Reduced Graphene Oxide-Polydopamine-Gold Nanoparticles: A Ternary Nanocomposite-Based Electrochemical Genosensor for Rapid and Early Mycobacterium tuberculosis Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of rGO-PDA/GCE Nanocomposite
2.3. Preparation of rGO-PDA-AuNP/GCE Nanocomposite
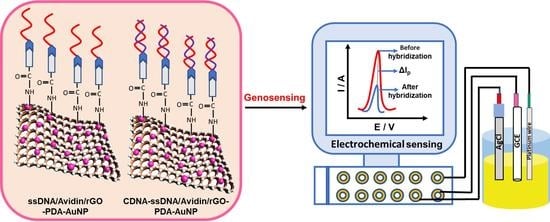
2.4. Electrode Modification
2.5. Fabrication of Nuclei-Acid-Functionalized Modified GCE
2.6. Instrumentation
3. Results and Discussion
3.1. Ultraviolet-Visible (UV-Vis) Spectroscopy
3.2. Raman Spectroscopy
3.3. X-ray Diffraction (XRD) Spectroscopy
3.4. Transmission Electron Microscopy (TEM)
3.5. Field Emission Scanning Electron Microscopy (FESEM)
3.6. Fourier Transform Infrared Spectroscopy (FT-IR)
3.7. Electrochemical Response Studies
3.8. Impact of Scan Rate
3.9. Electrochemical Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, Z.; Sun, S.; Salim, T.; Wu, S.; Huang, X.; He, Q.; Lam, Y.M.; Zhang, H. Organic photovoltaic devices using highly flexible reduced graphene oxide films as transparent electrodes. ACS Nano 2010, 4, 5263–5268. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarker, B.K.; Chunder, A.; Zhai, L.; Khondaker, S.I. Position dependent photodetector from large area reduced graphene oxide thin films. Appl. Phys. Lett. 2010, 96, 163109. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kundu, C.K.; Li, Z.; Li, X.; Zhang, Z. Layer-by-layer-assembled flame-retardant coatings from polydopamine-induced in situ functionalized and reduced graphene oxide. J. Mater. Sci. 2019, 54, 13848–13862. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.-M.; Wang, Y.-T.; Velusamy, V.; Ramaraj, S.K. A Facile Electrochemical Preparation of Reduced Graphene Oxide@Polydopamine Composite: A Novel Electrochemical Sensing Platform for Amperometric Detection of Chlorpromazine. Sci. Rep. 2016, 6, 33599. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zheng, Q.; Bai, G.; Song, L.; Yao, Y.; Cao, X.; Liu, S.; Yao, C. Polydopamine induced in-situ growth of Au nanoparticles on reduced graphene oxide as an efficient biosensing platform for ultrasensitive detection of bisphenol A. Electrochim. Acta 2017, 242, 56–65. [Google Scholar] [CrossRef]
- Ruan, C.; Shi, W.; Jiang, H.; Sun, Y.; Liu, X.; Zhang, X.; Sun, Z.; Dai, L.; Ge, D. One-pot preparation of glucose biosensor based on polydopamine–graphene composite film modified enzyme electrode. Sens. Actuators B Chem. 2013, 177, 826–832. [Google Scholar] [CrossRef]
- Das, M.; Dhand, C.; Sumana, G.; Srivastava, A.; Nagarajan, R.; Nain, L.; Iwamoto, M.; Manaka, T.; Malhotra, B. Electrophoretic fabrication of chitosan− zirconium-oxide nanobiocomposite platform for nucleic acid detection. Biomacromolecules 2011, 12, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, R.; Du, J.; Fan, J.; Peng, X. Gold nanoparticle-based colorimetric recognition of creatinine with good selectivity and sensitivity. Ind. Eng. Chem. Res. 2016, 55, 12334–12340. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, R.; Li, M.; Min, R.; Wu, Q.; Kaushik, B.K.; Jha, R.; Zhang, B.; Kumar, S. Cardiac Troponin I Detection using Gold/Cerium-Oxide Nanoparticles assisted Hetro-Core Fiber Structure. IEEE Trans. NanoBiosci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gao, J.; Yang, W.; Liu, R.; Shafi, M.; Zha, Z.; Liu, C.; Xu, S.; Ning, T.; Jiang, S. LSPR optical fiber sensor based on 3D gold nanoparticles with monolayer graphene as a spacer. Opt. Express 2022, 30, 10187–10198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, Y.; Wang, Z.; Singh, R.; Marques, C.; Wu, Q.; Kaushik, B.K.; Jha, R.; Zhang, B.; Kumar, S. Localized plasmon-based multicore fiber biosensor for acetylcholine detection. IEEE Trans. Instrum. Meas. 2021, 71, 1–9. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.-K.; Yang, Q.S.; Zhang, X.; Wang, W.; Zhang, B. LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160. [Google Scholar] [CrossRef]
- Singh, L.; Singh, R.; Zhang, B.; Cheng, S.; Kaushik, B.K.; Kumar, S. LSPR based uric acid sensor using graphene oxide and gold nanoparticles functionalized tapered fiber. Opt. Fiber Technol. 2019, 53, 102043. [Google Scholar] [CrossRef]
- Zhou, Z.; Wada, K.; Tong, L. Nanophotonics and Micro/Nano Optics VII; SPIE/International Society for Optical Engineering: Bellingham, WA, USA, 2021. [Google Scholar]
- Huang, K.-J.; Liu, Y.-J.; Wang, H.-B.; Wang, Y.-Y. A sensitive electrochemical DNA biosensor based on silver nanoparticles-polydopamine@ graphene composite. Electrochim. Acta 2014, 118, 130–137. [Google Scholar] [CrossRef]
- Li, D.-W.; Luo, L.; Lv, P.-F.; Wang, Q.-Q.; Lu, K.-Y.; Wei, A.-F.; Wei, Q.-F. Synthesis of polydopamine functionalized reduced graphene oxide-palladium nanocomposite for laccase based biosensor. Bioinorg. Chem. Appl. 2016, 2016, 5360361. [Google Scholar] [CrossRef] [Green Version]
- Leva-Bueno, J.; Peyman, S.A.; Millner, P. A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 2020, 209, 343–362. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, G.; Conteduca, D.; Armenise, M.N.; Ciminelli, C. Novel micro-nano optoelectronic biosensor for label-free real-time biofilm monitoring. Biosensors 2021, 11, 361. [Google Scholar] [CrossRef]
- Wang, Y.; Reardon, C.P.; Read, N.; Thorpe, S.; Evans, A.; Todd, N.; Van Der Woude, M.; Krauss, T.F. Attachment and antibiotic response of early-stage biofilms studied using resonant hyperspectral imaging. NPJ Biofilms Microbiomes 2020, 6, 57. [Google Scholar] [CrossRef]
- Therisod, R.; Tardif, M.; Marcoux, P.R.; Picard, E.; Jager, J.-B.; Hadji, E.; Peyrade, D.; Houdré, R. Gram-type differentiation of bacteria with 2D hollow photonic crystal cavities. Appl. Phys. Lett. 2018, 113, 111101. [Google Scholar] [CrossRef] [Green Version]
- Conteduca, D.; Brunetti, G.; Dell’Olio, F.; Armenise, M.N.; Krauss, T.F.; Ciminelli, C. Monitoring of individual bacteria using electro-photonic traps. Biomed. Opt. Express 2019, 10, 3463–3471. [Google Scholar] [CrossRef]
- Kumar, P.; Shahzad, F.; Yu, S.; Hong, S.M.; Kim, Y.-H.; Koo, C.M. Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon 2015, 94, 494–500. [Google Scholar] [CrossRef]
- Patel, M.; Bisht, N.; Prabhakar, P.; Sen, R.K.; Kumar, P.; Dwivedi, N.; Ashiq, M.; Mondal, D.; Srivastava, A.K.; Dhand, C. Ternary nanocomposite-based smart sensor: Reduced graphene oxide/polydopamine/alanine nanocomposite for simultaneous electrochemical detection of Cd2+, Pb2+, Fe2+, and Cu2+ ions. Environ. Res. 2023, 221, 115317. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Zhang, S.; Yang, L.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Multifunctional electrochemical platforms based on the Michael addition/Schiff base reaction of polydopamine modified reduced graphene oxide: Construction and application. ACS Appl. Mater. Interfaces 2015, 7, 17935–17946. [Google Scholar] [CrossRef]
- Wang, Z.; Gou, G.; Shi, L.; Yang, J.; Xu, C.; Zhang, L.; Fan, A.; Min, Y. Gold nanoparticle ensemble on polydopamine/graphene nanohybrid as a novel electrocatalyst for determination of baicalein. J. Appl. Polym. Sci. 2018, 135, 46720. [Google Scholar] [CrossRef]
- Hessein, A.; El-Moneim, A. Developing Cost Effective Graphene Conductive Coating and its Application as Counter Electrode for CdS Quantum Dot Sensitized Solar Cell. Proc. World Congr. New Technol. (NewTech 2015) 2015, 307. [Google Scholar]
- Li, Y.; Li, Y.; Yu, X.; Sun, Y. Electrochemical determination of carbofuran in tomatoes by a concanavalin A (con A) polydopamine (PDA)-reduced graphene oxide (RGO)-gold nanoparticle (GNP) glassy carbon electrode (GCE) with immobilized acetylcholinesterase (AChE). Anal. Lett. 2019, 52, 2283–2299. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Gurunathan, S. Combination of graphene oxide–silver nanoparticle nanocomposites and cisplatin enhances apoptosis and autophagy in human cervical cancer cells. Int. J. Nanomed. 2017, 12, 6537. [Google Scholar] [CrossRef] [Green Version]
- Weng, C.-H.; Huang, C.-C.; Yeh, C.-S.; Lei, H.-Y.; Lee, G.-B. Synthesis of hexagonal gold nanoparticles using a microfluidic reaction system. J. Micromech. Microeng. 2008, 18, 035019. [Google Scholar] [CrossRef]
- Silva, C.; Simon, F.; Friedel, P.; Pötschke, P.; Zimmerer, C. Elucidating the chemistry behind the reduction of graphene oxide using a green approach with polydopamine. Nanomaterials 2019, 9, 902. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, J.; Rao, Y.; Li, B.; Zhao, S.; Bai, H.; Cui, J. Polydopamine modified graphene oxide-TiO2 nanofiller for reinforcing physical properties and anticorrosion performance of waterborne epoxy coatings. Appl. Sci. 2019, 9, 3760. [Google Scholar] [CrossRef] [Green Version]
- Ha, Y.-M.; Kim, Y.N.; Jung, Y.C. Rapid and local self-healing ability of polyurethane nanocomposites using photothermal polydopamine-coated graphene oxide triggered by near-infrared laser. Polymers 2021, 13, 1274. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, N.; Liu, W.-W.; Lai, C.-W.; Noriman, N.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2017; p. 150002. [Google Scholar]
- Faiz, M.A.; Azurahanim, C.C.; Raba’ah, S.A.; Ruzniza, M.Z. Low cost and green approach in the reduction of graphene oxide (GO) using palm oil leaves extract for potential in industrial applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thangavelu, K.; Chen, S.-M.; Thirumalraj, B.; Liu, X.-H. Preparation and characterization of gold nanoparticles decorated on graphene oxide@ polydopamine composite: Application for sensitive and low potential detection of catechol. Sens. Actuators B Chem. 2016, 233, 298–306. [Google Scholar] [CrossRef]
- Banumathi, S.; Uma, J.; Ravi, A.; Balraj, B.; Siva, C.; Ilanchezhiyan, P.; Kumar, G.M. Rapid sun-light driven photocatalytic functions of 3D rGO/ZnO/Ag heterostructures via improved charge transfer kinetics. J. Mater. Res. Technol. 2021, 10, 1301–1309. [Google Scholar] [CrossRef]
- Ci, L.; Song, L.; Jin, C.; Jariwala, D.; Wu, D.; Li, Y.; Srivastava, A.; Wang, Z.; Storr, K.; Balicas, L. Atomic layers of hybridized boron nitride and graphene domains. Nat. Mater. 2010, 9, 430–435. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Esterle, A.; Sharma, N.C.; Sahi, S.V. Yucca-derived synthesis of gold nanomaterial and their catalytic potential. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Zhi, N.; Yang, L.; Xu, G.; Feng, Q.; Zhang, Q.; Sun, S. A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Jing, Y.; Yuan, X.; Yuan, Q.; He, K.; Liu, Y.; Lu, P.; Li, H.; Li, B.; Zhan, H.; Li, G. Determination of nicotine in tobacco products based on mussel-inspired reduced graphene oxide-supported gold nanoparticles. Sci. Rep. 2016, 6, 29230. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Lai, G.; Yu, A. Electroanalysis of Dopamine Using Polydopamine Functionalized Reduced Graphene Oxide-Gold Nanocomposite. In Proceedings of the 1st International Electronic Conference on Materials, online, 26 May–10 June 2014. [Google Scholar]
- Shi, L.; Wang, Z.; Chen, X.; Yang, G.; Liu, W. Reduced Graphene Oxide/Polydopamine/Gold Electrode as Elecrochemical Sensor for Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid. Int. J. Electrochem. Sci 2019, 14, 8882–8891. [Google Scholar] [CrossRef]
- Duoc, P.N.D.; Binh, N.H.; Van Hau, T.; Thanh, C.T.; Van Trinh, P.; Tuyen, N.V.; Van Quynh, N.; Van Tu, N.; Chinh, V.D.; Thu, V.T. A novel electrochemical sensor based on double-walled carbon nanotubes and graphene hybrid thin film for arsenic (V) detection. J. Hazard. Mater. 2020, 400, 123185. [Google Scholar] [CrossRef]
- Bonanni, A.; Pumera, M.; Miyahara, Y. Influence of gold nanoparticle size (2–50 nm) upon its electrochemical behavior: An electrochemical impedance spectroscopic and voltammetric study. Phys. Chem. Chem. Phys. 2011, 13, 4980–4986. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Z.; Li, J. Gold nanoparticles with special shapes: Controlled synthesis, surface-enhanced Raman scattering, and the application in biodetection. Sensors 2007, 7, 3299–3311. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Jiang, D.; Xiang, G.; Liu, L.; Liu, F.; Pu, X. An electrochemical DNA biosensor for the detection of Mycobacterium tuberculosis, based on signal amplification of graphene and a gold nanoparticle–polyaniline nanocomposite. Analyst 2014, 139, 5460–5465. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, F.S.; Mat Zaid, M.H.; Abdullah, J.; Zawawi, R.M.; Lim, H.N.; Sulaiman, Y.; Abdul Rahman, N. Synthesis and characterization of polyaniline/graphene composite nanofiber and its application as an electrochemical DNA biosensor for the detection of Mycobacterium tuberculosis. Sensors 2017, 17, 2789. [Google Scholar] [CrossRef] [Green Version]
- Torati, S.R.; Reddy, V.; Yoon, S.S.; Kim, C. Electrochemical biosensor for Mycobacterium tuberculosis DNA detection based on gold nanotubes array electrode platform. Biosens. Bioelectron. 2016, 78, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.D.; Dhand, C.; Dwivedi, N.; Singh, B.P.; Sumana, G.; Agarwal, V.V.; Tawale, J.S.; Malhotra, B.D. Facile synthesis of 2-dimensional transparent graphene flakes for nucleic acid detection. Sens. Actuators B Chem. 2015, 210, 281–289. [Google Scholar] [CrossRef]
- Mogha, N.K.; Sahu, V.; Sharma, R.K.; Masram, D.T. Reduced graphene oxide nanoribbon immobilized gold nanoparticle based electrochemical DNA biosensor for the detection of Mycobacterium tuberculosis. J. Mater. Chem. B 2018, 6, 5181–5187. [Google Scholar] [CrossRef] [PubMed]






| Matrix Used | Method of Immobilization | Detection Limit | Linear Range of Detection | Response Time | Reference |
|---|---|---|---|---|---|
| Probe/ZrO2-CHIT/ITO | Covalent immobilization | 7.8 × 10−10 M | From 7.8 × 10−10 to 5 × 10–8 M | 1 min | [9] |
| rGO/Au/PANI nanocomposite | Covalent binding | 1.0 × 10−15 M | From 1.0 × 10−15 to 1.0 × 10−9 M | - | [48] |
| PANI/GP composite nanofibers | Covalent binding via Avidin-Biotin coupling | 7.853 × 10−7 M | From 10−6 to 10−9 M | 30 min | [49] |
| AuNTsA (gold nanotube array) | Covalent binding | 1.0 × 10−12 M | From 1.0 × 10−12 to 1.46 × 10−8 M | - | [50] |
| GF/ITO | Covalent binding | 4.2 × 10−13 M | From 4.2 × 10−13 M to 4.2 × 10−5 M | - | [51] |
| Au/RGONR | Covalent binding | 1 × 10−16 M | From 1 × 10−16 to 10−6 M | - | [52] |
| rGO-PDA-AuNP/GCEs | Covalent binding via Avidin-Biotin coupling | 1.0 × 10−14 M | From 1.0 × 10−14 M to 1.0 × 10−10 M | 5 s | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaturvedi, M.; Patel, M.; Bisht, N.; Shruti; Das Mukherjee, M.; Tiwari, A.; Mondal, D.P.; Srivastava, A.K.; Dwivedi, N.; Dhand, C. Reduced Graphene Oxide-Polydopamine-Gold Nanoparticles: A Ternary Nanocomposite-Based Electrochemical Genosensor for Rapid and Early Mycobacterium tuberculosis Detection. Biosensors 2023, 13, 342. https://doi.org/10.3390/bios13030342
Chaturvedi M, Patel M, Bisht N, Shruti, Das Mukherjee M, Tiwari A, Mondal DP, Srivastava AK, Dwivedi N, Dhand C. Reduced Graphene Oxide-Polydopamine-Gold Nanoparticles: A Ternary Nanocomposite-Based Electrochemical Genosensor for Rapid and Early Mycobacterium tuberculosis Detection. Biosensors. 2023; 13(3):342. https://doi.org/10.3390/bios13030342
Chicago/Turabian StyleChaturvedi, Mansi, Monika Patel, Neha Bisht, Shruti, Maumita Das Mukherjee, Archana Tiwari, D. P. Mondal, Avanish Kumar Srivastava, Neeraj Dwivedi, and Chetna Dhand. 2023. "Reduced Graphene Oxide-Polydopamine-Gold Nanoparticles: A Ternary Nanocomposite-Based Electrochemical Genosensor for Rapid and Early Mycobacterium tuberculosis Detection" Biosensors 13, no. 3: 342. https://doi.org/10.3390/bios13030342
APA StyleChaturvedi, M., Patel, M., Bisht, N., Shruti, Das Mukherjee, M., Tiwari, A., Mondal, D. P., Srivastava, A. K., Dwivedi, N., & Dhand, C. (2023). Reduced Graphene Oxide-Polydopamine-Gold Nanoparticles: A Ternary Nanocomposite-Based Electrochemical Genosensor for Rapid and Early Mycobacterium tuberculosis Detection. Biosensors, 13(3), 342. https://doi.org/10.3390/bios13030342