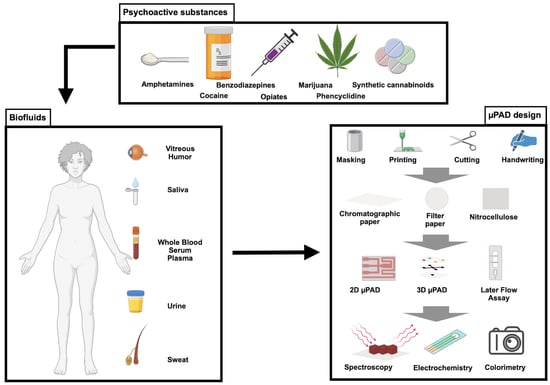
Application of Paper-Based Microfluidic Analytical Devices (µPAD) in Forensic and Clinical Toxicology: A Review
Abstract
:1. Introduction
2. Paper-Based Analytical Devices
2.1. Selection of Paper
2.2. Fabrication Procedures
2.3. Detection
2.3.1. Colorimetric Detection
2.3.2. Fluorimetric Detection
2.3.3. Electrochemical Detection
2.3.4. Spectroscopic Detection with a Focus on Surface-Enhanced Raman Scattering (SERS) Spectroscopy
3. Applications
- Toxic compounds (arsenic, cyanide, ethanol, and nitrite);
- Drugs and drugs of abuse (benzodiazepines, cathinones, cocaine, fentanyl, ketamine, MDMA, morphine, synthetic cannabinoids, and tetrahydrocannabinol);
- Psychoactive substances used for drug-facilitated crimes (flunitrazepam, GHB, ketamine, metamizole, midazolam, and scopolamine).
3.1. Toxic Compounds
3.2. Drugs (D) and Drugs of Abuse (DoA)
3.3. Psychoactive Substances Used for Drug-Facilitated Crimes (DFCs)
| Category | Analyte | Matrix | Detection (Sensing Molecule) | Equipment (Detection System) | Limit of Detection | Linearity | Comparison with a Different Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| Detection of Toxic Compounds in Biofluids | Arsenic | Urine | Colorimetric (AgNPr) | Qualitative determination with the naked eye | 0.5 µg/L (6.7 nmol/L) | 0.5–1000 µg/L (6.7–13,000 nmol/L) | Spectrophotometric method (UV-Vis) | [125] |
| Cyanide | Liquid samples | Colorimetry (Pd dimethylglioximate) | Homemade device integrating air flow module, addition of acid and LED-based detector (LED detector and laptop for signal elaboration) | 10 µg/L (0.4 µmol/L) | n/a | GC-MS | [129] | |
| Cyanide | Blood (plasma) | Colorimetry [Pt complex ([Pt(p-MeC6H4)2(phen)])] | Centrifuge (smartphone and laptop for signal elaboration) | 10 µg/L (0.4 µmol/L) | 26–2600 µg/L (1.0–100 µmol/L) | GC-MS | [130] | |
| Thiocyanate (metabolite of cyanide) | Urine | Colorimetric (cobalt porphyrin derivative) | (smartphone and laptop for signal elaboration) | 73 µg/L (1.26 µmol/L) (smartphone); 2.9 mg/L (50 µmol/L) (naked eye) | 1–100 µmol/L | Ion chromatography | [131] | |
| Ethanol | Blood (mice blood, sheep blood) | Colorimetric (ABTS) | (camera and laptop for signal elaboration) | 0.12 g/L (2.6 mmol/L) | 0.12–1.2 g/L (2.6–26 mmol/L) | HS-GC-MS | [132] | |
| Breath | Colorimetric (cerium nanoparticles) | (smartphone and laptop for signal elaboration) | 0.01 g/L (0.2 mmol/L) | 0.2–1.2 g/L (4–26 mmol/L) | Electronic breathalyzer | [162] | ||
| Nitrite | Saliva | Colorimetric (Griess reagent) | Centrifuge, circuit for generating voltage (smartphone) | 75 µg/L (1.6 μmol/L) | 75–1000 µg/L (1.6–21 μmol/L) | n/a | [134] | |
| Artificial urine | Colorimetric (Griess reagent) | (smartphone and laptop for signal elaboration) | 2.3 mg/L (50 µmol/L) | n/a | n/a | [135] | ||
| Saliva | Colorimetric (modified Griess reagent—phosphoric acid) | Nitrogen to store the device (scanner and laptop for signal elaboration) | 0.46 mg/L (10 µmol/L) | 0.46–46 mg/L (10–1000 µmol/L) | Spectrophotometric method (UV-Vis) | [139] | ||
| Saliva | Colorimetric (modified Griess reagent—phosphoric acid) | (scanner and laptop for signal elaboration) | 7.8 µg/L (0.17 µmol/L) (nitrite); 16.7 mg/L (0.27 mmol/L) (nitrate) | 0.23–11.5 mg/L (5–250 µmol/L) (nitrite); 9.2–55.2 mg/L (0.2–1.2 mmol/L) (nitrate) | n/a | [140] | ||
| Saliva | Colorimetric (Griess reagent—citric acid) | (smartphone, photo box equipped with 84LEDs and laptop for signal elaboration) | 0.46 mg/L (9.6 µmol/L); 3.4 mg/L (74 µmol/L) | 0.92 mg/L (0.02–5 mmol/L) | n/a | [136] | ||
| Saliva | Colorimetric (modified Griess reagent—hydrochloric acid) | (scanner and laptop for signal elaboration) | 0.26 mg/L (5.6 µmol/L) | 0.26–1.15 mg/L (5.6–25 mmol/L) | Spectrophotometric method | [141] | ||
| Saliva (artificial) | Colorimetric (modified Griess reagent—hydrochloric acid) | (smartphone and laptop for signal elaboration) | 0.26 mg/L (5.7 µmol/L) | 0.26–46 mg/L (5.7–1000 µmol/L) | HPLC-UV | [142] | ||
| Urine/serum | Colorimetric (Griess reagent—citric acid) | (smartphone and laptop for signal elaboration) | 0.2 mg/L (4.3 µmol/L) (serum); 0.1 mg/L (2.3 µmol/L) (urine) | 0.23–27.6 mg/L (5–600 µmol/L) (serum); 0.23–4.6 mg/L (5–100 µmol/L) (urine) | n/a | [137] | ||
| Saliva | Colorimetric (Griess reagent—citric acid) | (smartphone and laptop for signal elaboration) | 1.15 mg/L (25 µmol/L) | 1.15–11.5 mg/L (25–250 µmol/L) | n/a | [138] | ||
| Saliva | Colorimetric (Griess reagent—citric acid) | (scanner and laptop for signal elaboration) | 0.69 mg/L (0.015 µmol/L) | 0.04–1 mM (1.8–46 mg/L) | n/a | [36] | ||
| Whole blood | Chemiluminescence (Cu-MOF) | Ring-oven approach for MOF synthesis | 0.09 µg/L (2 nmol/L) | 0.09–4.6 µg/L (2–100 nmol/L) | n/a | [143] | ||
| Detection of Drugs and Illicit Drugs in Biofluids | Alprazolam | Blood; vitreous humor | Colorimetry (silver nanoparticles) | Plastic cassette cabinet (smartphone and laptop for signal elaboration) | smartphone: 10 µg/L (32 nmol/L) | 1–10 μg/L (3.2–32 nmol/L) | UV-Vis | [144] |
| Urine | Cyclic voltammetry and amperometry | Potentiostat, two-electrode configuration | 0.025 µg/L (0.08 nmol/L) | 1–300 µg/L (3.2–980 nmol/L) | n/a | [82] | ||
| Diazepam | Urine | Cyclic voltammetry | Potentiostat, two-electrode configuration | 0.42 µg/L (1.5 nmol/L) | 1 µg/L–1 g/L; (3.5 nmol/L–3.5 mmol/L) | n/a | [83] | |
| Cathinone mephedrone | Urine | Colorimetry (TMB) | (smartphone and laptop for signal elaboration) | 4.34 ng/mL (0.03 μmol/L) | n/a | n/a | [90] | |
| Cocaine | Urine | Colorimetry (iodine) | (smartphone and laptop for elaborating pictures) | 2.3 ng/mL (4.5 µmol/L) | 3–15 ng/mL (10–50 µmol/L) | n/a | [64] | |
| Urine | Colorimetry | Qualitative determination with the naked eye | 15 ng/L (50 µmol/L) | n/a | n/a | [145] | ||
| Saliva; blood (rats) | Luminescence (ACA-UCNPs) | (Smartphone) | 15.15 µg/L (50 nmol/L) (saliva) | n/a | n/a | [147] | ||
| Oral fluid (spiked) | Surface-enhanced Raman scattering spectroscopy | Raman microscope with 785 nm laser and 1200 line/mm grating | 1 ng/mL (3.29 nmol/L) | n/a | n/a | [163] | ||
| Fentanyl | Artificial urine; serum (rat) | Surface-enhanced Raman scattering spectroscopy | Portable Raman spectrometer with a 785 nm excitation laser | 0.59 μg/mL (1.75 μmol/L) (urine); 2.78 μg/mL (8.26 μmol/L) (serum) | 4–20 μg/mL (12–60 nmol/L) | n/a | [150] | |
| Ketamine | Saliva | Colorimetry (3,3′,5′,5-tetramethylbenzidine (TMB)) | (scanner, smartphone, and laptop for signal elaboration) | 0.03 ng/mL (0.1 pmol/L) | 1–1000 ng/mL (4.2–4200 pM) | GC-MS | [153] | |
| MDMA * | Urine; sweat | Differential pulse voltammetry | Potentiostat, two-electrode configuration | 19 ng/mL (0.1 µmol/L) | 0.19–190 mg/L (1–1000 µmol/L) | n/a | [100] | |
| Morphine | Saliva | Colorimetry (gold-conjugated anti-immunocomplex Fab) | Piezo-electric inkjet printer (scanner and laptop for signal elaboration) | 20 ng/mL (0.07 µmol/L) | 20–2000 ng/mL (0.07–7 µmol/L) | n/a | [155] | |
| Synthetic cannabinoid JWH-073 | Saliva | Colorimetry (rhodamine B-loaded polymersomes); Fluorescence | (smartphone and laptop for signal elaboration); fluorescence | 0.53 ng/mL (1.6 nmol/L) (sandwich); 0.31 ng/mL (0.9 nmol/L) (competitive); 0.16 ng/mL (0.5 nmol/L) (fluorescence) | 5–1000 ng/mL (15–3058 nmol/L) | n/a | [157] | |
| Tetrahydrocabinol | Saliva | Differential pulse voltammetry | Potentiostat, three-electrode configuration | 1.4 µg/L (4.5 nmol/L) | 0.01–1.5 mg/L (32–4700 nmol/L) | HPLC-UV/vis | [86] | |
| Detection of Compounds Added to Beverages for Drug-Facilitated Crimes (DFCs) | Flunitrazepam | Carbonated and noncarbonated soft drinks | Potentiometry | Digital ion analyzer | 0.17 mg/L (0.55 µmol/L) | 0.31 mg/L–3.1 g/L (1 µmol/L–0.01 mol/L) | Titrimetric method | [96] |
| GHB * | Beverages | Colorimetric (pentacosadiynoic acid–gabazine) | (Smartphone and laptop for signal elaboration) | 9.6 mg/L | n/a | n/a | [158] | |
| Ketamine | Cola, rum, whiskey | Colorimetric (bromocresol) | (Smartphone and dedicated smartphone app) | 2.4 g/L (0.01 mmol/L) | n/a | n/a | [63] | |
| Beverages | Colorimetric (cobalt thiocyanate); fluorescence (carbon dots–gold nanoparticles); potentiometry | Digital ion analyzer Smartphone and ultraviolet-LED torch with 395 nm light for the fluorimetric and colorimetric detection | 10 g/L (42 mmol/L) (colorimetry); 0.048 g/L (0.2 mmol/L) (potentiometry); 0.0008 g/L (0.003 mmol/L) (fluorimetric) | 0.04–0.4 mmol/L (colorimetry); 3.2 µmol/L 0.01 mol/L (potentiometry); 0.2–1 mmol/L (fluorimetric) | n/a | [159] | ||
| Alcoholic (whiskeys) and nonalcoholic drinks (real juices) | Cyclic voltammetry | Potentiostat, two-electrode configuration | 0.24 µg/L (1 nmol/L) | 0.24–1.2 µg/L (1 nmol/L–5 µmol/L) | n/a | [85] | ||
| Metamizole | Whiskey | Square wave voltammetry | Potentiostat, three-electrode configuration | 20 mg/L (0.064 mmol/L) | 50–250 mg/L (0.16–0.8 mmol/L) | n/a | [77] | |
| Midazolam | 4.8 mg/L (0.015 mmol/L) | 25–1000 mg/L (0.077–3.1 mmol/L) | 4.8 mg/L (0.015 mmol/L) | |||||
| Scopolamine | Alcohol beverages | Colorimetric (ZnTPP, Methyl orange, Bromocresol green, Iodoplatinate, Dragendorff’s, and Chen’s) | (Scanner and laptop for signal elaboration) | 0.6 g/L (2 mmol/L) | n/a | Spectrophotometric method | [161] | |
| Xylazine | Beverages | Electrochemical | Lab-built portable device | 0.06 mg/L (0.27 µmol/L) | 0.2 mg/L–0.1 g/L (0.9 µmol/L–0.45 mol/L) | n/a | [84] |
| Analyte | Matrix | Detection (Sensing Molecule) | Explanation of the Approach | Equipment (Detection System) | Limit of Detection | Linearity | Comparison with a Different Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cocaine | Seized materials | Cyclic voltammetry–Colorimetry | The device consisted of a first PAD to accommodate the electrodes for EC detection and a second paper layer containing cobalt(II) thiocyanate attached to the electrochemical device for colorimetric detection. A graphite lead modified with an electrochemically grown gold film (graphite–gold) was used as a working electrode to improve electron transfer. Silver tracks were painted with silver ink onto the PAD to create the auxiliary and the reference electrodes. Then, the Ag track was oxidized to AgCl. PCA was also used for sample discrimination. | Potentiostat, three-electrode configuration and smartphone | n/a | n/a | GC-FID | [164] |
| Fentanyl | Swab from plastic, glass, and metallic surfaces | Surface-enhanced Raman scattering–paper spray mass spectrometry | Commercially available pSERS substrates made by paper inkjet-printed with AgNPs. | Hand-held portable Raman spectrometer with a 785 nm excitation laser | n/a | n/a | n/a | [165] |
| Cocaine | MeOH | Surface-enhanced Raman scattering–paper spray mass spectrometry | Paper-based SERS substrate made of Whatman grade 1 filter paper immersed in a colloidal suspension of AuNPs. | Raman spectrometer equipped with a 785 nm excitation laser | 0.6 ng | n/a | n/a | [149] |
| Hydrocodone | 3.7 ng | n/a | n/a | |||||
| JWH-018 | 26 ng | n/a | n/a | |||||
| 2C-B | 3.8 ng | n/a | n/a |
4. Challenges
5. Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paracelso (Philippus Aureolus Theophrastus Bombastus von Hohenheim). Opera Omnia Medico-Chemico-Chirurgica, Tribus Voluminibus Comprehensa. 1658. Available online: https://www.forumauctions.co.uk/101/Paracelsus-Theophrastus-Bombastus-Opera-Omnia-Medico-Chemico-Chirurgica-tribus-voluminibus/1?view=lot_detail&auction_no=1002 (accessed on 2 May 2023).
- Negrusz, A.; Cooper, G. Clarke’s Analytical Forensic Toxicology; Pharmaceutical Press: London, UK, 2013; ISBN 978-0-85711-054-1. [Google Scholar]
- Riafi, N.; Horvath, A.R.; Wittwer, C.T. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-323-35921-4. [Google Scholar]
- Convery, N.; Gadegaard, N. 30 Years of Microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Performance Evaluation of Handheld Raman Spectroscopy for Cocaine Detection in Forensic Case Samples. Kranenburg. Drug Testing and Analysis. Wiley Online Library. 2021. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/dta.2993 (accessed on 14 June 2023).
- A Calibration Friendly Approach to Identify Drugs of Abuse Mixtures with a Portable Near-infrared Analyzer—Kranenburg. Drug Testing and Analysis. Wiley Online Library. 2022. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/dta.3231 (accessed on 14 June 2023).
- Paixão, T.R.; Coltro, W.K.T.; Salles, M.O. Forensic Analytical Methods; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Clegg, D.L. Automatic Paper Chromatography. Anal. Chem. 1949, 21, 1123–1125. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low Volume, Portable Bioassays. Angew. Chem. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kung, C.-T.; Hou, C.-Y.; Wang, Y.-N.; Fu, L.-M. Microfluidic Paper-Based Analytical Devices for Environmental Analysis of Soil, Air, Ecology and River Water. Sens. Actuators B Chem. 2019, 301, 126855. [Google Scholar] [CrossRef]
- Mahato, K.; Srivastava, A.; Chandra, P. Paper Based Diagnostics for Personalized Health Care: Emerging Technologies and Commercial Aspects. Biosens. Bioelectron. 2017, 96, 246–259. [Google Scholar] [CrossRef]
- Musile, G.; Agard, Y.; Wang, L.; De Palo, E.F.; McCord, B.; Tagliaro, F. Paper-Based Microfluidic Devices: On-Site Tools for Crime Scene Investigation. TrAC Trends Anal. Chem. 2021, 143, 116406. [Google Scholar] [CrossRef]
- Ray, R.; Prabhu, A.; Prasad, D.; Garlapati, V.K.; Aminabhavi, T.M.; Mani, N.K.; Simal-Gandara, J. Paper-Based Microfluidic Devices for Food Adulterants: Cost-Effective Technological Monitoring Systems. Food Chem. 2022, 390, 133173. [Google Scholar] [CrossRef]
- Noviana, E.; Carrão, D.B.; Pratiwi, R.; Henry, C.S. Emerging Applications of Paper-Based Analytical Devices for Drug Analysis: A Review. Anal. Chim. Acta 2020, 1116, 70–90. [Google Scholar] [CrossRef]
- Land, K.J.; Boeras, D.I.; Chen, X.-S.; Ramsay, A.R.; Peeling, R.W. Reassured Diagnostics to Inform Disease Control Strategies, Strengthen Health Systems and Improve Patient Outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef]
- Lu, J.; Ge, S.; Ge, L.; Yan, M.; Yu, J. Electrochemical DNA Sensor Based on Three-Dimensional Folding Paper Device for Specific and Sensitive Point-of-Care Testing. Electrochim. Acta 2012, 80, 334–341. [Google Scholar] [CrossRef]
- Musile, G.; Agard, Y.; De Palo, E.F.; Shestakova, K.; Bortolotti, F.; Tagliaro, F. Thanatochemistry at the Crime Scene: A Microfluidic Paper-Based Device for Ammonium Analysis in the Vitreous Humor. Anal. Chim. Acta 2019, 1083, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.L.; Corbin, I.; Kaufman, L.M.; Zreibe, K.; Blanes, L.; McCord, B.R. Simultaneous Colorimetric Detection of Improvised Explosive Compounds Using Microfluidic Paper-Based Analytical Devices (ΜPADs). Anal. Methods 2015, 7, 63–70. [Google Scholar] [CrossRef]
- Cromartie, R.L.; Wardlow, A.; Duncan, G.; McCord, B.R. Development of a Microfluidic Device (ΜPADs) for Forensic Serological Analysis. Anal. Methods 2019, 11, 587–595. [Google Scholar] [CrossRef]
- Musile, G.; Agard, Y.; Pesavento, S.; De Palo, E.F.; Dorizzi, R.M.; Bortolotti, F. An Origami Microfluidic Paper Device for On-Site Assessment of Urine Tampering. First Use of Nessler’s Reagent for the Colorimetric Determination of Creatinine. Anal. Chim. Acta 2023, 1237, 340610. [Google Scholar] [CrossRef] [PubMed]
- Musile, G.; Wang, L.; Bottoms, J.; Tagliaro, F.; McCord, B. The Development of Paper Microfluidic Devices for Presumptive Drug Detection. Anal. Methods 2015, 7, 8025–8033. [Google Scholar] [CrossRef]
- Silva, T.G.; de Araujo, W.R.; Muñoz, R.A.A.; Richter, E.M.; Santana, M.H.P.; Coltro, W.K.T.; Paixão, T.R.L.C. Simple and Sensitive Paper-Based Device Coupling Electrochemical Sample Pretreatment and Colorimetric Detection. Anal. Chem. 2016, 88, 5145–5151. [Google Scholar] [CrossRef]
- Schulz, M.; Schmoldt, A.; Andresen-Streichert, H.; Iwersen-Bergmann, S. Revisited: Therapeutic and Toxic Blood Concentrations of More than 1100 Drugs and Other Xenobiotics. Crit. Care 2020, 24, 195. [Google Scholar] [CrossRef]
- Baselt, R. Disposition of Toxic Drugs and Chemicals in Man, 11th ed.; Biomedical Publications: Seal Beach, CA, USA, 2017; ISBN 978-0-692-77499-1. [Google Scholar]
- Kim, M.; Kim, S.; Yang, W.; Sim, J. Determination of Nitrite and Nitrate in Postmortem Whole Blood Samples of 10 Sodium Nitrite Poisoning Cases: The Importance of Nitrate in Determining Nitrite Poisoning. Forensic Sci. Int. 2022, 335, 111279. [Google Scholar] [CrossRef]
- Ozturk, S.; Ozturk, Y.E.; Yeter, O.; Alpertunga, B. Application of a Validated LC–MS/MS Method for JWH-073 and Its Metabolites in Blood and Urine in Real Forensic Cases. Forensic Sci. Int. 2015, 257, 165–171. [Google Scholar] [CrossRef]
- De Araujo, W.R.; Paixão, T.R.L.C. Paper-Based Analytical Devices for Chemical Analysis and Diagnostics, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-820534-1. [Google Scholar]
- Araujo, W.R.d.; Paixão, T.R.L.C. Fabrication of Disposable Electrochemical Devices Using Silver Ink and Office Paper. Analyst 2014, 139, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R. Bioactive Paper Provides a Low-Cost Platform for Diagnostics. TrAC Trends Anal. Chem. 2009, 28, 925–942. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, W.R.; Frasson, C.M.R.; Ameku, W.A.; Silva, J.R.; Angnes, L.; Paixão, T.R.L.C. Single-Step Reagentless Laser Scribing Fabrication of Electrochemical Paper-Based Analytical Devices. Angew. Chem. 2017, 56, 15113–15117. [Google Scholar] [CrossRef] [PubMed]
- Fridley, G.E.; Holstein, C.A.; Oza, S.B.; Yager, P. The Evolution of Nitrocellulose as a Material for Bioassays. MRS Bull. 2013, 38, 326–330. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. Paper-Based Microfluidic Devices by Plasma Treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid Prototyping of Paper-Based Microfluidics with Wax for Low-Cost, Portable Bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef]
- Chiang, C.-K.; Kurniawan, A.; Kao, C.-Y.; Wang, M.-J. Single Step and Mask-Free 3D Wax Printing of Microfluidic Paper-Based Analytical Devices for Glucose and Nitrite Assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef]
- Le, S.; Zhou, H.; Nie, J.; Cao, C.; Yang, J.; Pan, H.; Li, J.; Zhang, Y. Fabrication of Paper Devices via Laser-Heating-Wax-Printing for High-Tech Enzyme-Linked Immunosorbent Assays with Low-Tech Pen-Type PH Meter Readout. Analyst 2017, 142, 511–516. [Google Scholar] [CrossRef]
- Olkkonen, J.; Lehtinen, K.; Erho, T. Flexographically Printed Fluidic Structures in Paper. Anal. Chem. 2010, 82, 10246–10250. [Google Scholar] [CrossRef]
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-Printed Microfluidic Multianalyte Chemical Sensing Paper. Anal. Chem. 2008, 80, 6928–6934. [Google Scholar] [CrossRef] [PubMed]
- Maejima, K.; Tomikawa, S.; Suzuki, K.; Citterio, D. Inkjet Printing: An Integrated and Green Chemical Approach to Microfluidic Paper-Based Analytical Devices. RSC Adv. 2013, 3, 9258–9263. [Google Scholar] [CrossRef]
- Wang, J.; Monton, M.R.N.; Zhang, X.; Filipe, C.D.M.; Pelton, R.; Brennan, J.D. Hydrophobic Sol-Gel Channel Patterning Strategies for Paper-Based Microfluidics. Lab Chip 2014, 14, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Gopalakrishnan, S.; Savitha, R.; Renganathan, T.; Pushpavanam, S. Fabrication of Laser Printed Microfluidic Paper-Based Analytical Devices (LP-ΜPADs) for Point-of-Care Applications. Sci. Rep. 2019, 9, 7896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallibu, C.; Gallibu, C.; Avoundjian, A.; Gomez, F.A. Easily Fabricated Microfluidic Devices Using Permanent Marker Inks for Enzyme Assays. Micromachines 2016, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Ostad, M.A.; Hajinia, A.; Heidari, T. A Novel Direct and Cost Effective Method for Fabricating Paper-Based Microfluidic Device by Commercial Eye Pencil and Its Application for Determining Simultaneous Calcium and Magnesium. Microchem. J. 2017, 133, 545–550. [Google Scholar] [CrossRef]
- Sousa, L.R.; Duarte, L.C.; Coltro, W.K.T. Instrument-Free Fabrication of Microfluidic Paper-Based Analytical Devices through 3D Pen Drawing. Sens. Actuators B Chem. 2020, 312, 128018. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, Y.; Lin, L.; Zhou, C.; Li, S.; Zhang, L.; Li, J. Low-Cost Fabrication of Paper-Based Microfluidic Devices by One-Step Plotting. Anal. Chem. 2012, 84, 6331–6335. [Google Scholar] [CrossRef]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Laiwattanapaisal, W. Novel, Simple and Low-Cost Alternative Method for Fabrication of Paper-Based Microfluidics by Wax Dipping. Talanta 2011, 85, 2587–2593. [Google Scholar] [CrossRef]
- Cardoso, T.M.G.; de Souza, F.R.; Garcia, P.T.; Rabelo, D.; Henry, C.S.; Coltro, W.K.T. Versatile Fabrication of Paper-Based Microfluidic Devices with High Chemical Resistance Using Scholar Glue and Magnetic Masks. Anal. Chim. Acta 2017, 974, 63–68. [Google Scholar] [CrossRef]
- Chitnis, G.; Ding, Z.; Chang, C.-L.; Savran, C.A.; Ziaie, B. Laser-Treated Hydrophobic Paper: An Inexpensive Microfluidic Platform. Lab Chip 2011, 11, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Curto, V.F.; Lopez-Ruiz, N.; Capitan-Vallvey, L.F.; Palma, A.J.; Benito-Lopez, F.; Diamond, D. Fast Prototyping of Paper-Based Microfluidic Devices by Contact Stamping Using Indelible Ink. RSC Adv. 2013, 3, 18811–18816. [Google Scholar] [CrossRef]
- De Freitas, S.V.; de Souza, F.R.; Rodrigues Neto, J.C.; Vasconcelos, G.A.; Abdelnur, P.V.; Vaz, B.G.; Henry, C.S.; Coltro, W.K.T. Uncovering the Formation of Color Gradients for Glucose Colorimetric Assays on Microfluidic Paper-Based Analytical Devices by Mass Spectrometry Imaging. Anal. Chem. 2018, 90, 11949–11954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, H.; Yan, L.; Li, N.; Shi, J.; Jiang, C. Recent Developments in Detection Using Noble Metal Nanoparticles. Crit. Rev. Anal. Chem. 2020, 50, 97–110. [Google Scholar] [CrossRef]
- Yamada, K.; Takaki, S.; Komuro, N.; Suzuki, K.; Citterio, D. An Antibody-Free Microfluidic Paper-Based Analytical Device for the Determination of Tear Fluid Lactoferrin by Fluorescence Sensitization of Tb3+. Analyst 2014, 139, 1637–1643. [Google Scholar] [CrossRef] [Green Version]
- De Tarso Garcia, P.; Garcia Cardoso, T.M.; Garcia, C.D.; Carrilho, E.; Tomazelli Coltro, W.K. A Handheld Stamping Process to Fabricate Microfluidic Paper-Based Analytical Devices with Chemically Modified Surface for Clinical Assays. RSC Adv. 2014, 4, 37637–37644. [Google Scholar] [CrossRef]
- Evans, E.; Moreira Gabriel, E.F.; Benavidez, T.E.; Tomazelli Coltro, W.K.; Garcia, C.D. Modification of Microfluidic Paper-Based Devices with Silica Nanoparticles. Analyst 2014, 139, 5560–5567. [Google Scholar] [CrossRef] [Green Version]
- Figueredo, F.; Garcia, P.T.; Cortón, E.; Coltro, W.K.T. Enhanced Analytical Performance of Paper Microfluidic Devices by Using Fe3O4 Nanoparticles, MWCNT, and Graphene Oxide. ACS Appl. Mater. Interfaces 2016, 8, 11–15. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, B.; Han, M.-Y.; Zhang, Z. Fluorescent Nanomaterials for Color-Multiplexing Test Papers toward Qualitative/Quantitative Assays. Small Methods 2018, 2, 1700379. [Google Scholar] [CrossRef]
- Gabriel, E.F.M.; Garcia, P.T.; Cardoso, T.M.G.; Lopes, F.M.; Martins, F.T.; Coltro, W.K.T. Highly Sensitive Colorimetric Detection of Glucose and Uric Acid in Biological Fluids Using Chitosan-Modified Paper Microfluidic Devices. Analyst 2016, 141, 4749–4756. [Google Scholar] [CrossRef]
- Evans, E.; Gabriel, E.F.M.; Coltro, W.K.T.; Garcia, C.D. Rational Selection of Substrates to Improve Color Intensity and Uniformity on Microfluidic Paper-Based Analytical Devices. Analyst 2014, 139, 2127–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.; Sindi, H.; Whitesides, G.M. Simple Telemedicine for Developing Regions: Camera Phones and Paper-Based Microfluidic Devices for Real-Time, Off-Site Diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morbioli, G.G.; Mazzu-Nascimento, T.; Milan, L.A.; Stockton, A.M.; Carrilho, E. Improving Sample Distribution Homogeneity in Three-Dimensional Microfluidic Paper-Based Analytical Devices by Rational Device Design. Anal. Chem. 2017, 89, 4786–4792. [Google Scholar] [CrossRef] [PubMed]
- Kaneta, T.; Alahmad, W.; Varanusupakul, P. Microfluidic Paper-Based Analytical Devices with Instrument-Free Detection and Miniaturized Portable Detectors. Appl. Spectrosc. Rev. 2019, 54, 117–141. [Google Scholar] [CrossRef] [Green Version]
- Narang, J.; Singhal, C.; Mathur, A.; Dubey, A.K.; Krishna, A.; Anil, A.; Pundir, C.S. Naked-Eye Quantitative Assay on Paper Device for Date Rape Drug Sensing via Smart Phone APP. Vacuum 2018, 153, 300–305. [Google Scholar] [CrossRef]
- Tian, T.; An, Y.; Wu, Y.; Song, Y.; Zhu, Z.; Yang, C. Integrated Distance-Based Origami Paper Analytical Device for One-Step Visualized Analysis. ACS Appl. Mater. Interfaces 2017, 9, 30480–30487. [Google Scholar] [CrossRef]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-Care Colorimetric Detection with a Smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, F.; Yang, L.; Yang, W.; Zhang, L.; Wang, Z. Paper-Based Multicolor Sensor for on-Site Quantitative Detection of 2,4-Dichlorophenoxyacetic Acid Based on Alkaline Phosphatase-Mediated Gold Nanobipyramids Growth and Colorimeter-Assisted Method for Quantifying Color. Talanta 2022, 245, 123489. [Google Scholar] [CrossRef]
- Lopez-Ruiz, N.; Curto, V.F.; Erenas, M.M.; Benito-Lopez, F.; Diamond, D.; Palma, A.J.; Capitan-Vallvey, L.F. Smartphone-Based Simultaneous PH and Nitrite Colorimetric Determination for Paper Microfluidic Devices. Anal. Chem. 2014, 86, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, W.; Podrażka, M.; Jarosińska, E.; Kappalakandy Valapil, K.; Wiloch, M.; Jönsson-Niedziółka, M.; Witkowska Nery, E. Paper-Based Electrochemical Sensors and How to Make Them (Work). ChemElectroChem 2020, 7, 2939–2956. [Google Scholar] [CrossRef]
- Shen, L.-L.; Zhang, G.-R.; Etzold, B.J.M. Paper-Based Microfluidics for Electrochemical Applications. ChemElectroChem 2020, 7, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, Q.-Y.; Chen, F.; Cao, Y. Paper-Based Electrochemical Sensor. Electrochem. Sci. Adv. 2022, 2, e2100057. [Google Scholar] [CrossRef]
- Ataide, V.N.; Mendes, L.F.; Gama, L.I.L.M.; de Araujo, W.R.; Paixão, T.R.L.C. Electrochemical Paper-Based Analytical Devices: Ten Years of Development. Anal. Methods 2020, 12, 1030–1054. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical Detection for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Moscone, D.; Arduini, F. Preparation of Paper-Based Devices for Reagentless Electrochemical (Bio)Sensor Strips. Nat. Protoc. 2019, 14, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-Based Electrochemical Biosensors for Food Safety Analysis. Biosensors 2022, 12, 1088. [Google Scholar] [CrossRef]
- Dossi, N.; Toniolo, R.; Pizzariello, A.; Impellizzieri, F.; Piccin, E.; Bontempelli, G. Pencil-Drawn Paper Supported Electrodes as Simple Electrochemical Detectors for Paper-Based Fluidic Devices. Electrophoresis 2013, 34, 2085–2091. [Google Scholar] [CrossRef]
- Ameku, W.A.; De Araujo, W.R.; Rangel, C.J.; Ando, R.A.; Paixão, T.R.L.C. Gold Nanoparticle Paper-Based Dual-Detection Device for Forensics Applications. ACS Appl. Nano Mater. 2019, 2, 5460–5468. [Google Scholar] [CrossRef]
- Dias, A.A.; Cardoso, T.M.G.; Chagas, C.L.S.; Oliveira, V.X.G.; Munoz, R.A.A.; Henry, C.S.; Santana, M.H.P.; Paixão, T.R.L.C.; Coltro, W.K.T. Detection of Analgesics and Sedation Drugs in Whiskey Using Electrochemical Paper-Based Analytical Devices. Electroanalysis 2018, 30, 2250–2257. [Google Scholar] [CrossRef]
- Rocha, D.S.; Duarte, L.C.; Silva-Neto, H.A.; Chagas, C.L.S.; Santana, M.H.P.; Antoniosi Filho, N.R.; Coltro, W.K.T. Sandpaper-Based Electrochemical Devices Assembled on a Reusable 3D-Printed Holder to Detect Date Rape Drug in Beverages. Talanta 2021, 232, 122408. [Google Scholar] [CrossRef]
- Dossi, N.; Terzi, F.; Piccin, E.; Toniolo, R.; Bontempelli, G. Rapid Prototyping of Sensors and Conductive Elements by Day-to-Day Writing Tools and Emerging Manufacturing Technologies. Electroanalysis 2016, 28, 250–264. [Google Scholar] [CrossRef]
- Dossi, N.; Toniolo, R.; Impellizzieri, F.; Bontempelli, G. Doped Pencil Leads for Drawing Modified Electrodes on Paper-Based Electrochemical Devices. J. Electroanal. Chem. 2014, 722, 90–94. [Google Scholar] [CrossRef]
- Jangid, A.R.; Strong, E.B.; Chuang, J.; Martinez, A.W.; Martinez, N.W. Evaluation of Commercially-Available Conductive Filaments for 3D Printing Flexible Circuits on Paper. PeerJ Mater. Sci. 2022, 4, e21. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; Krishna, A.K.; Pundir, C.S. Detection of Alprazolam with a Lab on Paper Economical Device Integrated with Urchin like Ag@ Pd Shell Nano-Hybrids. Mater. Sci. Eng. C 2017, 80, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Singhal, C.; Mathur, A.; Khanuja, M.; Varshney, A.; Garg, K.; Dahiya, T.; Pundir, C.S. Lab on Paper Chip Integrated with Si@GNRs for Electroanalysis of Diazepam. Anal. Chim. Acta 2017, 980, 50–57. [Google Scholar] [CrossRef]
- Saisahas, K.; Soleh, A.; Promsuwan, K.; Saichanapan, J.; Phonchai, A.; Sadiq, N.S.M.; Teoh, W.K.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. Nanocoral-like Polyaniline-Modified Graphene-Based Electrochemical Paper-Based Analytical Device for a Portable Electrochemical Sensor for Xylazine Detection. ACS Omega 2022, 7, 13913–13924. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; Chakraborty, D.; Anil, A.; Ingle, A.; Pundir, C.S. Point of Care with Micro Fluidic Paper Based Device Integrated with Nano Zeolite–Graphene Oxide Nanoflakes for Electrochemical Sensing of Ketamine. Biosens. Bioelectron. 2017, 88, 249–257. [Google Scholar] [CrossRef]
- Pholsiri, T.; Khamcharoen, W.; Vimolmangkang, S.; Siangproh, W.; Chailapakul, O. Paper-Based Electrochemical Sensor for Simultaneous Detection of Salivary Δ9-Tetrahydrocannabinol and Thiocyanate to Differentiate Illegal Cannabis Smokers. Sens. Actuators B Chem. 2023, 383, 133571. [Google Scholar] [CrossRef]
- Pang, R.; Zhu, Q.; Wei, J.; Meng, X.; Wang, Z. Enhancement of the Detection Performance of Paper-Based Analytical Devices by Nanomaterials. Molecules 2022, 27, 508. [Google Scholar] [CrossRef]
- Holman, J.B.; Shi, Z.; Fadahunsi, A.A.; Li, C.; Ding, W. Advances on Microfluidic Paper-Based Electroanalytical Devices. Biotechnol. Adv. 2023, 63, 108093. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Yang, L.; Zhao, Y.; Li, F. Metal-Organic Framework-Functionalized Paper-Based Electrochemical Biosensor for Ultrasensitive Exosome Assay. Anal. Chem. 2021, 93, 11792–11799. [Google Scholar] [CrossRef] [PubMed]
- McNeill, L.; Pearson, C.; Megson, D.; Norrey, J.; Watson, D.; Ashworth, D.; Linton, P.E.; Sutcliffe, O.B.; Shaw, K.J. Origami Chips: Development and Validation of a Paper-Based Lab-on-a-Chip Device for the Rapid and Cost-Effective Detection of 4-Methylmethcathinone (Mephedrone) and Its Metabolite, 4-Methylephedrine in Urine. Forensic Chem. 2021, 22, 100293. [Google Scholar] [CrossRef]
- Oh, J.M.; Chow, K.F. Recent Developments in Electrochemical Paper-Based Analytical Devices. Anal. Methods 2015, 7, 7951–7960. [Google Scholar] [CrossRef]
- Renault, C.; Anderson, M.J.; Crooks, R.M. Electrochemistry in Hollow-Channel Paper Analytical Devices. J. Am. Chem. Soc. 2014, 136, 4616–4623. [Google Scholar] [CrossRef]
- Marques, A.C.; Pinheiro, T.; Martins, G.V.; Cardoso, A.R.; Martins, R.; Sales, M.G.; Fortunato, E. Non-Enzymatic Lab-on-Paper Devices for Biosensing Applications. Compr. Anal. Chem. 2020, 89, 189–237. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; El-Galil, E.; Amr, A.; Elsayed, E.A.; Sayed, A.Y.A.; Kamel, A.H. Paper-Based Potentiometric Sensing Devices Modified with Chemically Reduced Graphene Oxide (CRGO) for Trace Level Determination of Pholcodine (Opiate Derivative Drug). RSC Adv. 2021, 11, 12227–12234. [Google Scholar] [CrossRef]
- Özbek, O.; Berkel, C. Recent Advances in Potentiometric Analysis: Paper–Based Devices. Sens. Int. 2022, 3, 100189. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Mohamed, E.H.; Yehia, A.M. All Solid-State Miniaturized Potentiometric Sensors for Flunitrazepam Determination in Beverages. Microchim. Acta 2021, 188, 192. [Google Scholar] [CrossRef]
- Arantes, I.V.S.; Paixão, T.R.L.C. Couple Batch-Injection Analysis and Microfluidic Paper-Based Analytical Device: A Simple and Disposable Alternative to Conventional BIA Apparatus. Talanta 2022, 240, 123201. [Google Scholar] [CrossRef]
- Pholsiri, T.; Lomae, A.; Pungjunun, K.; Vimolmangkang, S.; Siangproh, W.; Chailapakul, O. A Chromatographic Paper-Based Electrochemical Device to Determine Δ9-Tetrahydrocannabinol and Cannabidiol in Cannabis Oil. Sens. Actuators B Chem. 2022, 355, 131353. [Google Scholar] [CrossRef]
- Lan, W.-J.; Maxwell, E.J.; Parolo, C.; Bwambok, D.K.; Subramaniam, A.B.; Whitesides, G.M. Paper-Based Electroanalytical Devices with an Integrated, Stable Reference Electrode. Lab Chip 2013, 13, 4103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narang, J.; Singhal, C.; Khanuja, M.; Mathur, A.; Jain, A.; Pundir, C.S. Hydrothermally Synthesized Zinc Oxide Nanorods Incorporated on Lab-on-Paper Device for Electrochemical Detection of Recreational Drug. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fava, E.L.; Martimiano do Prado, T.; Almeida Silva, T.; Cruz de Moraes, F.; Censi Faria, R.; Fatibello-Filho, O. New Disposable Electrochemical Paper-Based Microfluidic Device with Multiplexed Electrodes for Biomarkers Determination in Urine Sample. Electroanalysis 2020, 32, 1075–1083. [Google Scholar] [CrossRef]
- Dossi, N.; Toniolo, R.; Impellizzieri, F.; Tubaro, F.; Bontempelli, G.; Terzi, F.; Piccin, E. A Paper-Based Platform with a Pencil-Drawn Dual Amperometric Detector for the Rapid Quantification of Ortho-Diphenols in Extravirgin Olive Oil. Anal. Chim. Acta 2017, 950, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dossi, N.; Toniolo, R.; Terzi, F.; Sdrigotti, N.; Tubaro, F.; Bontempelli, G. A Cotton Thread Fluidic Device with a Wall-Jet Pencil-Drawn Paper Based Dual Electrode Detector. Anal. Chim. Acta 2018, 1040, 74–80. [Google Scholar] [CrossRef]
- Yamamoto, S.; Uno, S. Redox Cycling Realized in Paper-Based Biochemical Sensor for Selective Detection of Reversible Redox Molecules Without Micro/Nano Fabrication Process. Sensors 2018, 18, 730. [Google Scholar] [CrossRef] [Green Version]
- Noviana, E.; Klunder, K.J.; Channon, R.B.; Henry, C.S. Thermoplastic Electrode Arrays in Electrochemical Paper-Based Analytical Devices. Anal. Chem. 2019, 91, 2431–2438. [Google Scholar] [CrossRef]
- Santhiago, M.; Wydallis, J.B.; Kubota, L.T.; Henry, C.S. Construction and Electrochemical Characterization of Microelectrodes for Improved Sensitivity in Paper-Based Analytical Devices. Anal. Chem. 2013, 85, 5233–5239. [Google Scholar] [CrossRef] [Green Version]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Le Ru, E.C.; Etchegoin, P.G. A Quick Overview of Surface-Enhanced Raman Spectroscopy. In Principles of Surface-Enhanced Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–27. ISBN 978-0-444-52779-0. [Google Scholar]
- Bonifacio, A. Nanostrucured Substrates for Surface-Enhanced Raman Scattering Spectroscopy. In Nanomaterials for Theranostics and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–174. ISBN 978-0-12-817838-6. [Google Scholar]
- Betz, J.F.; Yu, W.W.; Cheng, Y.; White, I.M.; Rubloff, G.W. Simple SERS Substrates: Powerful, Portable, and Full of Potential. Phys. Chem. Chem. Phys. 2014, 16, 2224–2239. [Google Scholar] [CrossRef]
- Vicente, A.T.; Araújo, A.; Mendes, M.J.; Nunes, D.; Oliveira, M.J.; Sanchez-Sobrado, O.; Ferreira, M.P.; Águas, H.; Fortunato, E.; Martins, R. Multifunctional Cellulose-Paper for Light Harvesting and Smart Sensing Applications. J. Mater. Chem. C 2018, 6, 3143–3181. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogundare, S.A.; van Zyl, W.E. A Review of Cellulose-Based Substrates for SERS: Fundamentals, Design Principles, Applications. Cellulose 2019, 26, 6489–6528. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Cao, K. Flexible Surface-Enhanced Raman Scattering Substrates: A Review on Constructions, Applications, and Challenges. Adv. Mater. Interfaces 2021, 8, 2100982. [Google Scholar] [CrossRef]
- Yu, W.W.; White, I.M. Inkjet Printed Surface Enhanced Raman Spectroscopy Array on Cellulose Paper. Anal. Chem. 2010, 82, 9626–9630. [Google Scholar] [CrossRef] [Green Version]
- Tay, L.-L.; Poirier, S.; Ghaemi, A.; Hulse, J.; Wang, S. Iodide Functionalized Paper-Based SERS Sensors for Improved Detection of Narcotics. Front. Chem. 2021, 9, 680556. [Google Scholar] [CrossRef]
- Polavarapu, L.; Porta, A.L.; Novikov, S.M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Pen-on-Paper Approach Toward the Design of Universal Surface Enhanced Raman Scattering Substrates. Small 2014, 10, 3065–3071. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Quaresma, P.; Peixoto de Almeida, M.; Araújo, A.; Pereira, E.; Fortunato, E.; Martins, R.; Franco, R.; Águas, H. Office Paper Decorated with Silver Nanostars—An Alternative Cost Effective Platform for Trace Analyte Detection by SERS. Sci. Rep. 2017, 7, 2480. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.; Lee, M.; Lee, S.G.; Jung, D.H.; Lee, H.L. Cellulose Nanofibrils Coated Paper Substrate to Detect Trace Molecules Using Surface-Enhanced Raman Scattering. Cellulose 2018, 25, 3339–3350. [Google Scholar] [CrossRef]
- Laing, S.; Jamieson, L.E.; Faulds, K.; Graham, D. Surface-Enhanced Raman Spectroscopy for in Vivo Biosensing. Nat. Rev. Chem. 2017, 1, 60. [Google Scholar] [CrossRef] [Green Version]
- Goodacre, R.; Graham, D.; Faulds, K. Recent Developments in Quantitative SERS: Moving towards Absolute Quantification. TrAC Trends Anal. Chem. 2018, 102, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Lin, X.; Jiang, C.; Li, C.; Lin, H.; Huang, J.; Wang, S.; Liu, G.; Yan, X.; Zhong, Q.; et al. Reliable Quantitative SERS Analysis Facilitated by Core-Shell Nanoparticles with Embedded Internal Standards. Angew. Chem. 2015, 54, 7308–7312. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Taylor, A.; Leese, E.; Allen, S.; Morton, J.; McAdam, J. Homicidal Arsenic Poisoning. Ann. Clin. Biochem. 2015, 52, 510–515. [Google Scholar] [CrossRef]
- Dueñas-Laita, A.; Pérez-Miranda, M.; González-López, M.A.; Martín-Escudero, J.C.; Ruiz-Mambrilla, M.; Blanco-Varela, J. Acute Arsenic Poisoning. Lancet 2005, 365, 1982. [Google Scholar] [CrossRef]
- Saadati, A.; Farshchi, F.; Hasanzadeh, M.; Liu, Y.; Seidi, F. Colorimetric and Naked-Eye Detection of Arsenic(Iii) Using a Paper-Based Colorimetric Device Decorated with Silver Nanoparticles. RSC Adv. 2022, 12, 21836–21850. [Google Scholar] [CrossRef]
- Swezey, R.; Shinn, W.; Green, C.; Drover, D.R.; Hammer, G.B.; Schulman, S.R.; Zajicek, A.; Jett, D.A.; Boss, G.R. Comparison of a New Cobinamide-Based Method to a Standard Laboratory Method for Measuring Cyanide in Human Blood. J. Anal. Toxicol. 2013, 37, 382–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wachełko, O.; Chłopaś-Konowałek, A.; Zawadzki, M.; Szpot, P. Old Poison, New Problem: Cyanide Fatal Intoxications Associated with Internet Shopping. J. Anal. Toxicol. 2022, 46, e52–e59. [Google Scholar] [CrossRef]
- Felscher, D.; Wulfmeyer, M. A New Specific Method to Detect Cyanide in Body Fluids, Especially Whole Blood, by Fluorimetry. J. Anal. Toxicol. 1998, 22, 363–366. [Google Scholar] [CrossRef] [Green Version]
- Petruci, J.F.D.S.; Hauser, P.C.; Cardoso, A.A. Colorimetric Paper-Based Device for Gaseous Hydrogen Cyanide Quantification Based on Absorbance Measurements. Sens. Actuators B Chem. 2018, 268, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Sheini, A.; Aseman, M.D.; Bordbar, M.M. Origami Paper Analytical Assay Based on Metal Complex Sensor for Rapid Determination of Blood Cyanide Concentration in Fire Survivors. Sci. Rep. 2021, 11, 3521. [Google Scholar] [CrossRef]
- Wirojsaengthong, S.; Aryuwananon, D.; Aeungmaitrepirom, W.; Pulpoka, B.; Tuntulani, T. A Colorimetric Paper-Based Optode Sensor for Highly Sensitive and Selective Determination of Thiocyanate in Urine Sample Using Cobalt Porphyrin Derivative. Talanta 2021, 231, 122371. [Google Scholar] [CrossRef] [PubMed]
- Thepchuay, Y.; Sonsa-Ard, T.; Ratanawimarnwong, N.; Auparakkitanon, S.; Sitanurak, J.; Nacapricha, D. Paper-Based Colorimetric Biosensor of Blood Alcohol with in-Situ Headspace Separation of Ethanol from Whole Blood. Anal. Chim. Acta 2020, 1103, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hikin, L.J.; Ho, J.; Morley, S.R.; Ahluwalia, A.; Smith, P.R. Sodium Nitrite Poisoning: A Series of 20 Fatalities in Which Post-Mortem Blood Nitrite and Nitrate Concentrations Are Reported. Forensic Sci. Int. 2023, 345, 111610. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Song, Y.-Z.; Fang, F.; Wu, Z.-Y. Sensitive Paper-Based Analytical Device for Fast Colorimetric Detection of Nitrite with Smartphone. Anal. Bioanal. Chem. 2018, 410, 2665–2669. [Google Scholar] [CrossRef]
- Yu, P.; Deng, M.; Yang, Y. New Single-Layered Paper-Based Microfluidic Devices for the Analysis of Nitrite and Glucose Built via Deposition of Adhesive Tape. Sensors 2019, 19, 4082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.; Deng, M.; Yang, Y.; Nie, B.; Zhao, S. 3d Microfluidic Devices in a Single Piece of Paper for the Simultaneous Determination of Nitrite and Thiocyanate. Sensors 2020, 20, 4118. [Google Scholar] [CrossRef]
- De Oliveira, R.A.G.; Camargo, F.; Pesquero, N.C.; Faria, R.C. A Simple Method to Produce 2D and 3D Microfluidic Paper-Based Analytical Devices for Clinical Analysis. Anal. Chim. Acta 2017, 957, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klasner, S.A.; Price, A.K.; Hoeman, K.W.; Wilson, R.S.; Bell, K.J.; Culbertson, C.T. Paper-Based Microfluidic Devices for Analysis of Clinically Relevant Analytes Present in Urine and Saliva. Anal. Bioanal. Chem. 2010, 397, 1821–1829. [Google Scholar] [CrossRef]
- Bhakta, S.A.; Borba, R.; Taba, M.; Garcia, C.D.; Carrilho, E. Determination of Nitrite in Saliva Using Microfluidic Paper-Based Analytical Devices. Anal. Chim. Acta 2014, 809, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.T.S.M.; Mesquita, R.B.R.; Rangel, A.O.S.S. Novel Microfluidic Paper-Based Analytical Devices (ΜPADs) for the Determination of Nitrate and Nitrite in Human Saliva. Talanta 2020, 219, 121183. [Google Scholar] [CrossRef]
- Cardoso, T.M.G.; Garcia, P.T.; Coltro, W.K.T. Colorimetric Determination of Nitrite in Clinical, Food and Environmental Samples Using Microfluidic Devices Stamped in Paper Platforms. Anal. Methods 2015, 7, 7311–7317. [Google Scholar] [CrossRef]
- Mollaie, E.; Asiaei, S.; Aryan, H. Nitrite Enhanced Detection from Saliva by Simple Geometrical Modifications of Paper-Based Micromixers. Microfluid. Nanofluidics 2022, 26, 88. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Guo, Y.; Ma, X.; Lv, C.; Yang, M.; Yao, S.; Jin, Y.; Li, B.; Liu, W. Ring-Oven-Assisted In Situ Synthesis of Metal–Organic Frameworks on the Lab-On-Paper Device for Chemiluminescence Detection of Nitrite in Whole Blood. Anal. Chem. 2023, 95, 4362–4370. [Google Scholar] [CrossRef]
- Ansari, N.; Lodha, A.; Pandya, A.; Menon, S.K. Determination of Cause of Death Using Paper-Based Microfluidic Device as a Colorimetric Probe. Anal. Methods 2017, 9, 5632–5639. [Google Scholar] [CrossRef]
- Wei, X.; Tian, T.; Jia, S.; Zhu, Z.; Ma, Y.; Sun, J.; Lin, Z.; Yang, C.J. Target-Responsive DNA Hydrogel Mediated “Stop-Flow” Microfluidic Paper-Based Analytic Device for Rapid, Portable and Visual Detection of Multiple Targets. Anal. Chem. 2015, 87, 4275–4282. [Google Scholar] [CrossRef]
- Tian, T.; Wei, X.; Jia, S.; Zhang, R.; Li, J.; Zhu, Z.; Zhang, H.; Ma, Y.; Lin, Z.; Yang, C.J. Integration of Target Responsive Hydrogel with Cascaded Enzymatic Reactions and Microfluidic Paper-Based Analytic Devices (ΜPADs) for Point-of-Care Testing (POCT). Biosens. Bioelectron. 2016, 77, 537–542. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, Z.; Ge, Y.; Liu, Z. Portable Upconversion Nanoparticles-Based Paper Device for Field Testing of Drug Abuse. Anal. Chem. 2016, 88, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.W.; White, I.M. Inkjet-Printed Paper-Based SERS Dipsticks and Swabs for Trace Chemical Detection. Analyst 2013, 138, 1020–1025. [Google Scholar] [CrossRef]
- Burr, D.S.; Fatigante, W.L.; Lartey, J.A.; Jang, W.; Stelmack, A.R.; McClurg, N.W.; Standard, J.M.; Wieland, J.R.; Kim, J.-H.; Mulligan, C.C.; et al. Integrating SERS and PSI-MS with Dual Purpose Plasmonic Paper Substrates for On-Site Illicit Drug Confirmation. Anal. Chem. 2020, 92, 6676–6683. [Google Scholar] [CrossRef]
- Han, S.; Zhang, C.; Lin, S.; Sha, X.; Hasi, W. Sensitive and Reliable Identification of Fentanyl Citrate in Urine and Serum Using Chloride Ion-Treated Paper-Based SERS Substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 251, 119463. [Google Scholar] [CrossRef]
- Haddad, A.; Comanescu, M.A.; Green, O.; Kubic, T.A.; Lombardi, J.R. Detection and Quantitation of Trace Fentanyl in Heroin by Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2018, 90, 12678–12685. [Google Scholar] [CrossRef]
- Tay, L.-L.; Poirier, S.; Ghaemi, A.; Hulse, J. Inkjet-Printed Paper-Based Surface Enhanced Raman Scattering (SERS) Sensors for the Detection of Narcotics. MRS Adv. 2022, 7, 190–196. [Google Scholar] [CrossRef]
- Chen, C.-A.; Wang, P.-W.; Yen, Y.-C.; Lin, H.-L.; Fan, Y.-C.; Wu, S.-M.; Chen, C.-F. Fast Analysis of Ketamine Using a Colorimetric Immunosorbent Assay on a Paper-Based Analytical Device. Sens. Actuators B Chem. 2019, 282, 251–258. [Google Scholar] [CrossRef]
- Vieira, O.; Moal, A.; Milan, N.; Sibille, P.; Deffontaine, G.; Ghysel-Laporte, M.-H. Paradoxal Use of Stimulants in Drug-Facilitated Crime. Toxicol. Anal. Et Clin. 2014, 26, S39. [Google Scholar] [CrossRef]
- Teerinen, T.; Lappalainen, T.; Erho, T. A Paper-Based Lateral Flow Assay for Morphine. Anal. Bioanal. Chem. 2014, 406, 5955–5965. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Bioanalytical Method Validation Guidance for Industry. 2018. Available online: https://www.nebiolab.com/fda-bioanalytical-method-validation-guidance-for-industry-2018/ (accessed on 2 May 2023).
- Moulahoum, H.; Ghorbanizamani, F.; Timur, S. Paper-Based Lateral Flow Assay Using Rhodamine B–Loaded Polymersomes for the Colorimetric Determination of Synthetic Cannabinoids in Saliva. Microchim. Acta 2021, 188, 1–12. [Google Scholar] [CrossRef]
- Son, S.U.; Jang, S.; Kang, B.; Kim, J.; Lim, J.; Seo, S.; Kang, T.; Jung, J.; Lee, K.-S.; Kim, H.; et al. Colorimetric Paper Sensor for Visual Detection of Date-Rape Drug γ-Hydroxybutyric Acid (GHB). Sens. Actuators B Chem. 2021, 347, 130598. [Google Scholar] [CrossRef]
- Yehia, A.M.; Farag, M.A.; Tantawy, M.A. A Novel Trimodal System on a Paper-Based Microfluidic Device for on-Site Detection of the Date Rape Drug “Ketamine”. Anal. Chim. Acta 2020, 1104, 95–104. [Google Scholar] [CrossRef]
- Melchior, S.E.; Nielsen, M.K.K.; Oropeza, A.R.; Banner, J.; Johansen, S.S. Detection of Scopolamine in Urine and Hair in a Drug-Facilitated Sexual Assault. Forensic Sci. Int. 2023, 347, 111678. [Google Scholar] [CrossRef]
- Dias, B.C.; Batista, A.D.; da Silveira Petruci, J.F. ΜOPTO: A Microfluidic Paper-Based Optoelectronic Tongue as Presumptive Tests for the Discrimination of Alkaloid Drugs for Forensic Purposes. Anal. Chim. Acta 2021, 1187, 339141. [Google Scholar] [CrossRef]
- Mustafa, F.; Carhart, M.; Andreescu, S. A 3D-Printed Breath Analyzer Incorporating CeO2 Nanoparticles for Colorimetric Enzyme-Based Ethanol Sensing. ACS Appl. Nano Mater. 2021, 4, 9361–9369. [Google Scholar] [CrossRef]
- Alder, R.; Hong, J.; Chow, E.; Fang, J.; Isa, F.; Ashford, B.; Comte, C.; Bendavid, A.; Xiao, L.; Ostrikov, K.; et al. Application of Plasma-Printed Paper-Based SERS Substrate for Cocaine Detection. Sensors 2021, 21, 810. [Google Scholar] [CrossRef] [PubMed]
- Ameku, W.A.; Gonçalves, J.M.; Ataide, V.N.; Ferreira Santos, M.S.; Gutz, I.G.R.; Araki, K.; Paixão, T.R.L.C. Combined Colorimetric and Electrochemical Measurement Paper-Based Device for Chemometric Proof-of-Concept Analysis of Cocaine Samples. ACS Omega 2021, 6, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Fedick, P.W.; Pu, F.; Morato, N.M.; Cooks, R.G. Identification and Confirmation of Fentanyls on Paper Using Portable Surface Enhanced Raman Spectroscopy and Paper Spray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2020, 31, 735–741. [Google Scholar] [CrossRef]
- NASA Technology Readiness Levels Demystified. Available online: http://www.nasa.gov/topics/aeronautics/features/trl_demystified.html (accessed on 12 June 2023).
- Kunkel, H.G.; Tiselius, A. Electrophoresis of Proteins on Filter Paper. J. Gen. Physiol. 1951, 35, 89–118. [Google Scholar] [CrossRef]
- Silva-Neto, H.A.; Arantes, I.V.S.; Ferreira, A.L.; do Nascimento, G.H.M.; Meloni, G.N.; de Araujo, W.R.; Paixão, T.R.L.C.; Coltro, W.K.T. Recent Advances on Paper-Based Microfluidic Devices for Bioanalysis. TrAC Trends Anal. Chem. 2023, 158, 116893. [Google Scholar] [CrossRef]
- Li, T.; Liang, B.; Ye, Z.; Zhang, L.; Xu, S.; Tu, T.; Zhang, Y.; Cai, Y.; Zhang, B.; Fang, L.; et al. An Integrated and Conductive Hydrogel-Paper Patch for Simultaneous Sensing of Chemical–Electrophysiological Signals. Biosens. Bioelectron. 2022, 198, 113855. [Google Scholar] [CrossRef]
- Xu, Y.; Fei, Q.; Page, M.; Zhao, G.; Ling, Y.; Stoll, S.B.; Yan, Z. Paper-Based Wearable Electronics. iScience 2021, 24, 102736. [Google Scholar] [CrossRef]
- Criscuolo, F.; Cantu, F.; Taurino, I.; Carrara, S.; De Micheli, G. A Wearable Electrochemical Sensing System for Non-Invasive Monitoring of Lithium Drug in Bipolar Disorder. IEEE Sens. J. 2021, 21, 9649–9656. [Google Scholar] [CrossRef]

| Classification on the Basis of the Present Report | Analyte | Category | Effect [3] | Lowest Blood Concentration Causing Toxic Effect (mg/L) [24] |
|---|---|---|---|---|
| Toxic Compounds | Arsenic | Xenobiotic—Inorganic ion | Arsenic interferes with energy transfer mechanisms | 0.05–0.25 |
| Cyanide | Xenobiotic—Inorganic ion | Cyanide causes cellular hypoxia | 0.2–0.5 | |
| Ethanol | Xenobiotic—Small organic molecule | Ethanol acts as a central nervous system depressant | 1000–2000 | |
| Nitrite | Xenobiotic—Inorganic ion | Nitrite and derivative compounds (amyl nitrite, butyl nitrite, and isobutyl nitrite) cause methemoglobinemia | 32.4 *** | |
| Drugs and Drugs of Abuse | Alprazolam | Licit drug | Alprazolam is used for its anxiolytic action [25] | 0.1–0.4 |
| Diazepam | Licit drug | Diazepam is used as a muscle relaxant or anticonvulsant and for its anti-anxiety action [25] | 3–5 | |
| Cathinone. Mephedrone | Illicit drug | Stimulant effect on central and peripheral nervous systems | n/a | |
| Cocaine | Illicit drug | Cocaine produces a sympathomimetic response | 0.25 | |
| Fentanyl | Licit drug | Fentanyl has an antinociceptive activity | 0.003 * | |
| Ketamine | Licit drug | Ketamine is used for its anesthetic action (pediatric medicine) | 0.02 (abuse) | |
| MDMA ** | Illicit drug | Stimulant effect on central and peripheral nervous system | 0.35–0.50 | |
| Morphine | Illicit drug | Morphine acts as a central nervous system depressant | 0.1 | |
| Synthetic Cannabinoids. JWH-073 | Illicit drug | Assumption of synthetic cannabinoids results in anxiety, tremulous, and experiencing palpitations | 0.002–0.006 **** | |
| Tetrahydrocannabinol | Illicit drug | It causes sedation, euphoria, and hallucinations | 0.34 | |
| Psychoactive Substances Added to Beverages for Drug-Facilitated Crimes (DFCs) | Flunitrazepam | Licit drug | Flunitrazepam acts as a hypnotic and anesthetic agent [25] | 0.05 |
| GHB ** | Licit drug | GHB causes euphoria, increases sexual desire, and has a CNS depressant effect | 80–200 | |
| Ketamine | Licit drug | Ketamine is used for its anesthetic action (pediatric medicine) | 0.02 (abuse) | |
| Metamizole | Licit drug | Metamizole provokes an analgesic effect | 20 | |
| Midazolam | Licit drug | Midazolam acts as depressant of the CNS | 1–1.5 | |
| Scopolamine | Licit drug | It is illegally used as an incapacitating agent and for inducing submissive behavior in the victim | 0.02 (abuse) | |
| Xylazine | Licit drug | Xylazine is use as a sedative, analgesic, and anesthetic agent (veterinary medicine) | 2.3 [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musile, G.; Grazioli, C.; Fornasaro, S.; Dossi, N.; De Palo, E.F.; Tagliaro, F.; Bortolotti, F. Application of Paper-Based Microfluidic Analytical Devices (µPAD) in Forensic and Clinical Toxicology: A Review. Biosensors 2023, 13, 743. https://doi.org/10.3390/bios13070743
Musile G, Grazioli C, Fornasaro S, Dossi N, De Palo EF, Tagliaro F, Bortolotti F. Application of Paper-Based Microfluidic Analytical Devices (µPAD) in Forensic and Clinical Toxicology: A Review. Biosensors. 2023; 13(7):743. https://doi.org/10.3390/bios13070743
Chicago/Turabian StyleMusile, Giacomo, Cristian Grazioli, Stefano Fornasaro, Nicolò Dossi, Elio Franco De Palo, Franco Tagliaro, and Federica Bortolotti. 2023. "Application of Paper-Based Microfluidic Analytical Devices (µPAD) in Forensic and Clinical Toxicology: A Review" Biosensors 13, no. 7: 743. https://doi.org/10.3390/bios13070743
APA StyleMusile, G., Grazioli, C., Fornasaro, S., Dossi, N., De Palo, E. F., Tagliaro, F., & Bortolotti, F. (2023). Application of Paper-Based Microfluidic Analytical Devices (µPAD) in Forensic and Clinical Toxicology: A Review. Biosensors, 13(7), 743. https://doi.org/10.3390/bios13070743