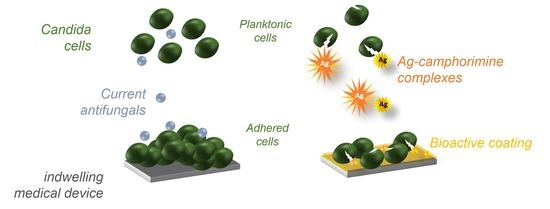
Bioactive Coatings with Ag-Camphorimine Complexes to Prevent Surface Colonization by the Pathogenic Yeast Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Camphorimine Complexes P and Q Inhibit the Growth of Azole-Susceptible and-Resistant C. albicans and C. glabrata Strains
2.2. Design of Bioactive Coatings with Silver Camphorimine Complexes
2.3. Effect of Bioactive Coating with P or Q in the Ability of C. albicans to Colonize Stainless Steel
2.4. Biocompatibility of the Coatings Functionalized with Silver Camphorimine Complexes
3. Materials and Methods
3.1. Synthesis of Silver Camphorimine Complexes: Compounds P and Q
3.2. Strains and Growth Media
3.3. Assessment of C. albicans and C. glabrata Susceptibility to Compounds P and Q
3.4. Bioactive Coating with Compounds P or Q Embedded in Polycaprolactone (PCL)
3.5. Assessment of the Bioactive Coating in the Ability of Candida to Colonize Stainless Steel
3.6. Cytotoxic Activity of the Bioactive Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Contributions of the Biofilm Matrix to Candida Pathogenesis. J. Fungi 2020, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Cuéllar-Cruz, M.; Vega-González, A.; Mendoza-Novelo, B.; López-Romero, E.; Ruiz-Baca, E.; Quintanar-Escorza, M.A.; Villagómez-Castro, J.C. The effect of biomaterials and antifungals on biofilm formation by Candida species: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2513–2527. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojic, E.M.; Darouiche, R.O. Candida Infections of Medical Devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera-González, N.; Shukla, A. Advances in Biomaterials for the Prevention and Disruption of Candida Biofilms. Front. Microbiol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peron, I.H.; Reichert-Lima, F.; Busso-Lopes, A.F.; Nagasako, C.K.; Lyra, L.; Moretti, M.L.; Schreiber, A.Z. Resistance Surveillance in Candida albicans: A Five-Year Antifungal Susceptibility Evaluation in a Brazilian University Hospital. PLoS ONE 2016, 11, e0158126. [Google Scholar] [CrossRef]
- Jung, J.; Li, L.; Yeh, C.-K.; Ren, X.; Sun, Y. Amphiphilic quaternary ammonium chitosan/sodium alginate multilayer coatings kill fungal cells and inhibit fungal biofilm on dental biomaterials. Mater. Sci. Eng. C 2019, 104, 109961. [Google Scholar] [CrossRef]
- Martins, N.; Rodrigues, C.F. Biomaterial-Related Infections. J. Clin. Med. 2020, 9, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-M.; Kim, D.-A.; Jo, J.-K.; Jun, S.-K.; Jang, T.-S.; Kim, H.-W.; Lee, J.-H.; Lee, H.-H. Ceria-Incorporated Biopolymer for Preventing Fungal Adhesion. ACS Biomater. Sci. Eng. 2021, 7, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yeh, C.-K.; Sun, Y. Salivary polypeptide/hyaluronic acid multilayer coatings act as "fungal repellents" and prevent biofilm formation on biomaterials. J. Mater. Chem. B 2018, 6, 1452–1457. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Rogachev, A.; Yarmolenko, V.; Rogachev, A.; Pyzh, A.; Jiang, X.; Yarmolenko, M. Features of electron beam deposition of polymer coatings with the prolonged release of the drug component. Mater. Sci. Eng. C 2020, 110, 110730. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species from 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [Green Version]
- Salazar, S.B.; Simões, R.S.; Pedro, N.A.; Pinheiro, M.J.; Carvalho, M.F.N.N.; Mira, N.P. An Overview on Conventional and Non-Conventional Therapeutic Approaches for the Treatment of Candidiasis and Underlying Resistance Mechanisms in Clinical Strains. J. Fungi 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.; Gupta, P.; Pruthi, V. Cinnamaldehyde incorporated gellan/PVA electrospun nanofibers for eradicating Candida biofilm. Mater. Sci. Eng. C 2021, 119, 111450. [Google Scholar] [CrossRef]
- Vaishampayan, A.; Ahmed, R.; Wagner, O.; de Jong, A.; Haag, R.; Kok, J.; Grohmann, E. Transcriptomic analysis of stress response to novel antimicrobial coatings in a clinical MRSA strain. Mater. Sci. Eng. C 2021, 119, 111578. [Google Scholar] [CrossRef]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, J.M.S.; Guerreiro, S.I.; Lourenço, A.; Alves, M.M.; Montemor, M.F.; Mira, N.P.; Leitão, J.H.; Carvalho, M.F.N.N. Ag(I) camphorimine complexes with antimicrobial activity towards clinically important bacteria and species of the Candida genus. PLoS ONE 2017, 12, e0177355. [Google Scholar] [CrossRef]
- Costa, J.P.; Pinheiro, M.J.F.; Sousa, S.A.; Rego, A.M.B.D.; Marques, F.; Oliveira, M.C.; Leitão, J.H.; Mira, N.P.; Carvalho, M.F.N.N. Antimicrobial Activity of Silver Camphorimine Complexes against Candida Strains. Antibiotics 2019, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Mondal, D.; Griffith, M.; Venkatraman, S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Guillaume, O.; Lavigne, J.-P.; Lefranc, O.; Nottelet, B.; Coudane, J.; Garric, X. New antibiotic-eluting mesh used for soft tissue reinforcement. Acta Biomater. 2011, 7, 3390–3397. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Khorasani, S.N.; Khalili, S.; Neisiany, R.E.; Ghomi, E.R.; Ejeian, F.; Das, O.; Nasr-Esfahani, M.H. Development of a Highly Proliferated Bilayer Coating on 316L Stainless Steel Implants. Polymers 2020, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Devadas, S.M.; Nayak, U.Y.; Narayan, R.; Hande, M.H.; Ballal, M. 2,5-Dimethyl-4-hydroxy-3(2H)-furanone as an Anti-biofilm Agent Against Non-Candida albicans Candida Species. Mycopathology 2019, 184, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- De Oliveira, S.M.M.; Da Silva, N.S.; Sene, A.; Gandra, R.F.; Junges, D.S.B.; Ramos, M.A.R.; Vieira, L. Comparative Study of Candida albicans Inactivation by Nonthermal Plasma on Stainless Steel with and without Diamond-like Carbon Film. ACS Omega 2019, 4, 6891–6902. [Google Scholar] [CrossRef] [Green Version]
- Tarabal, V.S.; Silva, F.G.; Sinisterra, R.D.; Gonçalves, D.; Silva, J.; Granjeiro, J.M.; Speziali, M.; Granjeiro, P.A. Impact of DMPEI on Biofilm Adhesion on Latex Urinary Catheter. Recent Pat. Biotechnol. 2021, 15, 51–66. [Google Scholar] [CrossRef]
- Alves, D.; Vaz, A.T.; Grainha, T.; Rodrigues, C.F.; Pereira, M.O. Design of an Antifungal Surface Embedding Liposomal Amphotericin B through a Mussel Adhesive-Inspired Coating Strategy. Front. Chem. 2019, 7, 431. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, K.G.U.R.; Silva, W.C.H.; Fernando, M.D.A.; Palliyaguru, L.; Jayawardena, P.S.; Shimomura, M.; Fernando, S.S.N.; Gunasekara, T.D.C.P.; Jayaweera, P.M. One-Pot Reducing Agent-Free Synthesis of Silver Nanoparticles/Nitrocellulose Composite Surface Coating with Antimicrobial and Antibiofilm Activities. BioMed Res. Int. 2021, 2021, 6666642. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J. Fibroblasts and the ground they walk on. Physiology 2013, 28, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO 10993-5; International Organization for Standardization: Geneve, Switzerland, 2009. [Google Scholar]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST)*. Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.M.; Cunha, D.V.; Santos, C.F.; Mira, N.P.; Montemor, M.F. In vitro corrosion behaviour and anti-Candida spp. activity of Zn coated with ZnO-nanostructured ‘Anastacia’ flowers. J. Mater. Chem. B 2016, 4, 4754–4761. [Google Scholar] [CrossRef]






| Strains | |
|---|---|
| C. albicans | SC5314 (reference strain) |
| 10A | |
| 22CL | |
| C. glabrata | CBS138 (reference strain) |
| FFUL29 | |
| FFUL412 | |
| FFUL443 | |
| FFUL830 | |
| FFUL866 | |
| FFUL874 | |
| 8estef | |
| 36estef | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, M.J.F.; Costa, J.P.; Marques, F.; Mira, N.P.; Carvalho, M.F.N.N.; Alves, M.M. Bioactive Coatings with Ag-Camphorimine Complexes to Prevent Surface Colonization by the Pathogenic Yeast Candida albicans. Antibiotics 2021, 10, 638. https://doi.org/10.3390/antibiotics10060638
Pinheiro MJF, Costa JP, Marques F, Mira NP, Carvalho MFNN, Alves MM. Bioactive Coatings with Ag-Camphorimine Complexes to Prevent Surface Colonization by the Pathogenic Yeast Candida albicans. Antibiotics. 2021; 10(6):638. https://doi.org/10.3390/antibiotics10060638
Chicago/Turabian StylePinheiro, M. Joana F., Joana P. Costa, Fernanda Marques, Nuno P. Mira, M. Fernanda N. N. Carvalho, and Marta M. Alves. 2021. "Bioactive Coatings with Ag-Camphorimine Complexes to Prevent Surface Colonization by the Pathogenic Yeast Candida albicans" Antibiotics 10, no. 6: 638. https://doi.org/10.3390/antibiotics10060638
APA StylePinheiro, M. J. F., Costa, J. P., Marques, F., Mira, N. P., Carvalho, M. F. N. N., & Alves, M. M. (2021). Bioactive Coatings with Ag-Camphorimine Complexes to Prevent Surface Colonization by the Pathogenic Yeast Candida albicans. Antibiotics, 10(6), 638. https://doi.org/10.3390/antibiotics10060638