Essential Oil and Hydrophilic Antibiotic Co-Encapsulation in Multiple Lipid Nanoparticles: Proof of Concept and In Vitro Activity against Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results and Discussion
2.1. REO Characterization
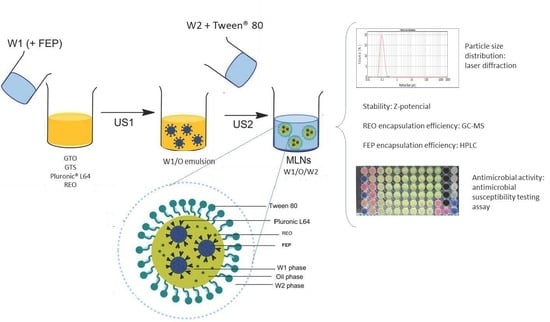
2.2. MLN Preparation and Characterization
2.2.1. Effect of MLN Composition on REO Encapsulation
2.2.2. Effect of MLN Composition on FEP Encapsulation
2.3. Antimicrobial Susceptibility Testing (AST) Assays
2.3.1. AST Assays of Pure Compound
2.3.2. AST Assays of Loaded MLNs
3. Materials and Methods
3.1. Materials
3.2. MLN Preparation
3.3. MLNs Characterization
3.3.1. PSD
3.3.2. MNL Stability: ZP Measurement
3.3.3. REO-EE and REO-LC
3.3.4. GC-MS
3.3.5. FEP-EE and FEP-LC
3.4. AST Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, S.B. Factors impacting on the problem of antibiotic resistance. J. Antimicrob. Chemother. 2002, 49, 25–30. [Google Scholar] [CrossRef]
- Chastre, J.; Trouillet, J.L. Problem pathogens (Pseudomonas aeruginosa and Acinetobacter). Semin. Respir. Infect. 2000, 15, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Vettoretti, L.; Floret, N.; Hocquet, D.; Dehecq, B.; Plésiat, P.; Talon, D.; Bertrand, X. Emergence of extensive-drug-resistant Pseudomonas aeruginosa in a French university hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1217–1222. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, E.B.; Tam, V.H. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev. Pharm. Outcomes Res. 2010, 10, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Wolter, D.J.; Lister, P.D. Mechanisms of β -lactam Resistance Among Pseudomonas aeruginosa. Curr. Pharm. Des. 2012, 19, 209–222. [Google Scholar] [CrossRef]
- Sader, H.S.; Fritsche, T.R.; Jones, R.N. Potency and spectrum trends for cefepime tested against 65 746 clinical bacterial isolates collected in North American medical centers: Results from the SENTRY Antimicrobial Surveillance Program (1998–2003). Diagn. Microbiol. Infect. Dis. 2005, 52, 265–273. [Google Scholar] [CrossRef]
- Palmer, A.C.; Kishony, R. Understanding, Predicting and manipulating the genotypic evolution of antibiotic resistance. Nat. Rev. Genet. 2013, 14, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Zengin, G.; Sinan, K.I.; Ak, G.; Ceylan, R.; Mahomoodally, M.F.; Uysal, A.; Sadeer, N.B.; Jekő, J.; Cziáky, Z.; et al. Chemical characterization, cytotoxic, antioxidant, antimicrobial, and enzyme inhibitory effects of different extracts from one sage (Salvia ceratophylla L.) from Turkey: Open a new window on industrial purposes. RSC Adv. 2021, 11, 5295–5310. [Google Scholar] [CrossRef]
- Donadu, M.; Usai, D.; Pinna, A.; Porcu, T.; Mazzarello, V.; Fiamma, M.; Marchetti, M.; Cannas, S.; Delogu, G.; Zanetti, S.; et al. In vitro activity of hybrid lavender essential oils against multidrug resistant strains of Pseudomonas aeruginosa. J. Infect. Dev. Ctries. 2018, 12, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elcocks, E.R.; Spencer-Phillips, P.T.N.; Adukwu, E.C. Rapid bactericidal effect of cinnamon bark essential oil against Pseudomonas aeruginosa. J. Appl. Microbiol. 2020, 128, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Doleski, P.H.; Santos, R.C.V.; Raffin, R.P.; Baldisserotto, B. Involvement of xanthine oxidase inhibition with the antioxidant property of nanoencapsulated Melaleuca alternifolia essential oil in fish experimentally infected with Pseudomonas aeruginosa. J. Fish Dis. 2018, 41, 791–796. [Google Scholar] [CrossRef]
- Araújo Silva, V.; Pereira da Sousa, J.; de Luna Freire Pessôa, H.; Fernanda Ramos de Freitas, A.; Douglas Melo Coutinho, H.; Beuttenmuller Nogueira Alves, L.; Oliveira Lima, E. Ocimum basilicum: Antibacterial activity and association study with antibiotics against bacteria of clinical importance. Pharm. Biol. 2016, 54, 863–867. [Google Scholar] [CrossRef] [Green Version]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielínska-Bliźniewska, H.; Dołegowska, B. The antibacterial activity of lavender essential oil alone and in combination with octenidine Dihydrochloride against MRSA strains. Molecules 2020, 25, 95. [Google Scholar] [CrossRef] [Green Version]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Vallejo, R.; Guirao, P.; Rodríguez-Rojo, S.; Cocero, M.J. Use of nanoemulsions of plant essential oils as aphid repellents. Ind. Crops Prod. 2017, 110, 45–57. [Google Scholar] [CrossRef]
- Pushparaj Selvadoss, P.; Nellore, J.; Balaraman Ravindrran, M.; Sekar, U.; Tippabathani, J. Enhancement of antimicrobial activity by liposomal oleic acid-loaded antibiotics for the treatment of multidrug-resistant Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2018, 46, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Drulis-Kawa, Z.; Gubernator, J.; Dorotkiewicz-Jach, A.; Doroszkiewicz, W.; Kozubek, A. In vitro antimicrobial activity of liposomal meropenem against Pseudomonas aeruginosa strains. Int. J. Pharm. 2006, 315, 59–66. [Google Scholar] [CrossRef]
- Wang, D.Y.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-Based Antimicrobial Delivery-Systems for the Treatment of Bacterial Infections. Front. Chem. 2020, 7, 872. [Google Scholar] [CrossRef]
- Ni, S.; Sun, R.; Zhao, G.; Xia, Q. Quercetin Loaded Nanostructured Lipid Carrier for Food Fortification: Preparation, Characterization and in vitro Study. J. Food Process Eng. 2015, 38, 93–106. [Google Scholar] [CrossRef]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wissing, S.; Kayser, O.; Müller, R. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Cavalcanti, S.M.T.; Nunes, C.; Lima, S.A.C.; Soares-Sobrinho, J.L.; Reis, S. Multiple Lipid Nanoparticles (MLN), a New Generation of Lipid Nanoparticles for Drug Delivery Systems: Lamivudine-MLN Experimental Design. Pharm. Res. 2017, 34, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Bruneton, J. Pharmacognosy, Phytochemistry, Medicinal Plants; Intercept Ltd.: Waterlooville, UK, 1999; pp. 539–540. [Google Scholar]
- Flamini, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Ceccarini, L. Main agronomic-productive characteristics of two ecotypes of Rosmarinus officinalis L. and chemical composition of their essential oils. J. Agric. Food Chem. 2002, 50, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Keivani Nahr, F.; Ghanbarzadeh, B.; Hamishehkar, H.; Samadi Kafil, H. Food grade nanostructured lipid carrier for cardamom essential oil: Preparation, characterization and antimicrobial activity. J. Funct. Foods 2018, 40, 1–8. [Google Scholar] [CrossRef]
- Shen, J.; Sun, M.; Ping, Q.; Ying, Z.; Liu, W. Incorporation of liquid lipid in lipid nanoparticles for ocular drug delivery enhancement. Nanotechnology 2010, 21, 025101. [Google Scholar] [CrossRef]
- Rodríguez-Rojo, S.; Varona, S.; Núñez, M.; Cocero, M.J. Characterization of rosemary essential oil for biodegradable emulsions. Ind. Crops Prod. 2012, 37, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Vitorino, C.; Almeida, J.; Gonçalves, L.M.; Almeida, A.J.; Sousa, J.J.; Pais, A.A.C.C. Co-encapsulating nanostructured lipid carriers for transdermal application: From experimental design to the molecular detail. J. Control. Release 2013, 167, 301–314. [Google Scholar] [CrossRef]
- Becker Peres, L.; Becker Peres, L.; de Araújo, P.H.H.; Sayer, C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surfaces B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef]
- Maeder, K.; Mehnert, W. Solid Lipid Nanoparticles: Concepts, Procedures, and Physicochemical Aspects. ChemInform 2005, 36. [Google Scholar] [CrossRef]
- Loo, C.; Basri, M.; Ismail, R.; Lau, H.; Tejo, B.A.; Kanthimathi, M.; Hassan, H.; Choo, Y. Effect of compositions in nanostructured lipid carriers (NLC) on skin hydration and occlusion. Int. J. Nanomed. 2012, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J. Phys. Sci. 2014, 25, 59–75. [Google Scholar]
- Lim, S.-J.; Kim, C.-K. Formulation parameters determining the physicochemical characteristics of solid lipid nanoparticles loaded with all-trans retinoic acid. Int. J. Pharm. 2002, 243, 135–146. [Google Scholar] [CrossRef]
- Schuh, R.S.; Bruxel, F.; Teixeira, H.F. Physicochemical properties of lecithin-based nanoemulsions obtained by spontaneous emulsification or high-pressure homogenization. Quim. Nova 2014, 37, 1193–1198. [Google Scholar] [CrossRef]
- Sun, D.; Kang, S.; Liu, C.; Lu, Q.; Cui, L.; Hu, B. Effect of zeta potential and particle size on the stability of SiO2 nanospheres as carrier for ultrasound imaging contrast agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Li, L.; Sultanbawa, Y. Formulation, Characterisation and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Moyá, M.L.; López-López, M.; Lebrón, J.A.; Ostos, F.J.; Pérez, D.; Camacho, V.; Beck, I.; Merino-Bohórquez, V.; Camean, M.; Madinabeitia, N.; et al. Preparation and characterization of new liposomes. Bactericidal activity of cefepime encapsulated into cationic liposomes. Pharmaceutics 2019, 11, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, I.M.S.; Bento, E.B.; Almeida, L.d.C.; de Sá, L.Z.C.M.; Lima, E.M. Preparation, Characterization and in vitro antimicrobial activity of liposomal ceftazidime and cefepime against Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2012, 43, 984–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim, M.; Sumnu, G.; Sahin, S. The effects of emulsifier type, phase ratio, and homogenization methods on stability of the double emulsion. J. Dispers. Sci. Technol. 2017, 38, 807–814. [Google Scholar] [CrossRef]
- Ramesh, D.K.V. Comparison of Oil-in-Oil, Water-in-Oil-in-Water and Melt Encapsulation Techniques for the Preparation of Controlled Release B12 Poly (ε-caprolactone) Microparticles. Trends Biomater. Artif. Organs. 2009, 23, 21–33. [Google Scholar]
- M100-S25: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 1-56230-990-4.
- Bilen Özyürek, S.; Seyis Bilkay, I.; Diken Gür, S. Antimicrobial Activity of Thyme and Rosemary Oils against Pseudomonas aeruginosa Strains. Hacettepe J. Biol. Chem. 2017, 3, 435–442. [Google Scholar] [CrossRef]
- Araby, E.; El-Tablawy, S.Y. Inhibitory effects of rosemary (Rosemarinus officinalis L.) essential oil on pathogenicity of irradiated and non-irradiated Pseudomonas aeruginosa. J. Photochem. Photobiol. B Biol. 2016, 159, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Saviuc, C.; Gheorghe, I.; Coban, S.; Drumea, V.; Chifiriuc, M.C.; Banu, O.; Bezirtzoglou, E.; Lazăr, V. Rosmarinus Officinalis essential oil and eucalyptol act as efflux pumps inhibitors and increase ciprofloxacin efficiency against Pseudomonas Aeruginosa and Acinetobacter Baumannii MDR strains. Rom. Biotechnol. Lett. 2016, 21, 11796–11804. [Google Scholar]
- Min, J.Y.; Ahn, S.I.; Lee, Y.K.; Kwak, H.S.; Chang, Y.H. Optimized conditions to produce water-in-oil-in-water nanoemulsion and spray-dried nanocapsule of red ginseng extract. Food Sci. Technol. 2018, 38, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Fazly Bazzaz, B.S.; Khameneh, B.; Namazi, N.; Iranshahi, M.; Davoodi, D.; Golmohammadzadeh, S. Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: The novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett. Appl. Microbiol. 2018, 66, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Gaysinsky, S.; Davidson, M.; McClements, J. Nanostructured Encapsulation Systems: Food Antimicrobials. Glob. Issues Food Sci. Technol. 2009, 425–479. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E.; Al Assiuty, B.A.; Moussa, E.A.; Mira, N.M. Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT-Food Sci. Technol. 2016, 69, 529–537. [Google Scholar] [CrossRef]
- Nasseri, M.; Golmohammadzadeh, S.; Arouiee, H.; Jaafari, M.R.; Neamati, H. Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition. Iran. J. Basic Med. Sci. 2016, 19, 1231–1237. [Google Scholar] [PubMed]
- Moreno-Sastre, M.; Pastor, M.; Salomon, C.J.; Esquisabel, A.; Pedraz, J.L. Pulmonary drug delivery: A review on nanocarriers for antibacterial chemotherapy. J. Antimicrob. Chemother. 2015, 70, 2945–2955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omri, A.; Ravaoarinoro, M. Preparation, properties and the effects of amikacin, netilmicin and tobramycin in free and liposomal formulations on Gram-negative and Gram-positive bacteria. Int. J. Antimicrob. Agents 1996, 7, 9–14. [Google Scholar] [CrossRef]
- Aelenei, P.; Miron, A.; Trifan, A.; Bujor, A.; Gille, E.; Aprotosoaie, A.C.; Nahar, L.; Basar, N.; Sarker, S.D. Essential Oils and Their Components as Modulators of Antibiotic Activity against Gram-Negative Bacteria. Medicines 2019, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- El-Hosseiny, L.; El-Shenawy, M.; Haroun, M.; Abdullah, F. Comparative Evaluation of the Inhibitory Effect of Some Essential Oils with Antibiotics against Pseudomonas aeruginosa. Int. J. Antibiot. 2014, 2014, 586252. [Google Scholar] [CrossRef] [Green Version]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B.; Burnhman, C.-A.; ZiImmer, B.L. M07 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1-56238-837-1. [Google Scholar]
| Component | % Area | Retention Time (min) |
|---|---|---|
| α-pinene | 16.2 | 9.07 |
| camphene | 9.8 | 9.55 |
| β-pinene | 6.0 | 10.58 |
| 1,8-cineole | 23.8 | 12.99 |
| camphor | 15.3 | 21.06 |
| borneol | 3.5 | 22.72 |
| 2-pinen-4-one | 1.6 | 25.40 |
| bornyl acetate | 4.9 | 29.55 |
| trans-caryophyllene | 2.4 | 35.62 |
| Global Composition | Oil Phase Composition | Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MLN# | W1:O (w:w) | (W1:O):W2 (w:w) | GTO (%) | GTS (%) | REO (%) | PSD | ZP (mV) | REO-EE (%) | REO-LC (mg/mL) | |
| d(3,2) (nm) | Span (-) | |||||||||
| 1 | 0.4:1.6 | 2:8 | 0 | 10 | 80 | 103 ± 4 a | 0.9 ± 0.1 a | −40 ±3 d | 51.2 ± 0.8 a | 65.3 ± 1.3 a |
| 2 | 10 | 70 | 99.5 ± 0.7 a | 0.829 ± 0.007 a | −55 ± 6 b | 44.2 ± 1.1 b | 49.3 ± 1.1 b | |||
| 3 | 20 | 60 | 101 ± 3 a | 0.8 ± 0.1 a | −42 ± 5 d | 44 ± 3 b | 42 ± 3 c | |||
| 4 | 40 | 40 | 109 ± 4 a | 0.843 ± 0.002 a | −58.1 ± 0.7 b | 43 ± 4 bc | 27 ± 2 d | |||
| 5 | 60 | 20 | 111 ± 3 a | 0.9 ± 0.1 a | −48 ± 3 c | 31 ± 7 d | 10 ± 2 f | |||
| 6 | 80 | 0 | 112 ± 3 a | 1.18 ± 0.04 a | −55 ± 4 b | - | - | |||
| 7 | 70 | 0 | 110 ± 4 a | 0.90 ± 0.10 a | −66 ± 4 a | 31.3 ± 0.6 d | 9.9 ± 0.3 f | |||
| 8 | 0.4:1.6 | 2:8 | 50 | 20 | 20 | 115.5 ± 0.7 a | 0.92 ± 0.03 a | −44 ± 2 cd | 39 ± 2 bc | 12.5 ± 0.6 f |
| 9 | 40 | 30 | 219 ± 82 b | 2.0 ± 0.4 b | −58 ± 2 b | 31 ± 6 d | 10 ± 2 f | |||
| 10 | 0.9:2.1 | 3:7 | 60 | 10 | 20 | 183 ± 92 ab | 27 ± 4 c | - | - | - |
| 11 | 1.6:2.4 | 4:6 | 60 | 10 | 20 | 107.5 ± 0.7 a | 1.194 ± 0.005 a | −43.2 ± 0.9 cd | 37 ± 2 cd | 18.3 ± 1.1 e |
| Global Composition | Oil Phase Composition | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MLN# | W1:O (w:w) | (W1:O):W2 (w:w) | GTO (%) | GTS (%) | REO (%) | PSD | FEP-EE (%) | FEP-LC (μg/mL) | |
| d(3,2) (nm) | Span (-) | ||||||||
| 1 | 0.4:1.6 | 2:8 | 0 | 10 | 80 | 110 ± 7 ab | 0.8 ± 0.2 a | 32 ± 2 c | 128 ± 8 a |
| 3 | 20 | 60 | 102 ± 5 a | 0.82 ± 0.03 a | 27 ± 8 bc | 108 ± 32 a | |||
| 6 | 80 | 0 | 111 ± 2 b | 1.62 ± 0.03 b | 22 ± 3 b | 88 ± 11 a | |||
| 11 | 1.6:2.4 | 4:6 | 60 | 10 | 20 | 125 ± 21 ab | 1.3 ± 0.4 ab | 10 ± 1 a | 160 ± 16 b |
| P. aeruginosa Target Strains | MICmedian (MICn=1/MICn=2/MICn=3) | |
|---|---|---|
| REO (mg/mL) | FEP (µg/mL) | |
| ATCC 9027 | 80.0 (80.0/40.0/80.0) | 1.0 (<0.5/4.0/1.0) |
| PS16 | 80.0 (80.0/80.0/80.0) | 4.0 (1.0/4.0/4.0) |
| PT3087 | 40.0 (40.0/80.0/40.0) | 16.0 (8.0/16.0/16.0) |
| Formulation | FEP Total Dose (µg/mL) | FEP-EE (%) | FEP-LC (µg/mL) | REO-LC (mg/mL) | REO-EE (%) |
|---|---|---|---|---|---|
| MLN 3 (REO) | - | - | - | 42.0 | 44 ± 3 |
| MLN 6 (FEP) | 80.0 | 22 ± 3 | 17.6 | - | - |
| MLN 3 (REO-FEP) | 80.0 | 27 ± 8 | 21.6 | 42.0 | 44 ± 3 |
| P. aeruginosa Target Strains | Formulation | MICmedian (MICn=1/MICn=2/MICn=3) (µL sample/mL) | Active Compound Concentration at MIC Value | |
|---|---|---|---|---|
| REOmedian (n = 1/n = 2/n = 3) (mg/mL) | FEPmedian (n = 1/n = 2/n = 3) (µg/mL) | |||
| ATCC 9027 | MLN 6 (empty) | 62.5 (500.0/31.3/62.5) | - | - |
| MLN 3 (REO) | 15.6 (15.6/62.5/15.6) | 0.6 (0.6/2.2/0.6) | - | |
| MLN 6 (FEP) | 31.3 (31.3/31.3/15.6) | - | 2.5 (2.5/2.5/1.3) | |
| MLN 3 (REO-FEP) | 62.5 (62.5/31.3/62.5) | 1.2 (4.4/1.1/2.4) | 5.0 (5.0/2.5/5.0) | |
| PS16 | MLN 6 (empty) | 125.0 (500.0/125.0/125.0) | - | - |
| MLN 3 (REO) | 31.3 (125.0/31.3/31.3) | 1.1 (4.4/1.1/1.1) | - | |
| MLN 6 (FEP) | 31.3 (31.3/31.3/31.3) | - | 2.5 (2.5/2.5/2.5) | |
| MLN 3 (REO-FEP) | 125.0 (250.0/125.0/125.0) | 4.8 (1.5/4.8/4.8) | 10.0 (20.0/10.0/10.0) | |
| PT3087 | MLN 6 (empty) | >500.0 (>500.0/>500.0/>500.0) | - | - |
| MLN 3 (REO) | 125.0 (125.0/125.0/125.0) | 4.4 (4.4/4.4/4.4) | - | |
| MLN 6 (FEP) | 250.0 (250.0/250.0/125.0) | - | 20.0 (40.0/20.0/10.0) | |
| MLN 3 (REO– FEP) | 500.0 (500.0/500.0/125.0) | 19.3 (19.3/19.3/4.8) | 40.0 (40.0/40.0/10.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Khalifa, R.; Gaspar, F.B.; Pereira, C.; Chekir-Ghedira, L.; Rodríguez-Rojo, S. Essential Oil and Hydrophilic Antibiotic Co-Encapsulation in Multiple Lipid Nanoparticles: Proof of Concept and In Vitro Activity against Pseudomonas aeruginosa. Antibiotics 2021, 10, 1300. https://doi.org/10.3390/antibiotics10111300
Ben-Khalifa R, Gaspar FB, Pereira C, Chekir-Ghedira L, Rodríguez-Rojo S. Essential Oil and Hydrophilic Antibiotic Co-Encapsulation in Multiple Lipid Nanoparticles: Proof of Concept and In Vitro Activity against Pseudomonas aeruginosa. Antibiotics. 2021; 10(11):1300. https://doi.org/10.3390/antibiotics10111300
Chicago/Turabian StyleBen-Khalifa, Rayhane, Frédéric Bustos Gaspar, Cristina Pereira, Leila Chekir-Ghedira, and Soraya Rodríguez-Rojo. 2021. "Essential Oil and Hydrophilic Antibiotic Co-Encapsulation in Multiple Lipid Nanoparticles: Proof of Concept and In Vitro Activity against Pseudomonas aeruginosa" Antibiotics 10, no. 11: 1300. https://doi.org/10.3390/antibiotics10111300
APA StyleBen-Khalifa, R., Gaspar, F. B., Pereira, C., Chekir-Ghedira, L., & Rodríguez-Rojo, S. (2021). Essential Oil and Hydrophilic Antibiotic Co-Encapsulation in Multiple Lipid Nanoparticles: Proof of Concept and In Vitro Activity against Pseudomonas aeruginosa. Antibiotics, 10(11), 1300. https://doi.org/10.3390/antibiotics10111300