, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Coraux, J.; N’Diaye, A.T.; Busse, C.; Michely, T. Structural Coherency of Graphene on Ir (111). Nano Lett. 2008, 8, 565–570. [Google Scholar] [CrossRef]
- Lahiri, J.; Lin, Y.; Bozkurt, P.; Oleynik, I.I.; Batzill, M. An extended defect in graphene as a metallic wire. Nat. Nanotechnol. 2010, 5, 326–329. [Google Scholar] [CrossRef]
- Malola, S.; Häkkinen, H.; Koskinen, P. Structural, chemical, and dynamical trends in graphene grain boundaries. Phys. Rev. B 2010, 81, 165447. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Louie, S.G. Topological defects in graphene: Dislocations and grain boundaries. Phys. Rev. B 2010, 81, 195420. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y. Simulation of interstitial diffusion in graphite. Phys. Rev. B 2007, 76, 075419. [Google Scholar] [CrossRef]
- Tsetseris, L.; Pantelides, S.T. Adatom complexes and self-healing mechanisms on graphene and single-wall carbon nanotubes. Carbon 2009, 47, 901–908. [Google Scholar] [CrossRef]
- Lusk, M.T.; Carr, L.D. Nanoengineering Defect Structures on Graphene. Phys. Rev. Lett. 2008, 100, 175503. [Google Scholar] [CrossRef] [Green Version]
- Lusk, M.T.; Wu, D.T.; Carr, L.D. Graphene nanoengineering and the inverse Stone-Thrower-Wales defect. Phys. Rev. B 2010, 81, 155444. [Google Scholar] [CrossRef] [Green Version]
- Cretu, O.; Krasheninnikov, A.V.; Rodríguez-Manzo, J.A.; Sun, L.; Nieminen, R.M.; Banhart, F. Migration and Localization of Metal Atoms on Strained Graphene. Phys. Rev. Lett. 2010, 105, 196102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.T.; Neaton, J.B.; Cohen, M.L. First-principles study of metal adatom adsorption on graphene. Phys. Rev. B 2008, 77, 235430. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Gao, L.; Liu, B.; Jiang, C.; Cheng, H.M. Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 2009, 47, 493–499. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.H.; Tran, Q.T.; Chuang, J.S.; Shin, E.W.; Kim, J.S.; Kim, E.J. Optoelectronic properties of graphene thin films prepared by thermal reduction of graphene oxide. Mater. Lett. 2010, 64, 765–767. [Google Scholar] [CrossRef]
- Pham, T.A.; Kim, J.S.; Kim, S.; Jeong, Y.T. One-step reduction of graphene oxide with l-glutathione. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 543–548. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 5356. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, R.; Zhang, L.; Shi, Z.; Yang, W.; Wang, D.; Xie, G.; Shi, D.; Zhang, G. Restoration of graphene from graphene oxide by defect repair. Carbon 2012, 50, 2581–2587. [Google Scholar] [CrossRef] [Green Version]
- Ci, L.; Song, L.; Jin, C.; Jariwala, D.; Wu, D.; Li, Y.; Srivastava, A.; Wang, Z.F.; Storr, K.; Balicas, L.; et al. Atomic layers of hybridized boron nitride and graphene domains. Nat. Mater. 2010, 9, 430–435. [Google Scholar] [CrossRef]
- Bianco, G.V.; Sacchetti, A.; Grande, M.; D’Orazio, A.; Milella, A.; Bruno, G. Effective hole conductivity in nitrogen-doped CVD-graphene by singlet oxygen treatment under photoactivation conditions. Sci. Rep. 2022, 12, 8703. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.M. Doped Graphene Sheets as Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Terrones, M.; Botello-Méndez, A.R.; Campos-Delgado, J.; López-Urías, F.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J.; Elías, A.L.; Munoz-Sandoval, E.; Cano-Márquez, A.G.; Charlier, J.C.; et al. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today 2010, 5, 351–372. [Google Scholar] [CrossRef]
- Cao, C.; Daly, M.; Singh, C.V.; Sun, Y.; Filleter, T. High strength measurement of monolayer graphene oxide. Carbon 2015, 81, 497–504. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Sheng, Y.; Yang, L.; Su, Y. The strain-dependent interfacial thermal resistance at graphene-silicon interface under various deformation conditions. Int. J. Heat Mass Transf. 2022, 198, 123383. [Google Scholar] [CrossRef]
- Min, K.; Aluru, N.R. Mechanical properties of graphene under shear deformation. Appl. Phys. Lett. 2011, 98, 013113. [Google Scholar] [CrossRef] [Green Version]
- Ovid’ko, I. Mechanical properties of graphene. Rev. Adv. Mater. Sci. 2013, 34, 1–11. [Google Scholar]
- Frank, I.W.; Tanenbaum, D.M.; van der Zande, A.M.; McEuen, P.L. Mechanical properties of suspended graphene sheets. J. Vac. Sci. Technol. B 2007, 25, 2558–2561. [Google Scholar] [CrossRef] [Green Version]
- Uhl, F.M.; Wilkie, C.A. Preparation of nanocomposites from styrene and modified graphite oxides. Polym. Degrad. Stabil. 2004, 84, 215–226. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef]
- Tabarraei, A.; Shadalou, S.; Song, J.H. Mechanical properties of graphene nanoribbons with disordered edges. Comput. Mater. Sci. 2015, 96, 10–19. [Google Scholar] [CrossRef]
- Olowojoba, G.B.; Eslava, S.; Gutierrez, E.S.; Kinloch, A.J.; Mattevi, C.; Rocha, V.G.; Taylor, A.C. In situ thermally reduced graphene oxide/epoxy composites: Thermal and mechanical properties. Appl. Nanosci. 2016, 6, 1015–1022. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Liu, Y.; Leng, J. Nanocomposites of epoxy-based shape memory polymer and thermally reduced graphite oxide: Mechanical, thermal and shape memory characterizations. Compos. Part B Eng. 2016, 91, 75–82. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.M.; Zheng, Q.; Lin, X.; Shen, X.; Jia, J.; Sharif, F.; Kim, J.-K. Highly aligned, ultralarge-size reduced graphene oxide/polyurethane nanocomposites: Mechanical properties and moisture permeability. Compos. Part A Appl. Sci. Manuf. 2013, 49, 42–50. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Wang, J.; Ouyang, Z.; Li, J.; Wei, G.; Su, Z. Interactive oxidation-reduction reaction for the in situ synthesis of graphene-phenol formaldehyde composites with enhanced properties. ACS Appl. Mater. Interfaces 2013, 6, 4254–4263. [Google Scholar] [CrossRef]
- Potts, J.R.; Lee, S.H.; Alam, T.M.; An, J.; Stoller, M.D.; Piner, R.D.; Ruoff, R.S. Thermomechanical properties of chemically modified graphene/poly (methyl methacrylate) composites made by in situ polymerization. Carbon 2013, 49, 2615–2623. [Google Scholar] [CrossRef]
- Qian, Y.; Lan, Y.; Xu, J.; Ye, F.; Dai, S. Fabrication of polyimide-based nanocomposites containing functionalized graphene oxide nanosheets by in situ polymerization and their properties. Appl. Surf. Sci. 2014, 314, 991–999. [Google Scholar] [CrossRef]
- Zhang, Y.; Mark, J.E.; Zhu, Y.; Ruoff, R.S.; Schaefer, D.W. Mechanical properties of polybutadiene reinforced with octadecylamine modified graphene oxide. Polymer 2014, 55, 5389–5395. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Yang, X.; Feng, W.; Huang, H. Preparation and properties of novel sulfonic graphene oxide-epoxy resin nanocomposites by resin transfer molding process. J. Compos. Mater. 2017, 51, 1197–1208. [Google Scholar] [CrossRef]
- Ma, L.; Wang, G.; Dai, J. Preparation and properties of graphene oxide/polyimide composites by in situ polymerization and thermal imidization process. High Perform. Polym. 2017, 29, 187–196. [Google Scholar] [CrossRef]
- Kang, H.; Zuo, K.; Wang, Z.; Zhang, L.; Liu, L.; Guo, B. Using a green method to develop graphene oxide/elastomers nanocomposites with combination of high barrier and mechanical performance. Compos. Sci. Technol. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Deshmukh, K.; Joshi, G.M. Thermo-mechanical properties of poly (vinyl chloride)/graphene oxide as high performance nanocomposites. Polym. Test. 2014, 34, 211–219. [Google Scholar] [CrossRef]
- Dixon, D.; Lemonine, P.; Hamilton, J.; Lubarsky, G.; Archer, E. Graphene oxide-polyamide 6 nanocomposites produced via in situ polymerization. J. Thermoplast. Compos. Mater. 2015, 28, 372–389. [Google Scholar] [CrossRef]
- Mikhaylov, A.; Zakharova, M.; Vlnieska, V.; Khanda, A.; Bremer, S.; Zuber, M.; Pezzin, S.H.; Kunka, D. Inverted Hartmann mask made by deep X-ray lithography for single-shot multi-contrast X-ray imaging with laboratory setup. Opt. Express 2022, 30, 8494–8509. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.I.; Ansari, M. Mechanical properties of graphene oxide (GO)/epoxy composites. HBRC J. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, C.; Zhang, M.; Shang, X. Preparation of graphene/poly (vinyl alcohol) nanocomposites with enhanced mechanical properties and water resistance. Polym. Int. 2011, 60, 816–822. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Song, L.; Yang, H.; Hu, Y.; Yeoh, G.H. Fabrication and characterization of graphene-reinforced waterborne polyurethane nanocomposite coatings by the sol-gel method. Surf. Coat. Technol. 2012, 206, 4778–4784. [Google Scholar] [CrossRef]
- Yao, H.; Hawkins, S.A.; Sue, H.-J. Preparation of epoxy nanocomposites containing well-dispersed graphene nanosheets. Compos. Sci. Technol. 2017, 146, 161–168. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Kudus, M.H.A.; Akil, H.M.; Thirmizir, M.Z.M. Comparative study of graphene nanoparticle and multiwall carbon nanotube filled epoxy nanocomposites based on mechanical, thermal and dielectric properties. Compos. Part B Eng. 2017, 119, 57–66. [Google Scholar] [CrossRef]
- Yang, Z.; McElrath, K.; Bahr, J.; D’Souza, N.A. Effect of matrix glass transition on reinforcement efficiency of epoxy-matrix composites with single walled carbon nanotubes, multi-walled carbon nanotubes, carbon nanofibers and graphite. Compos. Part B Eng. 2012, 43, 2079–2086. [Google Scholar] [CrossRef]
- Gao, Y.; Picot, O.T.; Bilotti, E.; Peijs, T. Influence of filler size on the properties of poly (lactic acid)(PLA)/graphene nanoplatelet (GNP) nanocomposites. Eur. Polym. J. 2017, 86, 117–131. [Google Scholar] [CrossRef]
- Yadav, S.K.; Cho, J.W. Functionalized graphene nanoplatelets for enhanced mechanical and thermal properties of polyurethane nanocomposites. Appl. Surf. Sci. 2013, 266, 360–367. [Google Scholar] [CrossRef]
- Moosa, A.A.; Kubba, F.; Raad, M.; Ahmad Ramazani, S.A. Mechanical and thermal properties of graphene nanoplates and functionalized carbon-nanotubes hybrid epoxy nanocomposites. Am. J. Mater. Sci. 2016, 6, 125–134. [Google Scholar]
- Huang, C.-L.; Lou, C.-W.; Liu, C.-F.; Huang, C.-H.; Song, X.-M.; Lin, J.-H. Polypropylene/graphene and polypropylene/carbon fiber conductive composites: Mechanical, crystallization and electromagnetic properties. Appl. Sci. 2015, 5, 1196–1210. [Google Scholar] [CrossRef] [Green Version]
- Che Berhanuddin, N.I.; Mohd Rozlan, S.A.; Zaman, I.B.; Mustapa, M.S.; Abdullah, M.E.; Bahrudin, I.A. Effect of thermal expansion and sonication on mechanical properties and adhesive toughness measurement of polymer/graphene composite. Mater. Sci. Forum. Trans. Tech. Publ. 2013, 889, 14–18. [Google Scholar] [CrossRef]
- Layek, R.K.; Samanta, S.; Nandi, A.K. The physical properties of sulfonated graphene/poly (vinyl alcohol) composites. Carbon 2012, 50, 815–827. [Google Scholar] [CrossRef]
- Tian, M.; Qu, L.; Zhang, X.; Zhang, K.; Zhu, S.; Guo, X.; Han, G.; Tang, X.; Sun, Y. Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Z.; Ge, Y.; Wang, Y.; Fan, J.; Yin, J. Solvent exfoliated graphene for reinforcement of PMMA composites prepared by in situ polymerization. Mater. Chem. Phys. 2012, 136, 43–50. [Google Scholar] [CrossRef]
- Quiles-Diaz, S.; Enrique-Jimenez, P.; Papageorgiou, D.; Ania, F.; Flores, A.; Kinloch, I.; Gómez-Fatou, M.A.; Young, R.J.; Salavagione, H.J. Influence of the chemical functionalization of graphene on the properties of polypropylene-based nanocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 100, 31–39. [Google Scholar]
- Abaszade, R.G.; Mamedov, A.G.; Bayramov, I.Y.; Khanmamadova, E.A.; Kotsyubynsky, V.O.; Kapush, O.A.; Boychuk, V.M.; Gur, E.Y. Structural and electrical properties of sulfur-doped graphene oxide/graphite oxide composite. Phys. Chem. Solid State 2022, 23, 256–260. [Google Scholar] [CrossRef]
- Tang, H.; Ehlert, G.J.; Lin, Y.; Sodano, H.A. Highly efficient synthesis of graphene nanocomposites. Nano Lett. 2011, 12, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Qin, W.; Vautard, F.; Drzal, L.T.; Yu, J. Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber-matrix interphase. Compos. B Eng. 2015, 69, 335–341. [Google Scholar]
- Almajid, A.; Sorochynska, L.; Friedrich, K.; Wetzel, B. Effects of graphene and CNT on mechanical, thermal, electrical and corrosion properties of vinylester based nanocomposites. Plast. Rubber Compos. 2015, 44, 50–62. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Lopez-Manchado, M.A.; Brasero, J.; Avilés, F.; Yazdani-Pedram, M. Effect of the morphology of thermally reduced graphite oxide on the mechanical and electrical properties of natural rubber nanocomposites. Compos. B Eng. 2016, 87, 350–356. [Google Scholar] [CrossRef]
- Saafi, M.; Tang, L.; Fung, J.; Rahman, M.; Liggat, J. Enhanced properties of graphene/fly ash geopolymeric composite cement. Cement. Concr. Res. 2015, 67, 292–299. [Google Scholar] [CrossRef]
- Taipalus, R.; Harmia, T.; Zhang, M.Q.; Friedrich, K. The electrical conductivity of carbon-fibre-reinforced polypropylene/polyaniline complex-blends: Experimental characterisation and modelling. Compos. Sci. Technol. 2001, 61, 801–814. [Google Scholar] [CrossRef]
- Hyunwoo, K.; Ahmed, A.A.; Christopher, W.M. Graphene/Polymer Nanocomposites. In Graphite, Graphene, and Their Polymer Nanocomposites; CRC Press: Boca Raton, FL, USA, 2012; pp. 513–556. [Google Scholar]
- Wang, J.; Ma, F.; Liang, W.; Sun, M. Electrical properties and applications of graphene, hexagonal boron nitride (h-BN), and graphene/h-BN heterostructures. Mater. Today Phys. 2017, 2, 6–34. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef]
- Su, Q.; Pang, S.; Alijani, V.; Li, C.; Feng, X.; Müllen, K. Composites of graphene with large aromatic molecules. Adv. Mater. 2009, 21, 3191–3195. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhi, L.; Mullen, K. Transparent, conductive graphene electrodes for dyesensitized solar cells. Nano Lett. 2007, 8, 323–327. [Google Scholar] [CrossRef]
- Diez-Betriu, X.; Alvarez-Garcia, S.; Botas, C.; Alvarez, P.; Sanchez-Marcos, J.; Prieto, C.; Menéndez, R.; de Andrés, A. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nano 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Bao, C.; Song, L.; Xing, W.; Yuan, B.; Wilkie, C.A.; Huang, J.; Guo, Y.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Wang, S.J.; Geng, Y.; Zheng, Q.; Kim, J.-K. Fabrication of highly conducting and transparent graphene films. Carbon 2010, 48, 1815–1823. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterisation of reduced graphene oxide: Effects of reduction variables on electrical conductivity. Mater. Sci. Eng. B 2015, 193, 49–60. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.; Stoller, M.D.; Zhu, Y.; Ji, H.; Murali, S.; Wu, Y.; Perales, S.; Clevenger, B.; Ruoff, R.S. Highly conductive and porous activated reduced graphene oxide films for high-power supercapacitors. Nano Lett. 2012, 12, 1806–1812. [Google Scholar] [CrossRef]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, B.; Gao, X. Thermal properties of graphene-based polymer composite materials: A molecular dynamics study. Results Phys. 2020, 16, 102974. [Google Scholar] [CrossRef]
- Goli, P.; Legedza, S.; Dhar, A.; Salgado, R.; Renteria, J.; Balandin, A.A. Graphene-enhanced hybrid phase change materials for thermal management of Li-ion batteries. J. Power Sources 2014, 248, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, A.P.I.; Popoola, O.M. A review on recent advances on the mechanical and conductivity properties of epoxy nanocomposites for industrial applications. Polym. Bull. 2022, 1–39. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Yuan, W. Preparation of a poly (methyl methacrylate)-reduced graphene oxide composite with enhanced properties by a solution blending method. Eur. Polym. J. 2012, 48, 1674–1682. [Google Scholar] [CrossRef]
- Menbari, S.; Ashori, A.; Rahmani, H.; Bahrami, R. Viscoelastic response and interlaminar delamination resistance of epoxy/glass fiber/functionalized graphene oxide multi-scale composites. Polym. Test. 2016, 54, 186–195. [Google Scholar] [CrossRef]
- Li, P.; Zheng, Y.; Li, M.; Shi, T.; Li, D.; Zhang, A. Enhanced toughness and glass transition temperature of epoxy nanocomposites filled with solvent-free liquid-like nanocrystal-functionalized graphene oxide. Mater. Des. 2016, 89, 653–659. [Google Scholar] [CrossRef]
- Morimune, S.; Nishino, T.; Goto, T. Poly (vinyl alcohol)/graphene oxide nanocomposites prepared by a simple eco-process. Polym. J. 2012, 44, 1056. [Google Scholar] [CrossRef] [Green Version]
- Galpaya, D.; Wang, M.; George, G.; Motta, N.; Waclawik, E.; Yan, C. Preparation of graphene oxide/epoxy nanocomposites with significantly improved mechanical properties. J. Appl. Phys. 2014, 116, 053518. [Google Scholar] [CrossRef] [Green Version]
- Liem, H.; Choy, H. Superior thermal conductivity of polymer nanocomposites by using graphene and boron nitride as fillers. Solid State Commun. 2013, 163, 41–45. [Google Scholar] [CrossRef]
- Wang, S.; Tambraparni, M.; Qiu, J.; Tipton, J.; Dean, D. Thermal expansion of graphene composites. Macromolecules 2009, 42, 5251–5255. [Google Scholar] [CrossRef]
- Debelak, B.; Lafdi, K. Use of exfoliated graphite filler to enhance polymer physical properties. Carbon 2007, 45, 1727–1734. [Google Scholar] [CrossRef]
- Shi, J.-N.; Ger, M.-D.; Liu, Y.-M.; Fan, Y.-C.; Wen, N.-T.; Lin, C.-K.; Pu, N.W. Improving the thermal conductivity and shapestabilization of phase change materials using nanographite additives. Carbon 2013, 51, 365–372. [Google Scholar] [CrossRef]
- Song, P.; Cao, Z.; Cai, Y.; Zhao, L.; Fang, Z.; Fu, S. Fabrication of exfoliated graphene-based polypropylene nanocomposites with enhanced mechanical and thermal properties. Polymer 2011, 52, 4001–4010. [Google Scholar] [CrossRef]
- Yavari, F.; Fard, H.R.; Pashayi, K.; Rafiee, M.A.; Zamiri, A.; Yu, Z.; Ozisik, R.; Borca-Tasciuc, T.; Koratkar, N. Enhanced thermal conductivity in a nanostructured phase change composite due to low concentration graphene additives. J. Phys. Chem. C 2011, 115, 8753–8758. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite nanoplatelet-epoxy composite thermal interface materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric nanoparticles in brain cancer therapy: A review of current approaches. Polymers 2022, 14, 2963. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, Y.; Li, W.; Qiu, S.; Zhu, C.; Wei, X.; Chen, M.; Liu, C.; Liao, S.; Gong, Y.; et al. Ultrahigh thermal conductivity of assembled aligned multilayer graphene/epoxy composite. Chem. Mater. 2014, 26, 4459–4465. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, M.; Liu, Z.; Wu, Y.; Xiong, X.; Li, C.; Wang, X.; Jiang, N.; Yu, J.; Lin, C.-T. Enhanced thermal conductivity for poly (vinylidene fluoride) composites with nano-carbon fillers. Rsc. Adv. 2016, 6, 68357–68362. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Abrar, M.A.; Dong, Y.; Lee, P.K.; Kim, W.S. Bendable electro-chemical lactate sensor printed with silver nano-particles. Sci. Rep. 2016, 6, 30565. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Q.; Gong, S.; Dyson, J.M.; Cheng, W. Soft, Disruptive and Wearable Electrochemical Biosensors. Curr. Anal. Chem. 2022, 18, 689–704. [Google Scholar] [CrossRef]
- Yüzer, E.; Doğan, V.; Kılıç, V.; Şen, M. Smartphone embedded deep learning approach for highly accurate and automated colorimetric lactate analysis in sweat. Sens. Actuators B Chem. 2022, 371, 32489. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L. Wearable electrochemical sensors for monitoring of glucose and electroactive drugs. Int. J. Electrochem. Sci. 2022, 17, 2. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Arya, S. Human sweat-based wearable glucose sensor on cotton fabric for real-time monitoring. J. Anal. Sci. Technol. 2022, 13, 11. [Google Scholar] [CrossRef]
- Holmes, D. Sweat-sensing patch for glucose monitoring and drug delivery. Nat. Rev. Endocrinol. 2016, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, J.; Liu, Y.; Wong, T.; Su, J.; Yao, K.; Zhou, J.; Huang, Y.; Li, H.; Li, D.; et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Des. Manuf. 2022, 5, 201–209. [Google Scholar] [CrossRef]
- Kang, B.C.; Park, B.S.; Ha, T.J. Highly sensitive wearable glucose sensor systems based on functionalized single-wall carbon nanotubes with glucose oxidase-nafion composites. Appl. Surf. Sci. 2019, 470, 13–18. [Google Scholar] [CrossRef]
- Wang, Y.X.; Rinawati, M.; Zhan, J.D.; Lin, K.Y.; Huang, C.J.; Chen, K.J.; Mizuguchi, H.; Jiang, J.C.; Hwang, B.J.; Yeh, M.H. Boron-Doped Graphene Quantum Dots Anchored to Carbon Nanotubes as Noble Metal-Free Electrocatalysts of Uric Acid for a Wearable Sweat Sensor. ACS Appl. Nano Mater. 2022, 5, 11100–11110. [Google Scholar] [CrossRef]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; De Loyola e Silva, A.N.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal enzymatic biosensors for sweat vitamin C: Toward personalized nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Y.; Li, D.; Yue, R.; Xu, J.; He, H. A stable sandwich-type amperometric biosensor based on poly (3,4-ethylenedioxythiophene)—Single walled carbon nanotubes/ascorbate oxidase/nafion films for detection of L-ascorbic acid. Sens. Actuators B Chem. 2011, 159, 277–285. [Google Scholar] [CrossRef]
- Csiffáry, G.; Fűtő, P.; Adányi, N.; Kiss, A. Ascorbate oxidase-based amperometric biosensor for L-ascorbic acid determination in beverages. Food Technol. Biotechnol. 2016, 54, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Shahub, S.; Upasham, S.; Ganguly, A.; Prasad, S. Machine learning guided electrochemical sensor for passive sweat cortisol detection. Sens. Bio-Sens. Res. 2022, 38, 100527. [Google Scholar] [CrossRef]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Aryal, K.P.; Jeong, H.K. Functionalization of thermally reduced graphite oxide and carbon nanotubes by p-sulfonatocalix [4] arene and supramolecular recognition of tyrosine. Chem. Phys. Lett. 2019, 714, 69–73. [Google Scholar] [CrossRef]
- Beitollahi, H.; Garkani Nejad, F. Graphene oxide/ZnO nano composite for sensitive and selective electrochemical sensing of levodopa and tyrosine using modified graphite screen printed electrode. Electroanalysis 2016, 28, 2237–2244. [Google Scholar] [CrossRef]
- Panneer Selvam, A.; Muthukumar, S.; Kamakoti, V.; Prasad, S. A wearable biochemical sensor for monitoring alcohol consumption lifestyle through Ethyl glucuronide (EtG) detection in human sweat. Sci. Rep. 2016, 6, 23111. [Google Scholar] [CrossRef]
- Kilic, T.; Brunner, V.; Audoly, L.; Carrara, S. Smart e-Patch for drugs monitoring in schizophrenia. In Proceedings of the 2016 IEEE International Conference on Electronics, Circuits and Systems (ICECS), Monte Carlo, Monaco, 11–14 December 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 57–60. [Google Scholar]
- Parrilla, M.; Ferré, J.; Guinovart, T.; Andrade, F.J. Wearable potentiometric sensors based on commercial carbon fibres for monitoring sodium in sweat. Electroanalysis 2016, 28, 1267–1275. [Google Scholar] [CrossRef]
- Parrilla, M.; Cánovas, R.; Jeerapan, I.; Andrade, F.J.; Wang, J. A textile-based stretchable multi-ion potentiometric sensor. Adv. Healthc. Mater. 2016, 5, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Glennon, T.; O’Quigley, C.; McCaul, M.; Matzeu, G.; Beirne, S.; Wallace, G.G.; Stroiescu, F.; O’Mahoney, N.; White, P.; Diamond, D. ‘SWEATCH’: A wearable platform for harvesting and analysing sweat sodium content. Electroanalysis 2016, 28, 1283–1289. [Google Scholar] [CrossRef]
- McCaul, M.; Porter, A.; Barrett, R.; White, P.; Stroiescu, F.; Wallace, G.; Diamond, D. Wearable platform for real-time monitoring of sodium in sweat. Chem. Phys. Chem. 2018, 19, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, J.S.; Cutting, G.R.; Searson, P.C. Wearable potentiometric chloride sweat sensor: The critical role of the salt bridge. Anal. Chem. 2016, 88, 12241–12247. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Ruiz, J.; Mas, R.; de Haro, C.; Cabruja, E.; Camero, R.; Alonso-Lomillo, M.A.; Muñoz, F.J. Early determination of cystic fibrosis by electrochemical chloride quantification in sweat. Biosens. Bioelectron. 2009, 24, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion sensors. Trac. Trends Anal. Chem. 2019, 110, e303–e320. [Google Scholar] [CrossRef]
- Ozer, T.; Agir, I.; Henry, C.S. Low-cost Internet of Things (IoT)-enabled a wireless wearable device for detecting potassium ions at the point of care. Sens. Actuators B Chem. 2022, 365, 131961. [Google Scholar] [CrossRef]
- Guinovart, T.; Valdés-Ramírez, G.; Windmiller, J.R.; Andrade, F.J.; Wang, J. Bandage-based wearable potentiometric sensor for monitoring wound pH. Electroanalysis 2014, 26, 1345–1353. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Hung, V.W.; Jia, W.; Valdés-Ramírez, G.; Windmiller, J.R.; Martinez, A.G.; Ramírez, J.; Chan, G.; Kerman, K.; Wang, J. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 2013, 138, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Manjakkal, L.; Dervin, S.; Dahiya, R. Flexible potentiometric pH sensors for wearable systems. RSC Adv. 2020, 10, 8594–8617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, F.J.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.C.; Ota, H.; Davis, R.W.; et al. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; de Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixao, T.R.; Wang, J. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Yang, Z.; Xu, Q.; Hou, C.; Peng, J.; Hu, X. Simultaneous determination of ultratrace lead and cadmium by square wave stripping voltammetry with in situ depositing bismuth at Nafion-medical stone doped disposable electrode. J. Hazard. Mater. 2011, 191, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [Green Version]
- Bear, C.E.; Li, C.; Kartner, N.; Bridges, R.J.; Jensen, T.J.; Ramjeesingh, M.; Riordan, J.R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 1992, 68, 809–818. [Google Scholar] [CrossRef]
- Venosa, R.A. Hypo-osmotic stimulation of active Na+ transport in frog muscle: Apparent upregulation of Na+ pumps. J. Membr. Biol. 1991, 120, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Olarte, O.; Chilo, J.; Pelegri-Sebastia, J.; Barbé, K.; Van Moer, W. Glucose detection in human sweat using an electronic nose. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1462–1465. [Google Scholar]
- Yu, Q.; Chen, J.; Xu, J.; Chen, L.; Song, Y.; Xie, X.; Jin, J.; Liu, H.; Liu, J.; Zhang, F.; et al. Smartphone-Based and Miniaturized Electrochemical Biosensing System for L-Lactate Detection. J. Electrochem. Soc. 2022, 169, 047514. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, P.J.; Barr, H.; Davis, F.; Higson, S.P. Lactate in human sweat: A critical review of research to the present day. J. Physiol. Sci. 2012, 62, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.M.; Baum, M.; Quigley, R. Cystic fibrosis presenting with hypokalemia and metabolic alkalosis in a previously healthy adolescent. J. Am. Soc. Nephrol. 1997, 8, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Tachi, T.; Yokoi, T.; Goto, C.; Umeda, M.; Noguchi, Y.; Yasuda, M.; Minamitani, M.; Mizui, T.; Tsuchiya, T.; Teramachi, H. Hyponatremia and hypokalemia as risk factors for falls. Eur. J. Clin. Nutr. 2015, 69, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.R.; Wheeler, J.S.; Murray, H.E. Sodium and Potassium in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2013; pp. 29–47. [Google Scholar]
- Choi, D.H.; Thaxton, A.; Jeong, I.C.; Kim, K.; Sosnay, P.R.; Cutting, G.R.; Searson, P.C. Sweat test for cystic fibrosis: Wearable sweat sensor vs. standard laboratory test. J. Cyst. Fibros. 2018, 17, e35–e38. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Mishra, A.; Basu, S.; Mascarenhas, R.J.; Kakarla, R.R.; Aminabhavi, T.M. Skin-patchable electrodes for biosensor applications: A review. ACS Biomater. Sci. Eng. 2020, 6, 1823–1835. [Google Scholar] [CrossRef]
- Tadakaluru, S.; Thongsuwan, W.; Singjai, P. Stretchable and flexible high-strain sensors made using carbon nanotubes and graphite films on natural rubber. Sensors 2014, 14, 868–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Li, J.; Cao, D.; Zhang, G.; Li, J.; Li, K.; Yang, Y.; Wang, W.; Jin, Y.; Sun, R.; et al. Recent advancements in flexible and stretchable electrodes for electromechanical sensors: Strategies, materials, and features. ACS Appl. Mater. Interfaces 2017, 9, 12147–12164. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Hong, J.; Ha, M.; Jung, Y.-D.; Lim, H.; Kim, S.Y.; Ko, H. Giant tunneling piezoresistance of composite elastomers with interlocked microdome arrays for ultrasensitive and multimodal electronic skins. ACS Nano 2014, 8, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [Green Version]
- Bai, W.; Kuang, T.; Chitrakar, C.; Yang, R.; Li, S.; Zhu, D.; Chang, L. Patchable micro/nanodevices interacting with skin. Biosens. Bioelectron. 2018, 122, 189–204. [Google Scholar] [CrossRef]
- Jeong, J.W.; Yeo, W.H.; Akhtar, A.; Norton, J.J.; Kwack, Y.J.; Li, S.; Jung, S.Y.; Su, Y.; Lee, W.; Xia, J.; et al. Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 2013, 25, 6839–6846. [Google Scholar] [CrossRef]
- Sharma, A.; Mahajan, P.; Singh, A.; Arya, S. Detection Of Physiological Markers Via Wearable Devices For Human Healthcare. ECS Trans. 2022, 107, 20265. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Salvatore, G.A.; Münzenrieder, N.; Kinkeldei, T.; Petti, L.; Zysset, C.; Strebel, I.; Büthe, L.; Tröster, G. Wafer-scale design of lightweight and transparent electronics that wraps around hairs. Nat. Commun. 2014, 5, 2982. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwodiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ha, T.-J.; Li, H.; Parrish, K.N.; Holt, M.; Dodabalapur, A.; Ruoff, R.S.; Akinwande, D. 25 GHz embedded-gate graphene transistors with high-K dielectrics on extremely flexible plastic sheets. ACS Nano 2013, 7, 7744–7750. [Google Scholar] [CrossRef]
- Park, S.I.; Brenner, D.S.; Shin, G.; Morgan, C.D.; Copits, B.A.; Chung, H.U.; Pullen, M.Y.; Noh, K.N.; Davidson, S.; Oh, S.J. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015, 33, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Libanori, R.; Erb, R.M.; Reiser, A.; Le Ferrand, H.; Süess, M.J.; Spolenak, R.; Studart, A.R. Stretchable heterogeneous composites with extreme mechanical gradients. Nat. Commun. 2012, 3, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Singh, A.; Gupta, V.; Arya, S. Advancements and future prospects of wearable sensing technology for healthcare applications. Sens. Diagn. 2022, 1, 387–404. [Google Scholar] [CrossRef]
- Gupta, N.; Rao, K.D.M.; Srivastava, K.; Gupta, R.; Kumar, A.; Marconnet, A.; Fisher, T.S.; Kulkarni, G.U. Cosmetically Adaptable Transparent Strain Sensor for Sensitively Delineating Patterns in Small Movements of Vital Human Organs. ACS Appl. Mater. Interfaces 2018, 10, 44126–44133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Li, X.; Fan, J.A.; Xu, S.; Song, Y.M.; Choi, K.J.; Yeo, W.H.; Lee, W.; Nazaar, S.N.; et al. Experimental and theoretical studies of serpentine microstructures bonded to prestrained elastomers for stretchable electronics. Adv. Funct. Mater. 2014, 24, 2028–2037. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Inoue, D.; Zalar, P.; Jin, H.; Matsuba, Y.; Itoh, A.; Yokota, T.; Hashizume, D.; Someya, T. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 2017, 16, 834–840. [Google Scholar] [PubMed]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef] [PubMed]
- Bukkitgar, S.D.; Shetti, N.P.; Malladi, R.S.; Reddy, K.R.; Kalanur, S.S.; Aminabhavi, T.M. Novel ruthenium doped TiO2/reduced graphene oxide hybrid as highly selective sensor for the determination of ambroxol. J. Mol. Liq. 2020, 300, 112368. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Bukkitgar, S.D.; Bagihalli, G.B.; Kulkarni, R.M.; Reddy, K.R. Novel nanoclay-based electrochemical sensor for highly efficient electrochemical sensing nimesulide. J. Phys. Chem. Solids 2020, 137, 109210. [Google Scholar] [CrossRef]
- Valentine, A.D.; Busbee, T.A.; Boley, J.W.; Raney, J.R.; Chortos, A.; Kotikian, A.; Berrigan, J.D.; Durstock, M.F.; Lewis, J.A. Hybrid 3D printing of soft electronics. Adv. Mater. 2017, 29, 1703817. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuk, H.; Lin, S.; Parada, G.A.; Tang, T.C.; Tham, E.; de la Fuente-Nunez, C.; Lu, T.K.; Zhao, X. 3D printing of living responsive materials and devices. Adv. Mater. 2018, 30, 1704821. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Bagihalli, G.B.; Kalanur, S.S.; Malladi, R.S.; Reddy, C.V.; Aminabhavi, T.M.; Reddy, K.R. Fabrication of ZnO nanoparticles modified sensor for electrochemical oxidation of methdilazine. Appl. Surf. Sci. 2019, 496, 143656. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. Nanostructured titanium oxide hybrids-based electrochemical biosensors for healthcare applications. Colloids Surf. B 2019, 178, 385–394. [Google Scholar] [CrossRef]
- Lu, L.; Yang, Z.; Meacham, K.; Cvetkovic, C.; Corbin, E.A.; Vázquez-Guardado, A.; Xue, M.; Yin, L.; Boroumand, J.; Pakeltis, G.; et al. Biodegradable monocrystalline silicon photovoltaic microcells as power supplies for transient biomedical implants. Adv. Energy Mater. 2018, 8, 1703035. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, J.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable flexible and stretchable glove biosensor for on-site detection of organophosphorus chemical threats. ACS Sens. 2017, 2, 553–561. [Google Scholar] [CrossRef]
- Kim, J.-O.; Kwon, S.Y.; Kim, Y.; Choi, H.B.; Yang, J.C.; Oh, J.; Lee, H.S.; Sim, J.Y.; Ryu, S.; Park, S. Highly Ordered 3D Microstructure-Based Electronic Skin Capable of Differentiating Pressure, Temperature, and Proximity. ACS Appl. Mater. Interfaces 2019, 11, 1503–1511. [Google Scholar] [CrossRef]
- Mishra, R.K.; Martin, A.; Nakagawa, T.; Barfidokht, A.; Lu, X.; Sempionatto, J.R.; Lyu, K.M.; Karajic, A.; Musameh, M.M.; Kyratzis, I.L.; et al. Detection of vapor-phase organophosphate threats using wearable conformable integrated epidermal and textile wireless biosensor systems. Biosens. Bioelectron. 2018, 101, 227–234. [Google Scholar] [CrossRef]
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable potentiometric tattoo biosensor for onbody detection of G-type nerve agents simulants. Sens. Actuators B 2018, 273, 966–972. [Google Scholar] [CrossRef]
- Kang, J.; Son, D.; Wang, G.J.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater. 2018, 30, 1706846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zheng, R.; Chen, S.; Wu, Y.; Liu, H.; Wang, P.; Deng, Z.; Liu, L. A compliant, self-adhesive and self-healing wearable hydrogel as epidermal strain sensor. J. Mater. Chem. C 2018, 6, 4183–4190. [Google Scholar] [CrossRef]
- Son, D.; Bao, Z. Nanomaterials in skin-inspired electronics: Toward soft and robust skin-like electronic nanosystems. ACS Nano 2018, 12, 11731–11739. [Google Scholar] [CrossRef]
- Chortos, A.; Bao, Z. Skin-inspired electronic devices. Mater. Today 2014, 17, 321–331. [Google Scholar] [CrossRef]
- Benight, S.J.; Wang, C.; Tok, J.B.; Bao, Z. Stretchable and selfhealing polymers and devices for electronic skin. Prog. Polym. Sci. 2013, 38, 1961–1977. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Son, D.; Kang, J.; Vardoulis, O.; Kim, Y.; Matsuhisa, N.; Oh, J.Y.; To, J.W.; Mun, J.; Katsumata, T.; Liu, Y.; et al. An integrated selfhealable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 2018, 13, 1057. [Google Scholar] [CrossRef]
- Kar, E.; Bose, N.; Dutta, B.; Mukherjee, N.; Mukherjee, S. UV and Microwave Protecting, Self-cleaning e-Skin for Efficient Energy Harvesting and Tactile Mechanosensing. ACS Appl. Mater. Interfaces 2019, 11, 17501–17512. [Google Scholar] [CrossRef]
- Yuan, L.; Dai, J.; Fan, X.; Song, T.; Tao, Y.T.; Wang, K.; Xu, Z.; Zhang, J.; Bai, X.; Lu, P.; et al. Self-cleaning flexible infrared nanosensor based on carbon nanoparticles. ACS Nano 2011, 5, 4007–4013. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, S.H.; Lee, J.H.; Lee, S.-J.; Kim, H.-K. Hydrophobic and stretchable Ag nanowire network electrode passivated by a sputtered PTFE layer for self-cleaning transparent thin film heaters. RSC Adv. 2018, 8, 18508–18518. [Google Scholar] [CrossRef] [Green Version]
- Lan, W.; Chen, Y.; Yang, Z.; Han, W.; Zhou, J.; Zhang, Y.; Wang, J.; Tang, G.; Wei, Y.; Dou, W.; et al. Ultraflexible transparent film heater made of Ag nanowire/PVA composite for rapid-response thermotherapy pads. ACS Appl. Mater. Interfaces 2017, 9, 6644–6651. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Kim, D.W.; Kim, J.; Pang, C. A transparent, glue-free, Skin-attachable graphene pressure sensor with micropillars for skinelasticity measurement. Nanotechnology 2019, 30, 335501. [Google Scholar] [CrossRef]
- Song, W.; Gan, B.; Jiang, T.; Zhang, Y.; Yu, A.; Yuan, H.; Chen, N.; Sun, C.; Wang, Z.L. Nanopillar arrayed triboelectric nanogenerator as a self-powered sensitive sensor for a sleep monitoring system. ACS Nano 2016, 10, 8097–8103. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Huang, T.; Wang, H.; Yu, H.; Zhang, Q.; Li, Y. A strong and stretchable self-healing film with self-activated pressure sensitivity for potential artificial skin applications. Sci. Rep. 2013, 3, 3138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Salvatore, G.A.; Araki, H.; Chiarelli, A.M.; Xie, Z.; Banks, A.; Sheng, X.; Liu, Y.; Lee, J.W.; Jang, K.-I.; et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2016, 2, e1600418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Liu, Y.; Kong, G.W.; Seo, J.H.; Ma, Y.; Jang, K.-I.; Fan, J.A.; Mao, S.; Chen, Q.; Li, D.; et al. Epidermal radio frequency electronics for wireless power transfer. Microsyst. Nanoeng. 2016, 2, 16052. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Banks, A.; Cheng, H.; Xie, Z.; Xu, S.; Jang, K.I.; Lee, J.W.; Liu, Z.; Gutruf, P.; Huang, X.; et al. Epidermal electronics with advanced capabilities in near-field communication. Small 2015, 11, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Yi, F.; Zi, Y.; Lin, J.; Wang, X.; Xu, Y.; Wang, Z.L. Sustainably powering wearable electronics solely by biomechanical energy. Nat. Commun. 2016, 7, 12744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turgut, A.; Tuhin, M.O.; Toprakci, O.; Pasquinelli, M.A.; Spontak, R.J.; Toprakci, H.A. Thermoplastic elastomer systems containing carbon nanofibers as soft piezoresistive sensors. ACS Omega 2018, 3, 12648–12657. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; You, J.-M.; Kim, N.-H.; Gu, Y.; Kumar, R.; Mohan, A.V.; Kurniawan, J.; Imani, S.; Nakagawa, T.; Parish, B.; et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 2017, 10, 1581–1589. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, J.; Sun, X.; Li, H.; Peng, H. Stretchable, wearable dye-sensitized solar cells. Adv. Mater. 2014, 26, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Wang, Z.L. Triboelectric nanogenerators as flexible power sources. NPJ Flex. Electron. 2017, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Yang, G.; Lang, T.; Yong, K.-T. Nanogenerators for wearable bioelectronics and biodevices. J. Phys. D Appl. Phys. 2019, 52, 023002. [Google Scholar] [CrossRef]
- Hwang, B.-U.; Lee, J.-H.; Trung, T.Q.; Roh, E.; Kim, D.-I.; Kim, S.-W.; Lee, N.-E. Transparent stretchable self-powered patchable sensor platform with ultrasensitive recognition of human activities. ACS Nano 2015, 9, 8801–8810. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Liu, M.; Chen, X.; Sun, J.; Du, C.; Zhang, Y.; Zhai, J.; Hu, W.; Wang, Z.L. Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci. Adv. 2017, 3, e1700015. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.-I.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R.; et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 2014, 344, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Chen, K.; Shin, W.J.; Lu, C.J.; Kong, G.W.; Patnaik, D.; Lee, S.H.; Cortes, J.F.; Rogers, J.A. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 2014, 10, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.-I.; Han, S.Y.; Xu, S.; Mathewson, K.E.; Zhang, Y.; Jeong, J.-W.; Kim, G.-T.; Webb, R.C.; Lee, J.W.; Dawidczyk, T.J. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 2014, 5, 4779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, S.; Chandra, S. Wearable Sensors for Physiological Parameters Measurement: Physics, Characteristics, Design and Applications; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Mathew, M.; Radhakrishnan, S.; Vaidyanathan, A.; Chakraborty, B.; Rout, C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Nalytical Bioanal. Chem. 2021, 413, 727–762. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, A.; Ahmed, A.; Arya, S. Highly selective and efficient electrochemical sensing of ascorbic acid via CuO/rGO nanocomposites deposited on conductive fabric. Appl. Phys. A 2022, 128, 262. [Google Scholar] [CrossRef]
- Schazmann, B.; Morris, D.; Slater, C.; Beirne, S.; Fay, C.; Reuveny, R.; Moyna, N.; Diamond, D. A wearable electrochemical sensor for the real-time measurement of sweat sodium concentration. Anal. Methods 2010, 2, 342–348. [Google Scholar]
- Xu, J.; Zhang, Z.; Gan, S.; Gao, H.; Kong, H.; Song, Z.; Ge, X.; Bao, Y.; Niu, L. Highly stretchable fiber-based potentiometric ion sensors for multichannel real-time analysis of human sweat. ACS Sens. 2020, 5, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Curto, V.F.; Fay, C.; Coyle, S.; Byrne, R.; O’Toole, C.; Barry, C.; Hughes, S.; Moyna, N.; Diamond, D.; Benito-Lopez, F. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens. Actuators B Chem. 2012, 171, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdes-Ramirez, G.; Andrade, F.J.; Schoening, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. Acs Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully printed wearable microfluidic devices for high-throughput sweat sampling and multiplexed electrochemical analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Yin Yin Nyein, H.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, Y.; Xia, F.; Liu, F.; He, D.; Long, G.; Zeng, X.; Liang, X.; Jin, C.; Wang, Y.; et al. An epidermal electronic system for physiological information acquisition, processing, and storage with an integrated flash memory array. Sci. Adv. 2022, 8, eabp8075. [Google Scholar] [CrossRef] [PubMed]
- Reeder, J.T.; Choi, J.; Xue, Y.; Gutruf, P.; Hanson, J.; Liu, M.; Ray, T.; Avila, R.; Xia, W.; Krishnan, S.; et al. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 2019, 5, eaau6356. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.B.; Lee, K.; Raj, M.S.; Lee, B.; Reeder, J.T.; Koo, J.; Hourlier-Fargette, A.; Bandodkar, A.J.; Won, S.M.; Sekine, Y.; et al. Soft, skin-interfaced microfluidic systems with wireless, battery-free electronics for digital, real-time tracking of sweat loss and electrolyte composition. Small 2018, 14, 1802876. [Google Scholar] [CrossRef]
- Cheng, C.; Li, X.; Xu, G.; Lu, Y.; Low, S.S.; Liu, G.; Zhu, L.; Li, C.; Liu, Q. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens. Bioelectron. 2021, 172, 112782. [Google Scholar] [CrossRef] [PubMed]
- Juan, Z.H.O.U.; Dong, M.E.N.; Xian-En, Z.H.A.N.G. Progress in Wearable Sweat Sensors and Their Applications. Chin. J. Anal. Chem. 2021, 50, 87–96. [Google Scholar]
- Criscuolo, F.; Hanitra, I.N.; Aiassa, S.; Taurino, I.; Oliva, N.; Carrara, S.; De Micheli, G. Wearable multifunctional sweat-sensing system for efficient healthcare monitoring. Sens. Actuators B Chem. 2021, 328, 129017. [Google Scholar] [CrossRef]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-integrated wearable systems: A comprehensive review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ghaffari, R.; Baker, L.B.; Rogers, J.A. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 2018, 4, eaar3921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katchman, B.A.; Zhu, M.; Blain Christen, J.; Anderson, K.S. Eccrine sweat as a biofluid for profiling immune biomarkers. PROTEOMICS—Clin. Appl. 2018, 12, 1800010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, R.; Choi, J.; Raj, M.S.; Chen, S.; Lee, S.P.; Reeder, J.T.; Aranyosi, A.J.; Leech, A.; Li, W.; Schon, S.; et al. Soft wearable systems for colorimetric and electrochemical analysis of biofluids. Adv. Funct. Mater. 2020, 30, 1907269. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable sensors for biochemical sweat analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brothers, M.C.; DeBrosse, M.; Grigsby, C.C.; Naik, R.R.; Hussain, S.M.; Heikenfeld, J.; Kim, S.S. Achievements and challenges for real-time sensing of analytes in sweat within wearable platforms. Acc. Chem. Res. 2019, 52, 297–306. [Google Scholar] [CrossRef]
- Harris, J.; Bickford, J.; Cho, P.; Coppock, M.; Farrell, M.; Holthoff, E.; Ratcliff, E.L. Approaching single molecule sensing: Predictive sweat sensor design for ultra-low limits of detection. In Proceedings of the Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XX, Baltimore, MD, USA, 15–17 April 2019; SPIE: Washington, DC, USA, 2019; Volume 11010, pp. 82–89. [Google Scholar]
- Klimuntowski, M.; Alam, M.M.; Singh, G.; Howlader, M.M. Electrochemical sensing of cannabinoids in biofluids: A noninvasive tool for drug detection. ACS Sens. 2020, 5, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Cao, Z.; Pan, L.; Shi, Y. Advanced wearable microfluidic sensors for healthcare monitoring. Small 2020, 16, 1903822. [Google Scholar] [CrossRef]
- Shrivastava, S.; Trung, T.Q.; Lee, N.-E. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing. Chem. Soc. Rev. 2020, 49, 1812–1866. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Keller, K.; Burtscher, B.; Greco, F. Temporary tattoo as unconventional substrate for conformable and transferable electronics on skin and beyond. Multifunct. Mater. 2020, 3, 032003. [Google Scholar]
- Sreenilayam, S.P.; Ahad, I.U.; Nicolosi, V.; Garzon, V.A.; Brabazon, D. Advanced materials of printed wearables for physiological parameter monitoring. Mater. Today 2020, 32, 147–177. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, Z.; Cheung, C.F.; Yan, F.; Li, G. Digital manufacturing of functional materials for wearable electronics. J. Mater. Chem. C 2020, 8, 10587–10603. [Google Scholar] [CrossRef]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Irie, M.; Ray, T.R.; Kohli, P.; Kozai, N.; et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip 2018, 18, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, H.; Kim, S.B.; Wu, Y.; Ostojich, D.; Park, S.H.; Wang, X.; Weng, Z.; Li, R.; Bandodkar, A.J.; et al. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab Chip 2019, 19, 1545–1555. [Google Scholar] [CrossRef]
- Kim, S.B.; Koo, J.; Yoon, J.; Hourlier-Fargette, A.; Lee, B.; Chen, S.; Jo, S.; Choi, J.; Oh, Y.S.; Lee, G.; et al. Soft, skin-interfaced microfluidic systems with integrated enzymatic assays for measuring the concentration of ammonia and ethanol in sweat. Lab Chip 2020, 20, 84–92. [Google Scholar] [CrossRef]
- Bolat, G.; De la Paz, E.; Azeredo, N.F.; Kartolo, M.; Kim, J.; de Loyola e Silva, A.N.; Rueda, R.; Brown, C.; Angnes, L.; Wang, J.; et al. Wearable soft electrochemical microfluidic device integrated with iontophoresis for sweat biosensing. Anal. Bioanal. Chem. 2022, 414, 5411–5421. [Google Scholar] [CrossRef]
- Kaya, T.; Liu, G.; Ho, J.; Yelamarthi, K.; Miller, K.; Edwards, J.; Stannard, A. Wearable sweat sensors: Background and current trends. Electroanalysis 2019, 31, 411–421. [Google Scholar] [CrossRef]
- Francis, J.; Stamper, I.; Heikenfeld, J.; Gomez, E.F. Digital nanoliter to milliliter flow rate sensor with in vivo demonstration for continuous sweat rate measurement. Lab Chip 2019, 19, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rajaji, U.; Arumugam, R.; Chen, S.M.; Chen, T.W.; Tseng, T.W.; Chinnapaiyan, S.; Lee, S.Y.; Chang, W.H. Graphene nanoribbons in electrochemical sensors and biosensors: A review. Int. J. Electrochem. Sci. 2018, 13, 6643. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef] [PubMed]
- Beitollai, H.; Safaei, M.; Tajik, S. Application of Graphene and Graphene Oxide for modification of electrochemical sensors and biosensors: A review. Int. J. Nano Dimens. 2019, 10, 125–140. [Google Scholar]
- Szunerits, S.; Boukherroub, R. Graphene-based biosensors. Interface Focus 2018, 8, 20160132. [Google Scholar] [CrossRef]
- Wang, W.; Su, H.; Wu, Y.; Zhou, T.; Li, T. Biosensing and biomedical applications of graphene: A review of current progress and future prospect. J. Electrochem. Soc. 2019, 166, B505. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Recent developments on graphene-based electrochemical sensors toward nitrite. J. Electrochem. Soc. 2019, 166, B881. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Justino, C.I.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of nanomaterials: A crucial step in biosensor fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef] [Green Version]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio) sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, B.; Reeder, J.T.; Seo, S.H.; Lee, S.U.; Hourlier-Fargette, A.; Shin, J.; Sekine, Y.; Jeong, H.; Oh, Y.S.; et al. Soft, skin-interfaced microfluidic systems with integrated immunoassays, fluorometric sensors, and impedance measurement capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 27906–27915. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Lin, P.H.; Sheu, S.C.; Chen, C.W.; Huang, S.C.; Li, B.R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Reghunath, R.; Singh, K.K. Recent advances in graphene based electrochemical glucose sensor. Nano Struct. Nano Objects 2021, 26, 100750. [Google Scholar] [CrossRef]
- Le Barc’H, N.; Grossel, J.M.; Looten, P.; Mathlouthi, M. Kinetic study of the mutarotation of D-glucose in concentrated aqueous solution by gas-liquid chromatography. Food Chem. 2001, 74, 119–124. [Google Scholar] [CrossRef]
- RossiNi, A.A.; Soeldner, J.S. Insulin release is glucose anomeric specific in the human. J. Clin. Investig. 1976, 57, 1083–1088. [Google Scholar] [CrossRef]
- Lewis, B.E.; Choytun, N.; Schramm, V.L.; Bennet, A.J. Transition states for glucopyranose interconversion. J. Am. Chem. Soc. 2006, 128, 5049–5058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beden, B.; Largeaud, F.; Kokoh, K.B.; Lamy, C. Fourier transform infrared reflectance spectroscopic investigation of the electrocatalytic oxidation of D-glucose: Identification of reactive intermediates and reaction products. Electrochim. Acta 1996, 41, 701–709. [Google Scholar] [CrossRef]
- Seo, M.; Yeon, S.Y.; Yun, J.; Chung, T.D. Nanoporous ITO implemented bipolar electrode sensor for enhanced electrochemiluminescence. Electrochim. Acta 2019, 314, 89–95. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Pletcher, D. Electrocatalysis: Present and future. J. Appl. Electrochem. 1984, 14, 403–415. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Burke, L.D. Premonolayer oxidation and its role in electrocatalysis. Electrochim. Acta 1994, 39, 1841–1848. [Google Scholar] [CrossRef]
- Burke, L.D. Scope for new applications for gold arising from the electrocatalytic behaviour of its metastable surface states. Gold Bull. 2004, 37, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, M.W.; Adžić, R.R.; Yeager, E.B. Electrochemical oxidation of glucose on single crystal and polycrystalline gold surfaces in phosphate buffer. J. Electrochem. Soc. 1996, 143, 759. [Google Scholar] [CrossRef]
- Singh, R.; Ayyub, M.M. Atomic layer deposition of crystalline β-NiS for superior sensing in thin-film non-enzymatic electrochemical glucose sensors. ACS Appl. Electron. Mater. 2021, 3, 1912–1919. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Segal, M. Selling graphene by the ton. Nat. Nanotechnol. 2009, 4, 612–614. [Google Scholar] [CrossRef]
- Banks, C.E.; Crossley, A.; Salter, C.; Wilkins, S.J.; Compton, R.G. Carbon nanotubes contain metal impurities which are responsible for the “electrocatalysis” seen at some nanotube-modified electrodes. Angew. Chem. Int. Ed. 2006, 45, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Arya, S. Recent advances in tin oxide nanomaterials as electrochemical/chemiresistive sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Yang, Q.; Chen, W. Graphene-based Electrochemical Glucose Sensors: Fabrication and Sensing Properties. Electroanalysis 2018, 30, 2504–2524. [Google Scholar] [CrossRef]
- Boroujerdi, R.; Paul, R. Graphene-Based Electrochemical Sensors for Psychoactive Drugs. Nanomaterials 2022, 12, 2250. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Ju, H. Amperometric glucose sensor based on catalytic reduction of dissolved oxygen at soluble carbon nanofiber. Biosens. Bioelectron. 2007, 23, 479–484. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens. Bioelectron. 2010, 25, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sens. Actuators B Chem. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Cao, L.; Han, G.C.; Xiao, H.; Chen, Z.; Fang, C. A novel 3D paper-based microfluidic electrochemical glucose biosensor based on rGO-TEPA/PB sensitive film. Anal. Chim. Acta 2020, 1096, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas. International Diabetes Federation. 2021. Available online: https://idf.org/our-activities/advocacy-awareness/resources-and-tools/58:idf-annual-report-2015.html (accessed on 14 October 2022).
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2017, 138, 271–281. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [CrossRef]
- Guagneli, L.; Mousavi, Z.; Sokalski, T.; Leito, I.; Bobacka, J. Novel design of a planar flow-through potentiometric sensor. J. Electroanal. Chem. 2022, 923, 116785. [Google Scholar] [CrossRef]
- Pei, X.; Sun, M.; Wang, J.; Bai, J.; Bo, X.; Zhou, M. A Bifunctional Fully Integrated Wearable Tracker for Epidermal Sweat and Wound Exudate Multiple Biomarkers Monitoring. Small 2022, 2205061. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Hinestroza, J.P.; Rodthongkum, N. Non-invasive textile based colorimetric sensor for the simultaneous detection of sweat pH and lactate. Talanta 2019, 192, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Fang, G.; Kuang, Z.; Cheng, L.; Wu, H.; Guo, D.; Liu, A. 3D-printed low-cost fabrication and facile integration of flexible epidermal microfluidics platform. Sens. Actuators B Chem. 2022, 353, 131085. [Google Scholar] [CrossRef]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B Chem. 2022, 131876. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Ismail-Beigi, F. Glycemic management of type 2 diabetes mellitus. N. Engl. J. Med. 2012, 366, 1319–1327. [Google Scholar] [CrossRef]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef]
- Namkoong, M.; Guo, H.; Rahman, M.S.; Wang, D.; Pfeil, C.J.; Hager, S.; Tian, L. Moldable and transferrable conductive nanocomposites for epidermal electronics. NPJ Flex. Electron. 2022, 6, 41. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, E.; Kellner, R. Measuring glucose and urea by flow injection analysis with FTIR detection. J. Mol. Struct. 1993, 294, 9–12. [Google Scholar] [CrossRef]
- Gupta, J.; Arya, S.; Verma, S.; Singh, A.; Sharma, A.; Singh, B.; Sharma, R. Performance of template-assisted electrodeposited Copper/Cobalt bilayered nanowires as an efficient glucose and Uric acid senor. Mater. Chem. Phys. 2019, 238, 121969. [Google Scholar] [CrossRef]
- Witkowska Nery, E.; Kundys, M.; Jelen, P.S.; Jönsson-Niedziółka, M. Electrochemical glucose sensing: Is there still room for improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, S.; Lai, Y.; Zhu, Y.; Guo, R.; Xia, Y.; Huang, W.; Li, Z. Rapid electrochemical conversion of smooth Cu surfaces to urchin-like Cu nanowire arrays via flower-like Cu2Se nanosheets as an advanced nonenzymatic glucose sensor. Sens. Actuators B Chem. 2018, 262, 801–809. [Google Scholar] [CrossRef]
- Lee, W.C.; Kim, K.B.; Gurudatt, N.G.; Hussain, K.K.; Choi, C.S.; Park, D.S.; Shim, Y.B. Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer. Biosens. Bioelectron. 2019, 130, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, Z.; Shen, S.; Xu, L.; Luo, Z. Highly sensitive nonenzymatic glucose sensor based on reduced graphene oxide/ultrasmall Pt nanowire nanocomposites. Int. J. Electrochem. Sci. 2018, 13, 4817–4826. [Google Scholar] [CrossRef]
- Tyagi, C.; Lakshmi, G.B.V.S.; Jaiswal, V.; Avasthi, D.K.; Tripathi, A. Gold-graphene oxide nanocomposites for enzyme-less glucose monitoring. Biomed. Phys. Eng. Express 2018, 4, 065002. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Hwang, J.Y.; Kim, N.H.; Lee, J.H. In situ synthesis of graphene-encapsulated gold nanoparticle hybrid electrodes for non-enzymatic glucose sensing. Carbon 2016, 98, 90–98. [Google Scholar] [CrossRef]
- Singhal, A.; Anand, V.K.; Virdi, G.S. Graphene Oxide/Silver Nanocomposite Based Non-Enzymatic Glucose Sensor. J. Bionanosci. 2018, 12, 397–400. [Google Scholar] [CrossRef]
- Joshi, A.C.; Markad, G.B.; Haram, S.K. Rudimentary simple method for the decoration of graphene oxide with silver nanoparticles: Their application for the amperometric detection of glucose in the human blood samples. Electrochim. Acta 2015, 161, 108–114. [Google Scholar] [CrossRef]
- Ismail, N.S.; Le, Q.H.; Yoshikawa, H.; Saito, M.; Tamiya, E. Development of non-enzymatic electrochemical glucose sensor based on graphene oxide nanoribbon-gold nanoparticle hybrid. Electrochim. Acta 2014, 146, 98–105. [Google Scholar] [CrossRef]
- Janyasupab, M.; Promptmas, C. Development of non-enzymatic N-doped graphene supported cobalt/iron amperometric based sensor for glucose detection in urine. In Proceedings of the 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 577–582. [Google Scholar]
- Chaiyo, S.; Mehmeti, E.; Siangproh, W.; Hoang, T.L.; Nguyen, H.P.; Chailapakul, O.; Kalcher, K. Non-enzymatic electrochemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine-ionic liquid-graphene composite. Biosens. Bioelectron. 2018, 102, 113–120. [Google Scholar] [CrossRef]
- Vilian, A.E.; Dinesh, B.; Rethinasabapathy, M.; Hwang, S.K.; Jin, C.S.; Huh, Y.S.; Han, Y.K. Hexagonal Co3O4 anchored reduced graphene oxide sheets for high-performance supercapacitors and non-enzymatic glucose sensing. J. Mater. Chem. A 2018, 6, 14367–14379. [Google Scholar] [CrossRef]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. A non-enzymatic glucose sensor based on the CuS nanoflakes-reduced graphene oxide nanocomposite. Anal. Methods 2018, 10, 381–388. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Q.; Yin, W.; Jiang, T.; Zhao, D.; Jiang, L. A novel nonenzymatic glucose sensor based on functionalized PDDA-graphene/CuO nanocomposites. Sens. Actuators B Chem. 2017, 253, 1087–1095. [Google Scholar] [CrossRef]
- Tehrani, F.; Bavarian, B. Facile and scalable disposable sensor based on laser engraved graphene for electrochemical detection of glucose. Sci. Rep. 2016, 6, 27975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, E.; Zhang, X. Non-enzymatic glucose biosensor based on copper oxide-reduced graphene oxide nanocomposites synthesized from water-isopropanol solution. Electrochim. Acta 2014, 130, 253–260. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, P.; Dai, E.; Liu, J.; Tian, Z.; Liang, C.; Shao, G. A novel reduction approach to fabricate quantum-sized SnO2-conjugated reduced graphene oxide nanocomposites as non-enzymatic glucose sensors. Phys. Chem. Chem. Phys. 2014, 16, 8801–8807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasseghian, Y.; Dragoi, E.N.; Moradi, M.; Khaneghah, A.M. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2021, 148, 111931. [Google Scholar]
- Shackery, I.; Patil, U.; Pezeshki, A.; Shinde, N.M.; Kang, S.; Im, S.; Jun, S.C. Copper hydroxide nanorods decorated porous graphene foam electrodes for non-enzymatic glucose sensing. Electrochim. Acta 2016, 191, 954–961. [Google Scholar] [CrossRef]
- Deng, Z.P.; Sun, Y.; Wang, Y.C.; Gao, J.D. A NiFe alloy reduced on graphene oxide for electrochemical nonenzymatic glucose sensing. Sensors 2018, 18, 3972. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Liang, X.; Liu, H.; Cui, X.Z.L.; Liu, C. Non-enzymatic electrochemical glucose sensor based on monodispersed stone-like PtNi alloy nanoparticles. Microchim. Acta 2018, 185, 339. [Google Scholar]
- Amin, B.G.; Masud, J.; Nath, M. A non-enzymatic glucose sensor based on a CoNi2 Se4/rGO nanocomposite with ultrahigh sensitivity at low working potential. J. Mater. Chem. B 2019, 7, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Su, L.; Li, H.; Li, M.; Li, C.; Xu, S.; Qian, L.; Yang, B. Non-enzymatic glucose sensor based on micro-/nanostructured Cu/Ni deposited on graphene sheets. J. Electroanal. Chem. 2019, 838, 154–162. [Google Scholar] [CrossRef]
- Chung, J.S.; Hur, S.H. A highly sensitive enzyme-free glucose sensor based on Co3O4 nanoflowers and 3D graphene oxide hydrogel fabricated via hydrothermal synthesis. Sens. Actuators B Chem. 2016, 223, 76–82. [Google Scholar]
- Li, G.; Huo, H.; Xu, C. Ni0.31Co0.69S2 nanoparticles uniformly anchored on a porous reduced graphene oxide framework for a high-performance non-enzymatic glucose sensor. J. Mater. Chem. A 2015, 3, 4922–4930. [Google Scholar] [CrossRef]
- Dhara, K.; Thiagarajan, R.; Nair, B.G.; Thekkedath, G.S.B. Highly sensitive and wide-range nonenzymatic disposable glucose sensor based on a screen printed carbon electrode modified with reduced graphene oxide and Pd-CuO nanoparticles. Microchim. Acta 2015, 182, 2183–2192. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Malode, S.J.; Shetti, N.P. Graphene-based electrochemical immunosensors for early detection of oncomarker carcinoembryonic antigen. Biosens. Bioelectron. X 2022, 11, 100189. [Google Scholar] [CrossRef]
- Dhara, K.; Stanley, J.; Ramachandran, T.; Nair, B.G.; TG, S.B. Pt-CuO nanoparticles decorated reduced graphene oxide for the fabrication of highly sensitive non-enzymatic disposable glucose sensor. Sens. Actuators B Chem. 2014, 195, 197–205. [Google Scholar] [CrossRef]
- Li, M.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. One-pot ionic liquid-assisted synthesis of highly dispersed PtPd nanoparticles/reduced graphene oxide composites for nonenzymatic glucose detection. Biosens. Bioelectron. 2014, 56, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, F.; Ben, A.; Chen, C. Synthesis of hollow Pt-Ni-graphene nanostructures for nonenzymatic glucose detection. J. Electroanal. Chem. 2014, 726, 55–61. [Google Scholar] [CrossRef]
- Qu, F.; Sun, H.; Zhang, Y.; Lu, H.; Yang, M. Electrochemically deposited Pd nanorod array/sol-gel silica thin film for the fabrication of electrochemical sensors. Sens. Actuators B Chem. 2012, 166, 837–841. [Google Scholar] [CrossRef]
- Li, M.; Bo, X.; Mu, Z.; Zhang, Y.; Guo, L. Electrodeposition of nickel oxide and platinum nanoparticles on electrochemically reduced graphene oxide film as a nonenzymatic glucose sensor. Sens. Actuators B Chem. 2014, 192, 261–268. [Google Scholar] [CrossRef]
- Chen, X.; Tian, X.; Zhao, L.; Huang, Z.; Oyama, M. Nonenzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with graphene nanosheets and Pt-Pd bimetallic nanocubes. Microchim. Acta 2014, 181, 783–789. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Ye, Y.; Sun, L.; Song, Y. Nickel-cobalt nanostructures coated reduced graphene oxide nanocomposite electrode for nonenzymatic glucose biosensing. Electrochim. Acta 2013, 114, 484–493. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Wang, W.-; Zhang, X.; Peng, W. CuO nanoparticles on sulfur-doped graphene for nonenzymatic glucose sensing. Electrochim. Acta 2015, 156, 244–251. [Google Scholar]
- Yang, S.; Liu, L.; Wang, G.; Li, G.; Deng, D.; Qu, L. One-pot synthesis of Mn3O4 nanoparticles decorated with nitrogen-doped reduced graphene oxide for sensitive nonenzymatic glucose sensing. J. Electroanal. Chem. 2015, 755, 15–21. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Wang, G.; Zhao, J.; Gao, X.; Qu, L. Synthesis of Mn3O4 nanoparticles/nitrogen-doped graphene hybrid composite for nonenzymatic glucose sensor. Sens. Actuators B Chem. 2015, 221, 172–178. [Google Scholar] [CrossRef]
- Balamurugan, J.; Thanh, T.D.; Heo, S.B.; Kim, N.H.; Lee, J.H. Novel route to synthesis of N-doped graphene/Cu-Ni oxide composite for high electrochemical performance. Carbon 2015, 94, 962–970. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Q.; Wang, K.; Qian, J.; Dong, X.; Yang, Z.; Du, X.; Qiu, B. Enhanced non-enzymatic glucose sensing based on copper nanoparticles decorated nitrogen-doped graphene. Biosens. Bioelectron. 2014, 54, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.M.; Goudini, L.; Piri, F.; Zamani, A.; Saadati, F. Molecular imprinting method for fabricating novel glucose sensor: Polyvinyl acetate electrode reinforced by MnO2/CuO loaded on graphene oxide nanoparticles. Food Chem. 2016, 194, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hui, N.; Wang, S.; Xie, H.; Xu, S.; Niu, S.; Luo, X. Nickel nanoparticles modified conducting polymer composite of reduced graphene oxide doped poly (3, 4-ethylenedioxythiophene) for enhanced nonenzymatic glucose sensing. Sens. Actuators B Chem. 2015, 221, 606–613. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Lu, X.; Li, Z.; Sun, L.; Song, Y. Dendritic copper-cobalt nanostructures/reduced graphene oxide-chitosan modified glassy carbon electrode for glucose sensing. Sens. Actuators B Chem. 2014, 195, 1–7. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, J.; Qiang, X.; Xia, Y.; Shi, G.; Zhang, F.; Tang, J. Polymer-assisted in situ growth of copper nanoparticles on graphene surface for non-enzymatic electrochemical sensing of glucose. Int. J. Electrochem. Sci. 2013, 8, 6941–6950. [Google Scholar]
- Yang, J.; Yu, J.H.; Strickler, J.R.; Chang, W.J.; Gunasekaran, S. Nickel nanoparticle-chitosan-reduced graphene oxide-modified screen-printed electrodes for enzyme-free glucose sensing in portable microfluidic devices. Biosens. Bioelectron. 2013, 47, 530–538. [Google Scholar] [CrossRef]
- Lu, L.M.; Li, H.B.; Qu, F.; Zhang, X.B.; Shen, G.L.; Yu, R.Q. In situ synthesis of palladium nanoparticle-graphene nanohybrids and their application in nonenzymatic glucose biosensors. Biosens. Bioelectron. 2011, 26, 3500–3504. [Google Scholar] [CrossRef] [PubMed]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramírez, J.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef]
- Jo, S.; Sung, D.; Kim, S.; Koo, J. A review of wearable biosensors for sweat analysis. Biomed. Eng. Lett. 2021, 11, 117–129. [Google Scholar] [CrossRef]
- Thomas, N.; Lähdesmäki, I.; Parviz, B.A. A contact lens with an integrated lactate sensor. Sens. Actuators B Chem. 2012, 162, 128–134. [Google Scholar] [CrossRef]
- Onor, M.; Gufoni, S.; Lomonaco, T.; Ghimenti, S.; Salvo, P.; Sorrentino, F.; Bramanti, E. Potentiometric sensor for non invasive lactate determination in human sweat. Anal. Chim. Acta 2017, 989, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Tur-García, E.L.; Davis, F.; Collyer, S.D.; Holmes, J.L.; Barr, H.; Higson, S.P. Novel flexible enzyme laminate-based sensor for analysis of lactate in sweat. Sens. Actuators B Chem. 2017, 242, 502–510. [Google Scholar] [CrossRef]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, D.; Möller, B.; Klimes, N.; Szeponik, J.; Fischer, S. Amperometric lactate oxidase catheter for real-time lactate monitoring based on thin film technology. Biosens. Bioelectron. 1997, 12, 539–550. [Google Scholar] [CrossRef]
- Van Haeringen, N.J. Clinical biochemistry of tears. Surv. Ophthalmol. 1981, 26, 84–96. [Google Scholar] [CrossRef]
- Koyun, A.; Ahlatcolu, E.; Koca, Y.; Kara, S. Biosensors and their principles. Roadmap Biomed. Eng. Milest. 2012, 117–142. [Google Scholar]
- Scouten, W.H.; Luong, J.H.; Brown, R.S. Enzyme or protein immobilization techniques for applications in biosensor design. Trends Biotechnol. 1995, 13, 178–185. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B. Electrochemical biosensing in extreme environment. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2002, 14, 7–16. [Google Scholar] [CrossRef]
- Habermüller, K.; Mosbach, M.; Schuhmann, W. Electron-transfer mechanisms in amperometric biosensors. Fresenius’ J. Anal. Chem. 2000, 366, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Ghindilis, A.L.; Atanasov, P.; Wilkins, E. Enzyme-catalyzed direct electron transfer: Fundamentals and analytical applications. Electroanalysis 1997, 9, 661–674. [Google Scholar] [CrossRef]
- Montagné, M.; Marty, J.L. Bi-enzyme amperometric d-lactate sensor using macromolecular NAD+. Anal. Chim. Acta 1995, 315, 297–302. [Google Scholar] [CrossRef]
- Bridge, K.; Davis, F.; Collyer, S.D.; Higson, S.P. Flexible ultrathin polyDVB/EVB composite membranes for the optimization of a Lactate Sensor. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2007, 19, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Groegel, D.B.; Link, M.; Duerkop, A.; Wolfbeis, O.S. A New Fluorescent PET Probe for Hydrogen Peroxide and its Use in Enzymatic Assays for L-Lactate and D-Glucose. ChemBioChem 2011, 12, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; RoyChoudhury, S.; Jalal, A.H.; Umasankar, Y.; Forouzanfar, S.; Akter, N.; Bhansali, S.; Pala, N. Lactate biosensing: The emerging point-of-care and personal health monitoring. Biosens. Bioelectron. 2018, 117, 818–829. [Google Scholar] [CrossRef]
- Lin, K.C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible graphene oxide) sensor for electrochemical monitoring lactate in low-volume passive perspired human sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, D.; Ghanta, R.; Lin, K.C.; Muthukumar, S.; Prasad, S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, I.; Revenga-Parra, M.; Weber, K.; Popp, J.; Pariente, F.; Lorenzo, E. One-step reduced/quinone functionalized graphene oxide as reagentless lactate biosensing platform. Sens. Actuators B Chem. 2018, 267, 533–541. [Google Scholar] [CrossRef]
- Sainz, R.; Del Pozo, M.; Vázquez, L.; Vilas-Varela, M.; Castro-Esteban, J.; Blanco, E.; Petit-Domínguez, M.D.; Quintana, C.; Casero, E. Lactate biosensing based on covalent immobilization of lactate oxidase onto chevron-like graphene nanoribbons via diazotization-coupling reaction. Anal. Chim. Acta 2022, 1208, 339851. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Sharma, S.; Adak, B.; Hossain, M.M.; LaChance, A.M.; Mukhopadhyay, S.; Sun, L. Two-dimensional MXenes: New frontier of wearable and flexible electronics. InfoMat 2022, 4, e12295. [Google Scholar] [CrossRef]
- He, X.; Fan, C.; Luo, Y.; Xu, T.; Zhang, X. Flexible microfluidic nanoplasmonic sensors for refreshable and portable recognition of sweat biochemical fingerprint. NPJ Flex. Electron. 2022, 6, 60. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhu, M.; Li, J.; Liu, L.; Yu, J.; Li, Z.; Ding, B. Wearable biosensor for sensitive detection of uric acid in artificial sweat enabled by a fiber structured sensing interface. Nano Energy 2021, 85, 106031. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.; Diao, L.; Li, H.; Tong, Z.; Gu, Z.; Miao, B.; Xu, Z.; Zhang, H.; Wu, Y.; et al. Highly Sensitive Uric Acid Detection Based on a Graphene Chemoresistor and Magnetic Beads. Biosensors 2021, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Movlaee, K.; Norouzi, P.; Beitollahi, H.; Rezapour, M.; Larijani, B. Highly Selective Differential Pulse Voltammetric Determination of Uric Acid Using Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2017, 12, 3241–3251. [Google Scholar] [CrossRef]
- Pramoda, K.; Moses, K.; Maitra, U.; Rao, C.N.R. Superior Performance of a MoS2-RGO Composite and a Borocarbonitride in the Electrochemical Detection of Dopamine, Uric Acid and Adenine. Electroanalysis 2015, 27, 1892–1898. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, T.F.; Kong, W.Y.; Zhang, Z.X.; Qu, H.; Chen, W.; Wang, Y.B.; Luo, L.B.; Zheng, L. ZIF-67 Derived Porous Co3O4 Hollow Nanopolyhedron Functionalized Solution-Gated Graphene Transistors for Simultaneous Detection of Glucose and Uric Acid in Tears. Biosens. Bioelectron. 2018, 101, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, X.; Chai, H.; Diao, G.; Piao, Y. 3D Hybrids of Interconnected Porous Carbon Nanosheets/Vertically Aligned Polyaniline Nanowires for High-Performance Supercapacitors. Adv. Mater. Interfaces 2018, 5, 1800106. [Google Scholar] [CrossRef]
- Peng, B.; Cui, J.; Wang, Y.; Liu, J.; Zheng, H.; Jin, L.; Zhang, X.; Zhang, Y.; Wu, Y. CeO2-x/C/rGO Nanocomposites Derived from Ce-MOF and Graphene Oxide as a Robust Platform for Highly Sensitive Uric Acid Detection. Nanoscale 2018, 10, 1939–1945. [Google Scholar] [CrossRef]
- Jiang, J.; Du, X. Sensitive Electrochemical Sensors for Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid Based on Au@Pd-Reduced Graphene Oxide Nanocomposites. Nanoscale 2014, 6, 11303–11309. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.-C.; Ma, L.-X. One-Pot Facile Fabrication of Graphene-Zinc Oxide Composite and Its Enhanced Sensitivity for Simultaneous Electrochemical Detection of Ascorbic Acid, Dopamine and Uric Acid. Sens. Actuators B Chem. 2016, 227, 488–496. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Z.; Liu, L. Covalent Bonded Graphene/Neutral Red Nanocomposite Prepared by One-Step Electrochemical Method and Its Electrocatalytic Properties toward Uric Acid. Electroanalysis 2018, 30, 1017–1021. [Google Scholar] [CrossRef]
- Dai, H.; Wang, N.; Wang, D.; Zhang, X.; Ma, H.; Lin, M. Voltammetric Uric Acid Sensor Based on a Glassy Carbon Electrode Modified with a Nanocomposite Consisting of Polytetraphenylporphyrin, Polypyrrole, and Graphene Oxide. Microchim. Acta 2016, 183, 3053–3059. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical Characterization of Au/ZnO/PPy/RGO Nanocomposite and Its Application for Simultaneous Determination of Ascorbic Acid, Epinephrine, and Uric Acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Ghanbari, K.; Moloudi, M. Flower-like ZnO Decorated Polyaniline/Reduced Graphene Oxide Nanocomposites for Simultaneous Determination of Dopamine and Uric Acid. Anal. Biochem. 2016, 512, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.W.; Jeong, S.Y.; Kwon, Y.W.; Lee, J.U.; Cho, S.C.; Shin, B.S. Fabrication of laser-induced graphene-based multifunctional sensing platform for sweat ion and human motion monitoring. Sens. Actuators A Phys. 2022, 334, 113320. [Google Scholar] [CrossRef]
- An, Q.; Gan, S.; Xu, J.; Bao, Y.; Wu, T.; Kong, H.; Zhong, L.; Ma, Y.; Song, Z.; Niu, L. A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring. Electrochem. Commun. 2019, 107, 106553. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Serani, A.; Fiore, L.; Moscone, D.; Arduini, F. All-solid state ion-selective carbon black-modified printed electrode for sodium detection in sweat. Electrochim. Acta 2021, 394, 139050. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Biscay, J.; Findlay, E.; Dennany, L. Electrochemical monitoring of alcohol in sweat. Talanta 2021, 224, 121815. [Google Scholar] [CrossRef]
- Dias, A.A.; Chagas, C.L.; Silva-Neto, H.D.A.; Lobo-Junior, E.O.; Sgobbi, L.F.; de Araujo, W.R.; Paixão, T.R.; Coltro, W.K. Environmentally friendly manufacturing of flexible graphite electrodes for a wearable device monitoring zinc in sweat. ACS Appl. Mater. Interfaces 2019, 11, 39484–39492. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, S.; Jeong, R.A.; Boo, H.; Park, J.; Kim, H.C.; Chung, T.D. Nonenzymatic continuous glucose monitoring in human whole blood using electrified nanoporous Pt. Biosens. Bioelectron. 2012, 31, 284–291. [Google Scholar] [CrossRef] [PubMed]



































| Matrix | Synthesis Process | Filler | Fracture Strain (%) | Tensile Strength (MPa) | Reference |
|---|---|---|---|---|---|
| DGEBA | Three-roll mill | Thermally rGO | - | 44.1 ± 5.0 | [208] |
| Epoxy | In situ Polymerization (ISP) | Thermally rGO | 5.0 ± 0.5 | 63 ± 1.0 | [209] |
| WPU | ISP | rGO | - | 14.6 ± 3.8 | [210] |
| Phenol formaldehyde | ISP | rGO | - | 1400 ± 0.04 | [211] |
| PMMA | ISP | GO | - | 66.8 ± 3.05 | [212] |
| PI | ISP | FGO | 6.9 ± 2.1 | 179.79 ± 17.72 | [213] |
| Polybutadiene | ISP | Octadecylamine GO | 450 ± 25 | - | [214] |
| DGEBF | Resin transfer moulding | Sulfonic GO | - | 41 | [215] |
| PI | ISP | GO | 8.5 ± 2.4 | 137.8 ± 9.7 | [216] |
| Carboxylated Acrylonitrile butadiene rubber | Latex coagulation Method | GO | 206 ± 16 | 8.8 ± 0.5 | [217] |
| Polyvinylchloride (PVC) | Colloidal blending | GO | 73.95 | 54.42 | [218] |
| Polyamide 6 | ISP | GO | - | 64.9 | [219] |
| DGEBA | ISP | GO | - | 50 | [220] |
| Epoxy | Casting | GO | 55 | 13 | [221] |
| PVA | Solution mixing | Graphene | 19 ± 1 | 9.01 ± 0.3 | [222] |
| WPU | Sol–gel | f-GNS | 138 ± 30 | 20.2 ± 2.0 | [223] |
| DETDA | Solution mixing | Diazonium-FG | 3.9 ± 0.3 | 71.4 ± 0.8 | [224] |
| Epoxy | Solution blending | GNP | 3.61 ± 0.19 | 51.65 ± 1.43 | [225] |
| Glassy epoxy | Solution blending | Graphite | 4.75 | 60.76 | [226] |
| Poly (lactic acid) | Melt compounding | GNP-small | 10.9 ± 0.3 | 58.5 ± 0.7 | [227] |
| PU | Solvent casting | GNP | 5.7 ± 0.54 | 18.8 ± 1.95 | [228] |
| Epoxy | Direct mixing method | GNPs | 1.143 | 88.99 | [229] |
| PP | Melt Compounding method | GNS | 3.66 ± 0.75 | 30.16 ± 0.34 | [230] |
| Epoxy | Solution mixing | Expanded graphene | 25 (mm) | 2.25 | [231] |
| PVA | Solution mixing | Sulfonated graphene | - | 97 | [232] |
| Regenerated cellulose (RC) | Wet spinning | Graphene | - | 360 | [233] |
| PMMA | ISP | Graphene | 1.79 ± 0.18 | 49.15 ± 0.86 | [234] |
| Polypropylene (iPP) | Solution mixing | Graphene | 2.9 ± 0.5 | 16 ± 3 | [235] |
| Material | Synthesis Method | Reducing Agent | Electrical Conductivity (S cm−1) | Reference |
|---|---|---|---|---|
| TrGO | Liquid Exfoliation (LE) | Thermal reduction | 80 | [247] |
| fGO | LE | Hydrazine and Pyrene groups | ∼1000 | [248] |
| GNS | LE | Hydrazine | 24 | [249] |
| rGO | LE | Hydroiodic acid and acetic acid | 304 | [249] |
| TrGO | LE | Thermal | 727 | [250] |
| TrGO | LE | Hydrazine and thermal annealing | 298 | [251] |
| GNS | LE | Ammonia and hydrazine | 7.2 | [252] |
| Gr | LE | Ammonia and hydrazine | 5.5 | [253] |
| TrGO | LE | Thermal | 2.3 | [254] |
| GNS | LE | Hydroquinone | - | [255] |
| Gr | LE | Hydrazine hydrate | 1000 | [256] |
| rGO | LE | - | 72 | [257] |
| rGO | LE | Dextrose | 18 | [258] |
| rGO | LE | Sodium borohydride | 34 | [258] |
| rGO | LE | Hydrobromic acid | 36 | [258] |
| rGO | LE | Hydrazine hydrate | 58 | [258] |
| rGO | LE | Hydroiodic acid | 103 | [258] |
| rGO | LE | KOH | 60 | [259] |
| Matrix | Synthesis Process | Filler | CTE (°C) | k (W m−1 K−1) | Tg (°C) | Reference |
|---|---|---|---|---|---|---|
| DGEBA | Ball mill mixing | rGO | - | - | 157.4 ± 1.8 | [263] |
| DGEBA | Three-roll mill | rGO | - | - | 154.8 | [264] |
| PMMA | Solution blending | rGO | 4.59 × 10−5 | - | 135.23 | [265] |
| EP/GF | Hand lay-up process | Ethylenediamine (EDA)-FGO | - | - | 127.9 | [266] |
| Epoxy resin (CYD−128) | Solvent-free | Nanocrystal-f-GO | - | - | 131.42 | [267] |
| PVA | Casting method | GO | - | - | 76 | [268] |
| DGEBA | Polymerization | GO | - | - | 71.5 | [269] |
| Epoxy | Polymerization | Graphene-BN | - | 6.2–9.5 | - | [270] |
| EPON 862 | Polymerization | Graphite | 7.7 ± 0.1 × 10−5 | 1.0 | 135.3 ± 0.8 | [271] |
| EPON 862 | Solution blending | Exfoliated graphite | 57.73 µm/(m °C) | 5.0 | - | [272] |
| Paraffin | Solvent evaporation | xGnPs | - | 2.7 | - | [273] |
| Analytes | Recognition Component | Transduction Technique | Concentration in Sweat | References |
|---|---|---|---|---|
| Glucose | Glucose oxidase | Amperometry | 10–200 µM | [281,282,283,284,285,286,287,288,289,290] |
| Lactate | Lactate Oxidase | Amperometry | 5–20 mM | [282,283,284,285] |
| Uric acid | Uricase | Amperometry | 2–10 mM | [292] |
| Cortisol | 2D materials | Impedimetric sensor | 8–140 ng mL−1 | [296,297] |
| Ascorbic acid | Ascorbate oxidase | Amperometry | 10–50 µM | [293,294,295] |
| Caffeine | Nanomaterials | Voltammetry | - | [310] |
| Tyrosine | Nanomaterials | Amperometry | 6–240 µM | [298,299] |
| F17464 | Carbon | Voltammetry | - | [301] |
| Ethyl glucuronide | Monoclonal antibody | Immunosensor | 1.7–103 µg L−1 | [300] |
| Cd2+ | Bi | SWASV | <100 µg L−1 | [316] |
| Zn2+ | Bi | SWASV | 100–1560 µg L−1 | [315] |
| Ca2+ | Ca ion selective electrode | Potentiometry | 0.41–12.4 mM | [314] |
| NH4+ | Nonactin ionophore | Potentiometry | 0.1–1 mM | [313] |
| pH | Conducting polymer | Potentiometry | 3–8 | [310,311,312] |
| K+ | K Ion selective membrane | Potentiometry | 1–18.5 mM | [308] |
| Cl− | Ag/AgCl | Potentiometry | 10–100 mM | [306,307] |
| Na+ | Na Ion selective membrane | Potentiometry | 10–100 mM | [300,301,302,303,304,305] |
| Electrode | Sensitivity | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|
| PtNW/RGO | 56.11 μA mmol cm−2/L | 0.032–1.89 mmol/L | 4.6 μmol/L | [496] |
| Au–GO | 25 μA mM−1 cm−2 | 0.05 mM–10 mM | - | [497] |
| AuNP-FLG | 0.195 μA mM−1 cm−2 | 6 μM–28.5 mM | 1 μM | [498] |
| Pt/GOH | 137.4 μA mM−1 cm−2 | - | - | [499] |
| AgNP-GO | 11 μA mM−1 cm−2 | 1–14 mM | 4 μM | [500] |
| AuNPs/GONR | 59.1 μA mM−1 cm−2 | 0.005–4.92 mM | 5 μM | [501] |
| Co/Fe/N-doped graphene | 476.67 μA mM−1 cm−2 | 0–32.5 mM | 37.7 μM | [502] |
| CoPC/graphene/IL/SPCE | - | 0.01–13 mM | 0.67 μM | [503] |
| HexagonalCo3O4/rGO | 1.315 mA mM−1 cm−2 | - | 0.4 μM | [504] |
| CuO nanoflakes/rGO | 53.5 μA mM−1 cm−2 | 1–2000 μM | 0.19 μM | [505] |
| PDDAgraphene/CuO nanocomposite | 4982.2 μA mM−1 cm−2 | 0.4–4000 μM | 0.2 μM | [506] |
| CuNCs/graphene | 4532.2 μA mM−1 cm−2 | 25 μM–4 mM | 250 nM | [507] |
| CuO/rGO | 2221 μA mM−1 cm−2 | 0.4 μM–12 mM | 0.1 μM | [508] |
| SnO2/rGO | 1.93 AM−1 cm−2 | 50 μM–500 μM | 13.35 μM | [509] |
| NiNPs/graphene | 8652 μA mM−1 cm−2 | 5–550 μM | 1.85 μM | [510] |
| Cu(OH)2/PGF | 3.36 mA mM−1 cm−2 | 1.2 μM–6 mM | 1.2 μM | [511] |
| NiFe/GO | 173 μA mM−1 cm−2 | 0.05–5 mM | 9 μM | [512] |
| PtNi alloy/graphene glassy electrode | 40.17 μA mM−1 cm−2 | 0.5–40 mM | 0.355 μM | [513] |
| CoNi2Se4/rGO | 18.89 mA mM−1 cm−2 | 1 μM–4.0 mM | 0.65 μM | [514] |
| Cu/Ni/graphene/Ta | 17 857 μA mM−1 cm−2 | 0.24–2.33 mM | 0.0027 μM | [515] |
| Co3O4NF/GOHs | 492.8 A mM−1 cm−2 | 0.25 mM–10 mM | - | [516] |
| NiCoS2/rGO | 1753 μA mM−1 cm−2 | 0.001–5 mM | 0.078 μM | [517] |
| Pd–CuO/rGO/SPE | 3355 μA mM−1 cm−2 | 6 μM–22 mM | 30 nM | [518] |
| Pd/NiO@Nile-rGO | - | 0.020–20.0 mmol L−1 | 2.2 μmol L−1 | [519] |
| Pt–CuO/rGO | 3577 μA mM−1 cm−2 | Up to 12 mM | 0.01 μM | [520] |
| PtPd-IL-rGO | 1.47 μA mM−1 cm−2 | 0.1–22 mM | 2 μM | [521] |
| Pt-Ni/graphene | 30.32 μA mM−1 cm−2 | 0.5–20 mM | 2 μM | [522] |
| PdCu/GE | 48 μA mM−1 cm−2 | up to 18 mM | 20 μM | [523] |
| NiO/Pt/ERGO | 668.2 μA mM−1 cm−2 | 0.05–5.66 mM | 0.2 μM | [524] |
| PtPdNCs/GNs | 1.4 μA mM−1 cm−2 | Up to 24.5 mM | - | [525] |
| Ni–Co/rGO | 1773.6 μA mM−1 cm−2 | 0.01–2.65 mM | 3.79 μM | [526] |
| CuONPs/sulphur-doped graphene | 1298.6 μA mM−1 cm−2 | 0.1–10.5 mM | 80 nM | [527] |
| MnO3O4/N-doped rGO | 0.026 μA μM−1 cm−2 | 1.0–329.5 μM | 0.5 μM | [528] |
| MnO3O4/N-doped graphene/CPE | 0.1011 μA μM−1 cm−2 | 2.5–529.5 μM | 1.0 μM | [529] |
| CuNiO/N-doped graphene | 7.49 μA mM−1 cm−2 | 0.2 μM–0.3 mM | 50 nM | [530] |
| Cu/N-doped graphene | 43.13 μA mM−1 cm−2 | 0.004–4.5 mM | 1.3 μM | [531] |
| MnO2/CuO/GO | - | 0.55–4.4 mM | 53 μM | [532] |
| NiNPs/PEDOT/rGO/GCE | 36.15 μA μM−1 cm−2 | 1 μM–5.1 mM | 0.8 μM | [533] |
| Cu–Co/CS/rGO/GCE | 1921 μA μM−1 cm−2 | 0.015–6.96 mM | 10 μM | [534] |
| CuNPs/PAA/Graphene | - | 0.0003–0.6 mM | 0.08 μM | [535] |
| NiNPs/CS/rGO | 318.4 μ AμM−1 cm−2 | Up to 9 mM | 4.1 μM | [536] |
| PdNPs/Nafion/Graphene | - | 10 μM–5 mM | 1 μM | [537] |
| Materials | LoDuM | Range M | Reference |
|---|---|---|---|
| Fe3O4/SiO2/GO | 0.07 | 0.5 × 10−6–2.5 ×10−4 | [565] |
| MoS2−rGO | 3.8 | 4.0 × 10−6–4.0 × 10−5 | [566] |
| GOx−chitosan/Co3O4/Au | 0.1 | 3.0 × 10−7–3.0 × 10−6 | [567] |
| ZnO−graphene | 5.0 | 5.0 × 10−6–8.0 × 10−5 | [568] |
| CeO2−x/C/rGO | 2.0 | 4.98× 10−5–1.05 × 10−3 | [569] |
| Au/Pd−rGO | 5.0 | 2.0 × 10−8–5.0 × 10−4 | [570] |
| rGO−ZnO | 0.33 | 1.0 × 10−6–7.0 × 10−5 | [571] |
| Graphene/Neutral Red | 0.076 | 0.5 × 10−6–2.0 × 10−2 | [572] |
| Polytetraphenylporphyrin/PPy/GO | 1.15 | 5.0 × 10−6–2.0×10−4 | [573] |
| Au/ZnO/PPy/rGO | 0.09 | 1.0 × 10−6–6.8 × 10−6 | [574] |
| ZnO/PANI/rGO | 0.042 | 1.0 × 10−9–1.0 × 10−7 | [575] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Ahmed, A.; Sharma, A.; Arya, S. Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat. Biosensors 2022, 12, 910. https://doi.org/10.3390/bios12100910
Singh A, Ahmed A, Sharma A, Arya S. Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat. Biosensors. 2022; 12(10):910. https://doi.org/10.3390/bios12100910
Chicago/Turabian StyleSingh, Anoop, Aamir Ahmed, Asha Sharma, and Sandeep Arya. 2022. "Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat" Biosensors 12, no. 10: 910. https://doi.org/10.3390/bios12100910
APA StyleSingh, A., Ahmed, A., Sharma, A., & Arya, S. (2022). Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat. Biosensors, 12(10), 910. https://doi.org/10.3390/bios12100910