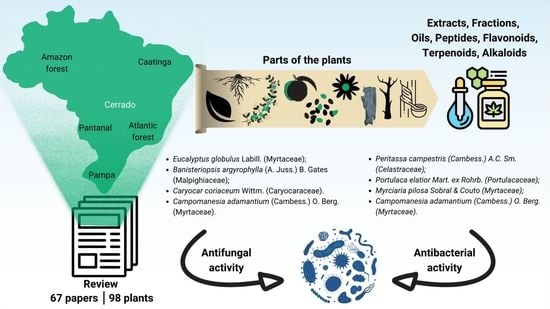
Prospecting Plant Extracts and Bioactive Molecules with Antimicrobial Activity in Brazilian Biomes: A Review
Abstract
:1. Introduction
2. Results and Discussion
2.1. Brazilian Biomes and Plants with Antimicrobial Activity
2.2. Antifungal Activity
| Strains | Citations | MIC (µg/mL) | Extract, Fraction, Bioactive Substance, and Plant |
|---|---|---|---|
| Candida albicans | 32 | 2.5 | Bioactive fractions F and J obtained from Eucalyptus globulus Labill. (Myrtaceae) stump wood methanolic extract [39]. |
| Candida tropicalis | 14 | 2.5 | Bioactive fractions A and F obtained from Eucalyptus globulus Labill. (Myrtaceae) stump wood methanolic extract [39]. |
| Candida glabrata | 7 | 2.83 | Fraction A1 (pure catechin) obtained from Banisteriopsis argyrophylla (A. Juss) B. Gates (Malpighiaceae) leaves [40]. |
| Microsporum canis | 2 | 4.1 | Crude ethanolic extract obtained from the bark and fruit pulp of Caryocar coriaceum Wittm. (Caryocaraceae) [41]. |
| Candida krusei | 10 | 7.81 | Aqueous fraction and aqueous Tannin of Campomanesia adamantium (Cambess) O. Berg. (Myrtaceae) leaves [37]. |
| Trichophyton mentagrophytes | 3 | 7.81 | Volatile oil obtained from Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) flowers [37]. |
| Sporotrichum schenckii | 3 | 10.35 | Aqueous extract of Dypsis decaryi (Jum.) Beentje & J. Dransf. (Arecaceae) seed tegument [42]. |
| Cryptococcus neoformans | 1 | 15.62 | Crude ethanolic extract and ethyl acetate fraction of Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) leaves [37]. |
| Trichophyton rubrum | 2 | 15.62 | Valoneic acid from Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) leaves [37]. |
| Malassezia spp. | 1 | 39.1 | Crude ethanolic extract of Caryocar coriaceum Wittm. (Caryocaraceae) fruit pulp and peel [41]. |
| Candida parapsilosis | 6 | 16.0 | Lectin extracted from a saline extract of Portulaca elatior Mart. ex Rohrb. (Portulacaceae) roots [37,38]. |
| Candida famata | 2 | 62.5 | Ethanolic extract of Senna rugosa (G. Don) H.S. Irwin & Barneby (Fabaceae) leaves [43]. |
| Paracoccidioides lutzii | 1 | 62.5 | Copaíba resin oil obtained from the stem of Copaifera langsdorffii Desf. (Fabaceae) [44]. |
| Paracoccidioides brasiliensis | 1 | 62.5 | Copaíba resin oil obtained from the stem of Copaifera langsdorffii Desf. (Fabaceae) [44]. |
| Paracoccidioides americana | 1 | 62.5 | Copaíba resin oil obtained from the stem of Copaifera langsdorffii Desf. (Fabaceae) [44]. |
| Paracoccidioides restrepiensis | 1 | 62.5 | Copaíba resin oil obtained from the stem of Copaifera langsdorffii Desf. (Fabaceae) [44]. |
| Saccharomyces cerevisae | 1 | 62.5 | Non-oxygenated and oxygenated sesquiterpenes fraction 1–6, obtained from the leaves of Casearia sylvestris Sw. (Salicaceae) [45]. |
| Candida guilliermondii | 1 | 500 μg/disc | Aqueous extract obtained from Eugenia dysenterica (Mart) DC. (Myrtaceae) leaves [46]. |
| Microsporum gypseum | 1 | 500 | Seed hexanic extract (soxhlet method) and seed ethanolic extract (maceration) of Artocarpus heterophyllus Lam. (Moraceae) [47]. |
| Epidermophyton floccosum | 1 | 1000 | Seed hexanic extract (soxhlet method) of Artocarpus heterophyllus Lam. (Moraceae) [47]. |
| Rhizopus sp. | 1 | 1000 | Seed hexanic extract (soxhlet method) of Artocarpus heterophyllus Lam. (Moraceae) [47]. |
| Malassezia furfur | 1 | 1800 | Essential oils of Piper augustum Rudge (Piperaceae) leaves [48]. |
| Candida dubliniensis | 1 | 2500 | Stem bark hexane extract of Guatteria blepharophylla Mart. (Annonaceae) [49]. |
| Aspergillus flavus | 1 | Indefinite MIC | Leaves ethanolic extract of de Davilla kunthii A. St. –Hil. (Dilleniaceae) caused 11.92% of growth inhibition at 250 µg/mL [50]. |
| Fusarium proliferatum | 1 | Indefinite MIC | Leaves ethanolic extract of de Davilla kunthii A. St. –Hil. (Dilleniaceae) caused 10.04% of growth inhibition at 250 µg/mL [50]. |
| Colletotrichum gloeosporioides | 4 | Indefinite MIC | Proteic fraction denominated IIFF7Ca peptide, obtained from Capsicum annuum L. (Solanaceae) immature fruit caused 73.94% of growth inhibition at 200 μg/mL [51]. |
| Fusarium solani | 4 | Indefinite MIC | Proteic fraction F5 (a peptide like Defensin) extracted from Capsicum chinense Jacq. (Solanaceae) fruits caused 44% of growth inhibition at 100 μg/mL [52]. |
| Fusarium oxysporum | 3 | Indefinite MIC | Proteic fraction (trypsin inhibitor peptide CaTI) obtained from the seeds of Capsicum annuum L. (Solanaceae) caused growth inhibition at 64 µg/mL [35]. |
| Colletotrichum lindemuthianum | 2 | Indefinite MIC | Proteic fraction (trypsin inhibitor peptide CaTI) obtained from the seeds of Capsicum annuum L. (Solanaceae) inhibited approximately 21% of growth inhibition at 64 µg/mL [53]. |
| Colletotrichum abscissum | 1 | Indefinite MIC | Endophytic fungi extracts belonging to the Diaporthe cf. heveae LGMF1631 strain cultivated with malt extract obtained from the leaves and petiole of Vochysia divergens Pohl (Vochysiaceae) and Stryphnodendron adstringens (Mart.) Coville (Fabaceae) had 72% of mycelial growth inhibition [54]. |
| Fusarium verticillioides | 1 | Indefinite MIC | Endophytic fungi extracts belonging to the Diaporthe cf. heveae LGMF1631 strain cultivated with malt extract obtained from the leaves and petiole of Vochysia divergens Pohl (Vochysiaceae) and Stryphnodendron adstringens (Mart.) Coville (Fabaceae) had 50% of mycelial growth inhibition [54]. |
| Phyllosticta citricarpa | 1 | Indefinite MIC | Endophytic fungi extracts belonging to the Diaporthe cf. heveae LGMF1631 strain cultivated with malt extract obtained from the leaves and petiole of Vochysia divergens Pohl (Vochysiaceae) and Stryphnodendron adstringens (Mart.) Coville (Fabaceae) had 88% of mycelial growth inhibition [54]. |
| Fusarium lateritium | 1 | Indefinite MIC | Proteic fraction Fa5 (antimicrobial peptide) obtained from Capsicum annuum L. (Solanaceae) fruits caused approximately 47% of growth inhibition [55]. |
| Sclerotinia sclerotiorum | 1 | Indefinite MIC | Ethanol extract from the barks of Byrsonima crassifolia (L.) Kunth (Malpighiaceae) had 37.5% of mycelial growth inhibition at 24 µg/mL [56]. |
| Aspergillus fumigatus | 2 | Inactive | Seed hexanic and ethanolic extracts of Artocarpus heterophyllus Lam. (Moraceae) at 1000 µg/mL [47]; Leaves ethanolic extract of Peumus boldus Molina (Monimiaceae); leaves hydro-alcoholic extracts of Psidium guajava L. (Myrtaceae) and Vernonia polysphaera Baker (Asteraceae) at 500 mg/mL; dry leaves methanolic extract of Persea americana Mill. (Lauraceae) and raw sap of Jatropha multifida L. (Euphorbiaceae) at 1000 µg/mL [57]; Methanolic extract of Simaba ferruginea A.St-Hil. (Simaroubaceae) rhizome [58]. |
| Aspergillus niger | 1 | Inactive | Leaves ethanolic extract of Senna rugosa (G. Don) H.S. Irwin & Barneby (Fabaceae) at 1000 µg/mL [43]; Methanolic extract of Simaba ferruginea A.St-Hil. (Simaroubaceae) rhizome [58]. |
| Aspergillus parasiticus | 1 | Inactive | Methanolic extract of Simaba ferruginea A.St-Hil. (Simaroubaceae) rhizome [58]. |
| Aspergillus terreus | 1 | Inactive | Methanolic extract of Simaba ferruginea A.St-Hil. (Simaroubaceae) rhizome [58]. |
| Penicillium expansum | 1 | Inactive | Leaves ethanolic extract of Senna rugosa (G. Don) H.S. Irwin & Barneby (Fabaceae) at 1000 µg/mL [43]. |
| Ceratocystis cacaofunesta | 1 | Inactive | Leaves aqueous and ethanolic solutions of Adiantum latifolium Lam. (Pteridaceae) [59]. |
2.3. Antibacterial Activity
| Strains | Citations | MIC (µg/mL) | Extract, Fraction, Bioactive Substance, and Plant |
|---|---|---|---|
| Bacillus megaterium | 1 | 0.78 | Maytenin and maytenol isolated from the dichloromethane extract of Peritassa campestris (Cambess.) A.C. Sm. (Celastraceae) roots [66]. |
| Pseudomonas aeruginosa | 33 | 4.06 | Lectin extracted from a saline extract of Portulaca elatior Mart. ex Rohrb. (Portulacaceae) roots [38]. |
| Staphylococcus aureus | 54 | 5.0 | Essential oils of Myrciaria pilosa Sobral & Couto (Myrtaceae) leaves [67]. |
| Staphylococcus aureus 679 * | 1 | 5.0 | Essential oils of Myrciaria pilosa Sobral & Couto (Myrtaceae) leaves [67]. |
| Staphylococcus aureus 683 ** | 1 | 5.0 | Essential oils of Myrciaria pilosa Sobral & Couto (Myrtaceae) leaves [67]. |
| Staphylococcus sp. 841 *** | 1 | 7.80 | Essential oils of Chromolaena squalida (DC.) RM King & H. Rob (Asteraceae) leaves [15]. |
| Enterococcus faecalis | 6 | 8.12 | Lectin extracted from saline extract of Portulaca elatior Mart. ex Rohrb. (Portulacaceae) roots [38]. |
| Bacillus thuringiensis | 1 | 9.08 | Crude extract from the roots of Passiflora alata Curtis (Passifloraceae) [68]. |
| Staphyloccocus epidermidis | 12 | 16 | Ethanolic extract and fractions from dichloromethane of Rosmarinus officinalis L. (Lamiaceae) leaves [69]. |
| Streptococcus sobrinus | 4 | 20 | Essential oils of Nectandra megapotamica (Spreng.) Mez (Lauraceae) leaves [70] |
| Escherichia coli | 49 | 25 | Methanolic extract of Simaba ferruginea A.St.-Hil. (Simaroubaceae) rhizome [58]. |
| Streptococcus pyogene | 2 | 28.98 | Crude extract from the roots of Passiflora alata Curtis (Passifloraceae) [68]. |
| Listeria monocytogenes | 6 | 31.25 | Volatile oile from the leaves of Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) [37]. |
| Acinetobacter baumannii | 2 | 31.25 | Isolated tannic acid-enriched fraction from the roots of Cochlospermum regium (Mart. Et. Schr.) Pilger (Bixaceae) [71]. |
| Listeria innocua | 1 | 31.25 | Dichloromethane fraction of Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) leaves [37]. |
| Bacteroides fragilis | 1 | 31.25 | Essential oils of Nectandra megapotamica (Spreng.) Mez (Lauraceae) leaves [70]. |
| Bacillus cereus | 6 | 32 | Ethanolic extract and fractions from dichloromethane of Rosmarinus officinalis L. (Lamiaceae) leaves [69]. |
| Streptococcus mutans | 10 | 50 | Essential oils of Eugenia klotzschiana O.Berg (Myrtaceae) leaves in natura, dry leaves, and flowers [72]; Essential oils of Nectandra megapotamica (Spreng.) Mez (Lauraceae) leaves [70]. |
| Prevotella nigrescens | 1 | 50 | Essential oils of Eugenia klotzschiana O.Berg (Myrtaceae) leaves in natura, dry leaves, and flowers [72]; Essential oils of Nectandra megapotamica (Spreng.) Mez (Lauraceae) leaves [70]. |
| Enterobacter cloacae | 2 | 62.5 | Hexane fraction of Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) leaves [37]. |
| Bacillus subtilis | 2 | 62.5 | Essential oils from Croton heliotropiifolius Kunth (Euphorbiaceae) aerial parts (leaves and stems) [73]. |
| Klebsiella pneumoniae | 9 | 80 | Bioactive fraction I of the methanolic extract from the stump wood of Eucalyptus globulus Labill. (Myrtaceae) [39]. |
| Porphyromonas gingivalis | 1 | 100 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Peptostreptococcus micros (Clinical isolate) | 1 | 100 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Micrococcus luteus | 3 | 125 | Hexane fraction of Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) leaves [37]. |
| Micrococcus roseus | 1 | 125 | Hexane fraction of Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) leaves [37]. |
| Streptococcus agalactiae | 2 | 125 | Essential oil of Mentha piperita L. (Lamiaceae) leaves [75]. |
| Methicillin-resistant Staphylococcus aureus | 3 | 156 | Bioactive fraction I obtained from the methanolic extract of Eucalyptus globulus Labill. (Myrtaceae) stump wood [39]. |
| Staphylococcus haemolyticus | 1 | 170 | Ethanolic extract of Schinopsis brasiliensis Engl. (Anacardiaceae) leaves [76]. |
| Streptococcus salivarius | 2 | 200 | Essential oils of Eugenia klotzschiana O.Berg (Myrtaceae) leaves in natura, dry leaves, and flowers [72]. |
| Streptococcus salivarius (Clinical isolate) | 1 | 200 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Shigella flexneri | 3 | 200 | Methanolic extract of Simaba ferruginea A.St.-Hil. (Simaroubaceae) rhizome [58]. |
| Enterobacter aerogenes | 1 | 250 | Dichloromethane fraction of Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) leaves [37]. |
| Staphylococcus sp. 873 **** | 1 | 250 | Essential oils from the leaves of Chromolaena squalida (DC.) RM King & H. Rob (Asteraceae); Campomanesia sessiliflora (O. Berg) Mattos (Myrtaceae); Ocotea minarum (Nees & Mart.) Mez (Lauraceae), and Endlicheria paniculata (Spreng.) JF Macbr. (Lauraceae) [15]. |
| Salmonella sp. ATCC 6017 | 2 | 200–400 | Essential oil GB2a of Ocimum basilicum L. aerial parts (Lamiaceae), known as Green Brazil [77]. |
| Salmonella enterica | 3 | 390 | Crude ethanolic extract from the Schinopsis brasiliensis Engl. (Anacardiaceae) barks [78]. |
| Streptococcus sanguinis | 3 | 400 | Essential oils of Eugenia klotzschiana O.Berg (Myrtaceae) leaves in natura, dry leaves, and flowers [72]. |
| Streptococcus sanguinis (Clinical isolate) | 1 | 200 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Actinomyces naeslundii (Clinical isolate) | 1 | 400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Fusobacterium nucleatum | 1 | 400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Streptococcus mitis | 1 | >400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Lactobacillus casei | 1 | >400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Prevotella intermedia | 1 | >400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Actinomyces viscosus (Clinical isolate) | 1 | >400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Prevotella buccae (Clinical isolate) | 1 | >400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Porphyromonas gingivalis (Clinical isolate) | 1 | >400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Actinomyces naeslundii | 1 | >400 | Hydroalcoholic extract of Copaifera trapezifolia Hayne (Fabaceae) leaves [74]. |
| Listeria grayi | 1 | 450 | Essential oils of Hedychium coronarium J.Koenig (Zingiberaceae) leaves [48]. |
| Salmonella typhi 905 ***** | 1 | 500 | Essential oils of Campomanesia sessiliflora (O. Berg) Mattos (Myrtaceae) leaves [15]. |
| Kocuria rhizophila | 1 | >500 | Galangin-3-methyl ether isolated from the aerial parts of Lychnophora markgravii G.M. Barroso (Asteraceae) [79]. |
| Providencia stuartii | 1 | 750 | DdeL—A new thermostable lecitin extracted from the seeds of Dypsis decaryi (Jum.) Beentje & J. Dransf. (Arecaceae) [42]. |
| Enterococcus hirae | 1 | 900 | Hydro-ethanolic extract of Myrcia fallax (Rich.) DC. (Myrtaceae) leaves [80]. |
| Klebsiella oxytoca | 2 | 900 | Essential oils of Hedychium coronarium J.Koenig (Zingiberaceae) leaves [48]. |
| Proteus vulgaris | 1 | 900 | Essential oils of Hedychium coronarium J.Koenig (Zingiberaceae) leaves [48]. |
| Rhodococcus equi | 1 | 1000 | Hydro-ethanolic extract of Myrcia guianensis (Aubl.) DC. (Myrtaceae) leaves [80]. |
| Enterococcus faecalis clinical isolate | 4 | 1500 | DdeL—A new thermostable lecitin extracted from the seeds of Dypsis decaryi (Jum.) Beentje & J. Dransf. (Arecaceae) [42]. |
| Extended spectrum beta-lactamase-producing (ESBL) Enterobacteriaceae (Clinical isolate) | 1 | 1500 | DdeL—A new thermostable lecitin extracted from the seeds of Dypsis decaryi (Jum.) Beentje & J. Dransf. (Arecaceae) [42]. |
| Morganella morganii | 1 | 20,000 | Hydro-ethanolic extract of Pereskia aculeata Mill. (Cactaceae) leaves [65]. |
| Proteus mirabilis | 2 | >20,000 | Hydro-ethanolic extract of Pereskia aculeata Mill. (Cactaceae) leaves [65]. |
| Salmonella typhimurium | 5 | 25,000 | Ethyl acetate extract of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves [81]. |
| Salmonella enteritidis | 3 | 25,000 | Alcoholic and ethyl acetate extracts of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves [81]. |
| Salmonella heidelberg | 1 | 25,000 | Alcoholic and ethyl acetate extracts of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves [81]. |
| Salmonella infantis | 1 | 25,000 | Alcoholic extract of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves [81]. |
| Salmonella ohio | 1 | 25,000 | Alcoholic extract of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves [81]. |
| Salmonella naintpaul | 1 | 25,000 | Alcoholic extract of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves [81]. |
| Salmonella agona | 1 | 50,000 | Aqueous, alcoholic, and ethyl acetate extracts of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves; Aqueous and ethyl acetate extracts of Ocotea diospyrifolia (Meisn.) Mez (Lauraceae) leaves [81]. |
| Salmonella mbandaka | 1 | 50,000 | Aqueous, alcoholic, and ethyl acetate extracts of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves; Aqueous and ethyl acetate extracts of Ocotea diospyrifolia (Meisn.) Mez (Lauraceae) leaves [81]. |
| Salmonella gallinarum | 1 | 50,000 | Alcoholic and ethyl acetate extracts of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves; ethyl acetate extract of Ocotea diospyrifolia (Meisn.) Mez (Lauraceae) leaves [81]. |
| Salmonella give | 1 | 50,000 | Alcoholic and ethyl acetate extracts of Ocotea silvestris Vattimo-Gil (Lauraceae) leaves; ethyl acetate extract of Ocotea diospyrifolia (Meisn.) Mez (Lauraceae) leaves [81]. |
| Serratia marcescens | 1 | 62,500 | Crude ethanolic extract from the Schinopsis brasiliensis Engl. (Anacardiaceae) barks [78]. |
| Staphylococcus sanguinis | 2 | Indeterminate MIC | Ethanolic extract of Davilla kunthii A. St. –Hil. (Dilleniaceae) leaves at 9.375 µg/mL caused 21,198% of growth inhibition [50]. |
| Stenotrophomonas maltophilia | 1 | Indeterminate MIC | Endophytic fungi extracts belonging to the Diaporthe cf. heveae LGMF1631strain cultivated with malt extract obtained from the leaves and petiole of Vochysia divergens Pohl (Vochysiaceae) and Stryphnodendron adstringens (Mart.) Coville (Fabaceae) showed an inhibition halo of 16 mm [54]. |
| Aeromonas caviae | 1 | Inactive | Crude extract from the leaves and roots of Passiflora alata Curtis (Passifloraceae); Passiflora foetida L. (Passifloraceae); Passiflora pohlii Mast. (Passifloraceae), and Passiflora suberosa L. (Passifloraceae) at 1000 µg/mL [68]. |
| Aeromonas hydrophila | 1 | Inactive | Crude extract from the leaves and roots of Passiflora alata Curtis (Passifloraceae); Passiflora foetida L. (Passifloraceae); Passiflora pohlii Mast. (Passifloraceae), and Passiflora suberosa L. (Passifloraceae) at 1000 µg/mL [68]. |
| Citrobacter freundii | 1 | Inactive | Passiflora alata Curtis (Passifloraceae); Passiflora foetida L. (Passifloraceae); Passiflora pohlii Mast. (Passifloraceae), and Passiflora suberosa L. (Passifloraceae) at 1000 µg/mL [68]. |
| Shigella sonnei | 1 | Inactive | Crude extract from the leaves and roots of Passiflora alata Curtis (Passifloraceae); Passiflora foetida L. (Passifloraceae); Passiflora pohlii Mast. (Passifloraceae), and Passiflora suberosa L. (Passifloraceae) at 1000 µg/mL [68]. |
| Staphylococcus saprophyticus | 1 | Inactive | Crude extract from the leaves and roots of Passiflora alata Curtis (Passifloraceae); Passiflora foetida L. (Passifloraceae); Passiflora pohlii Mast. (Passifloraceae), and Passiflora suberosa L. (Passifloraceae) at 1000 µg/mL [68]. |
| Helicobacter pylori | 1 | Inactive | Hydroethanolic extract of Piper umbellatum L.(Piperaceae) leaves [82]. |
2.4. Bioactive Molecules and Mechanisms of Action
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Antibiotic Resistentece. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 30 April 2021).
- de Souza, J.L.S.; da Silva Guimarães, V.B.; Campos, A.D.; Lund, R.G. Antimicrobial Potential of Pyroligneous Extracts–a Systematic Review and Technological Prospecting. Braz. J. Microbiol. 2018, 49, 128–139. [Google Scholar] [CrossRef] [PubMed]
- da Costa Monteiro, P.E.A. A bioeconomy-driven special economic zone in the Amazon region. In Handbook of Research on Special Economic Zones as Regional Development Enablers; IGI Global: Hershey, PA, USA, 2022; pp. 393–413. [Google Scholar]
- Brasil; Renisus. Relação Nacional de Plantas Medicinais de Interesse ao Sistema Único de Saúde. Available online: http://portalarquivos.saude.gov.br/images/pdf/2017/junho/06/renisus.pdf (accessed on 30 August 2021).
- Qadri, H.; Shah, A.H.; Andrabi, S.M.; Alshehri, B.; Almilaibary, A.; Mir, M.A. Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 103376. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Reis, L.K.; Borges, F.L.G.; Ojeda, P.T.A.; Pineda, D.A.M.; Miranda, C.O.; de Lima Maidana, D.P.F.; dos Santos, T.M.R.; Shibuya, P.S.; Marques, M.C.M.; et al. Ecological restoration in brazilian biomes: Identifying advances and gaps. For. Ecol. Manag. 2020, 458, 117802. [Google Scholar] [CrossRef]
- IBGE; Coordenação de Recursos Naturais e Estudos Ambientais. Biomas e Sistema Costeiro-Marinho do Brasil: Compatível com a Escala 1:250,000; IBGE: Rio de Janeiro, Brazil, 2019; Volume 45, ISBN 978-85-240-4510-3. [Google Scholar]
- BFG (The Brazil Flora Group). Flora do Brasil 2020. Available online: http://dspace.jbrj.gov.br/jspui/bitstream/doc/118/5/Flora%202020%20digital.pdf (accessed on 29 December 2021).
- Maracahipes-Santos, L.; Lenza, E.; Santos, J.O.; Mews, H.A.; Oliveira, B. Effects of soil and space on the woody species composition and vegetation structure of three Cerrado phytophysiognomies in the Cerrado-Amazon transition. Braz. J. Biol. 2017, 77, 830–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novaes, P.; Molinillo, J.M.G.; Varela, R.M.; Macías, F.A. Ecological phytochemistry of Cerrado (Brazilian savanna) plants. Phytochem. Rev. 2013, 12, 839–855. [Google Scholar] [CrossRef] [Green Version]
- Forzza, R.C.; Baumgratz, J.F.A.; Bicudo, C.E.M.; Canhos, D.A.; Carvalho Jr, A.A.; Coelho, M.A.N.; Costa, A.F.; Costa, D.P.; Hopkins, M.G.; Leitman, P.M. New brazilian floristic list highlights conservation challenges. BioScience 2012, 62, 39–45. [Google Scholar] [CrossRef]
- Marchese, C. Biodiversity hotspots: A shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015, 3, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Frederico, S. From subsistence to financial asset: The appropriation of the Brazilian Cerrado lands as a resource. Rev. NERA 2019, 239–260. [Google Scholar] [CrossRef]
- Fetzer, D.L.; Cruz, P.N.; Hamerski, F.; Corazza, M.L. Extraction of baru (Dipteryx alata vogel) seed oil using compressed solvents technology. J. Supercrit. Fluids 2018, 137, 23–33. [Google Scholar] [CrossRef]
- Jesus, G.S.; Micheletti, A.C.; Padilha, R.G.; de Souza Paula, J.; Alves, F.M.; Leal, C.R.B.; Garcez, F.R.; Garcez, W.S.; Yoshida, N.C. Antimicrobial Potential of Essential Oils from Cerrado Plants against Multidrug−Resistant Foodborne Microorganisms. Molecules 2020, 25, 3296. [Google Scholar] [CrossRef]
- Dick, M.; Abreu da Silva, M.; Franklin da Silva, R.R.; Lauz Ferreira, O.G.; de Souza Maia, M.; Ferreira de Lima, S.; Borges de Paiva Neto, V.; Dewes, H. Environmental impacts of Brazilian beef cattle production in the Amazon, Cerrado, Pampa, and Pantanal biomes. J. Clean. Prod. 2021, 311, 127750. [Google Scholar] [CrossRef]
- Scarano, F.R.; Ceotto, P. Brazilian Atlantic forest: Impact, vulnerability, and adaptation to climate change. Biodivers. Conserv. 2015, 24, 2319–2331. [Google Scholar] [CrossRef]
- Marques, M.C.M.; Trindade, W.; Bohn, A.; Grelle, C.E.V. The Atlantic Forest: An Introduction to the Megadiverse Forest of South America. In The Atlantic Forest; Marques, M.C.M., Grelle, C.E.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–23. ISBN 978-3-030-55321-0. [Google Scholar]
- Wilson, O.J.; Mayle, F.E.; Walters, R.J.; Lingner, D.V.; Vibrans, A.C. Floristic change in Brazil’s southern Atlantic Forest biodiversity hotspot: From the last glacial maximum to the late 21st century. Quat. Sci. Rev. 2021, 264, 107005. [Google Scholar] [CrossRef]
- Ricardo, S.D.F.; Coe, H.H.G.; Dias, R.R.; de Sousa, L.d.O.F.; Gomes, E. Reference collection of plant phytoliths from the Caatinga biome, Northeast Brazil. Flora 2018, 249, 1–8. [Google Scholar] [CrossRef]
- Moro, M.F.; Nic Lughadha, E.; de Araújo, F.S.; Martins, F.R. A Phytogeographical Metaanalysis of the Semiarid Caatinga Domain in Brazil. Bot. Rev. 2016, 82, 91–148. [Google Scholar] [CrossRef]
- Colman, C.; Oliveira, P.; Almagro, A.; Soares-Filho, B.; Rodrigues, D. Effects of Climate and Land-Cover Changes on Soil Erosion in Brazilian Pantanal. Sustainability 2019, 11, 7053. [Google Scholar] [CrossRef] [Green Version]
- Pessoa, J.B.; Pessoa, L.B.; de Almeida, F.O.; Moreno, K.G.T.; de Almeida, D.A.T.; Garcia, A.A.K.; Lourenço, E.L.B.; Gasparotto Junior, A. Ethnobotanical Practices Among Riverine People in the Brazilian Pantanal. SSRN J. 2022. [Google Scholar] [CrossRef]
- Farias, D.P.; Neri-Numa, I.A.; de Araújo, F.F.; Pastore, G.M. A critical review of some fruit trees from the Myrtaceae family as promising sources for food applications with functional claims. Food Chem. 2020, 306, 125630. [Google Scholar] [CrossRef]
- Asfaw, M.M.; Abebe, F.B. Traditional Medicinal Plant Species Belonging to Fabaceae Family in Ethiopia: A Systematic Review. IJPB Int. J. Plant Biol. 2022, 12, 8473. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Niciforovic, N.; Topuzovic, M.; Solujic, S. Total Phenolic Content, Flavonoid Concentrations and Antioxidant Activity, of The Whole Plant and Plant Parts Extracts from Teucrium montanum L. var. montanum, f. supinum (L.) Reichenb. Biotechnol. Biotechnol. Equip. 2011, 25, 2222–2227. [Google Scholar] [CrossRef] [Green Version]
- Liporacci, H.; Simão, D. Ethnobotanical survey of medicinal plants from home gardens of Bairro Novo Horizonte, Ituiutaba, MG. Rev. Bras. Plantas Med. 2013, 15, 529–540. [Google Scholar] [CrossRef] [Green Version]
- National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ethanol (accessed on 23 January 2023).
- Maleš, I.; Pedisić, S.; Zorić, Z.; Elez-Garofulić, I.; Repajić, M.; You, L.; Vladimir-Knežević, S.; Butorac, D.; Dragović-Uzelac, V. The medicinal and aromatic plants as ingredients in functional beverage production. J. Funct. Foods 2022, 96, 105210. [Google Scholar] [CrossRef]
- Okwu, M.U.; Olley, M.; Akpoka, A.O.; Izevbuwa, O.E. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol. 2019, 5, 117–137. [Google Scholar] [CrossRef]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-F.; Yang, Y.-L.; Yao, T.-J.; Lin, C.-Y.; Liu, J.-S.; Tang, R.-B.; Yu, K.-W.; Fan, Y.-H.; Hsieh, K.-S.; Ho, M. Risk factors for fatal candidemia caused by Candida albicans and non-albicans Candida species. BMC Infect. Dis. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Al-Hatmi, A.; de Hoog, G.S.; Meis, J.F. Multiresistant Fusarium Pathogens on Plants and Humans: Solutions in (from) the Antifungal Pipeline? IDR Infect. Drug Resist. 2019, 12, 3727–3737. [Google Scholar] [CrossRef] [Green Version]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [Green Version]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memórias Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [Green Version]
- Sá, S.; Chaul, L.T.; Alves, V.F.; Fiuza, T.S.; Tresvenzol, L.M.; Vaz, B.G.; Ferri, P.H.; Borges, L.L.; Paula, J.R. Phytochemistry and antimicrobial activity of Campomanesia adamantium. Rev. Bras. Farmacogn. 2018, 28, 303–311. [Google Scholar] [CrossRef]
- Silva, J.D.F.; Silva, S.P.; Silva, P.M.; Vieira, A.M.; de Araújo, L.C.C.; de Albuquerque Lima, T.; de Oliveira, A.P.S.; do Nascimento Carvalho, L.V.; da Rocha Pitta, M.G.; de Melo Rêgo, M.J.B.; et al. Portulaca elatior root contains a trehalose-binding lectin with antibacterial and antifungal activities. Int. J. Biol. Macromol. 2019, 126, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Neiva, D.M.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Bioassay-guided fractionation, GC–MS identification and in vitro evaluation of antioxidant and antimicrobial activities of bioactive compounds from Eucalyptus globulus stump wood methanolic extract. Ind. Crops Prod. 2016, 91, 97–103. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Silva, T.F.; Martins, M.M.; de Morais, S.A.; Chang, R.; de Aquino, F.J.; da Silva, C.V.; Teixeira, T.L.; Martins, C.H.; Moraes, T.S. Antifungal and cytotoxicity activities of Banisteriopsis argyrophylla leaves. J. Pharm. Pharmacol. 2018, 70, 1541–1552. [Google Scholar] [CrossRef]
- Alves, D.R.; Maia de Morais, S.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Vasconcelos, F.R.; da Silva, I.N.G.; Araujo de Sousa, H.; Assolini, J.P.; Conchon-Costa, I.; Pavanelli, W.R. Flavonoid Composition and Biological Activities of Ethanol Extracts of Caryocar coriaceum Wittm., a Native Plant from Caatinga Biome. Evid.-Based Complement. Altern. Med. 2017, 2017, 6834218. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.É.L.M.; Brandão-Costa, R.M.P.; de Oliveira Santos, J.V.; Cavalcanti, I.M.F.; da Silva, M.M.; Nascimento, T.P.; de Oliveira Nascimento, C.; Porto, A.L.F. DdeL, a novel thermostable lectin from Dypsis decaryi seeds: Biological properties. Process Biochem. 2019, 86, 169–176. [Google Scholar] [CrossRef]
- Cunha, L.F.; Costa, C.M.; Barroso, P.R.; Kato, K.C.; de Oliveira, F.; Mendonça Filho, C.V.; Grael, C.F.F.; Gregório, L.E.; Campos, F.F.; de Oliveira, P.M. Phytochemical screening and biological assays of ethanolic leaf extract of Senna rugosa. Rodriguésia 2020, 71. [Google Scholar] [CrossRef]
- Do Carmo Silva, L.; Miranda, M.A.C.M.; de Freitas, J.V.; Ferreira, S.F.A.; de Oliveira Lima, E.C.; de Oliveira, C.M.A.; Kato, L.; Terezan, A.P.; Rodriguez, A.F.R.; Faria, F.S.E.D.V.; et al. Antifungal activity of Copaíba resin oil in solution and nanoemulsion against Paracoccidioides spp. Braz. J. Microbiol. 2020, 51, 125–134. [Google Scholar] [CrossRef]
- Pereira, F.G.; Marquete, R.; Domingos, L.T.; Rocha, M.E.; Ferreira-Pereira, A.; Mansur, E.; Moreira, D.L. Antifungal activities of the essential oil and its fractions rich in sesquiterpenes from leaves of Casearia sylvestris Sw. An. Acad. Bras. Ciências 2017, 89, 2817–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, A.F.; Silveira, D.; Fonseca-Bazzo, Y.M.; Magalhães, P.O.; Fagg, C.W.; da Silva, E.C.; Gomes, S.M.; Gandolfi, L.; Pratesi, R.; de Medeiros Nóbrega, Y.K. Activity of crude extracts from Brazilian cerrado plants against clinically relevant Candida species. BMC Complement. Altern. Med. 2016, 16, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tramontin, D.P.; Cadena-Carrera, S.E.; Bella-Cruz, A.; Cruz, C.C.B.; Bolzan, A.; Quadri, M.B. Biological activity and chemical profile of Brazilian jackfruit seed extracts obtained by supercritical CO2 and low pressure techniques. J. Supercrit. Fluids 2019, 152, 104551. [Google Scholar] [CrossRef]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef] [Green Version]
- Andreazza, N.L.; de Lourenco, C.C.; Hernandez-Tasco, A.J.; Pinheiro, M.L.B.; Stefanello, M.É.A.; Costa, E.V.; Salvador, M.J. Antimicrobial photodynamic effect of extracts and oxoaporphine alkaloid isomoschatoline from Guatteria blepharophylla. J. Photochem. Photobiol. B Biol. 2016, 160, 154–162. [Google Scholar] [CrossRef]
- Nascimento, L.; Rabelo, S.; Silva, G.; Nascimento, F.; Santos, R. Biological activity of Davilla kunthii A. St. –Hil. (Dilleniaceae). Rev. Bras. Plantas Med. 2016, 18, 172–179. [Google Scholar] [CrossRef]
- Maracahipes, Á.C.; Taveira, G.B.; Sousa-Machado, L.Y.; Machado, O.L.T.; Rodrigues, R.; Carvalho, A.O.; Gomes, V.M. Characterization and antifungal activity of a plant peptide expressed in the interaction between Capsicum annuum fruits and the anthracnose fungus. Biosci. Rep. 2019, 39, BSR20192803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, L.d.A.; Taveira, G.B.; Da Silva, M.S.; da Silva Gebara, R.; da Silva Pereira, L.; Perales, J.; Teixeira-Ferreira, A.; de Oliveira Mello, É.; de Oliveira Carvalho, A.; Rodrigues, R. Antimicrobial peptides from Capsicum chinense fruits: Agronomic alternatives against phytopathogenic fungi. Biosci. Rep. 2020, 40, BSR20200950. [Google Scholar] [CrossRef]
- Silva, M.S.; Ribeiro, S.F.; Taveira, G.B.; Rodrigues, R.; Fernandes, K.V.; Carvalho, A.O.; Vasconcelos, I.M.; Mello, E.O.; Gomes, V.M. Application and bioactive properties of CaTI, a trypsin inhibitor from Capsicum annuum seeds: Membrane permeabilization, oxidative stress and intracellular target in phytopathogenic fungi cells. J. Sci. Food Agric. 2017, 97, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Noriler, S.A.; Savi, D.C.; Aluizio, R.; Palacio-Cortes, A.M.; Possiede, Y.M.; Glienke, C. Bioprospecting and Structure of Fungal Endophyte Communities Found in the Brazilian Biomes, Pantanal, and Cerrado. Front. Microbiol. 2018, 9, 1526. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, L.d.A.; Taveira, G.B.; Ribeiro, S.d.F.F.; da Silva Pereira, L.; Carvalho, A.d.O.; Rodrigues, R.; Oliveira, A.E.A.; Machado, O.L.T.; da Silva Araújo, J.; Vasconcelos, I.M. Purification and characterization of peptides from Capsicum annuum fruits which are α-amylase inhibitors and exhibit high antimicrobial activity against fungi of agronomic importance. Protein Expr. Purif. 2017, 132, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.S.; Matias, R.; Corrêa, B.O.; Oliveira, A.K.M.; Guidolin, D.G.F.; Roel, A.R. Phytochemistry, Antioxidant potential and antifungal of Byrsonima crassifolia on soil phytopathogen control. Braz. J. Biol. 2017, 78, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Da Cruz, J.E.R.; Da Costa Guerra, J.F.; De Souza Gomes, M.; Freitas, G.R.O.e.; Morais, E.R. Phytochemical Analysis and Evaluation of Antimicrobial Activity of Peumus boldus, Psidium guajava, Vernonia polysphaera, Persea americana, Eucalyptus citriodora Leaf Extracts and Jatropha multifida Raw Sap. CPB Curr. Pharm. Biotechnol. 2019, 20, 433–444. [Google Scholar] [CrossRef]
- Gazoni, V.F.; Balogun, S.O.; Arunachalam, K.; Oliveira, D.M.; Filho, V.C.; Lima, S.R.; Colodel, E.M.; Soares, I.M.; Ascêncio, S.D.; Martins, D.T.d.O. Assessment of toxicity and differential antimicrobial activity of methanol extract of rhizome of Simaba ferruginea A. St.-Hil. and its isolate canthin-6-one. J. Ethnopharmacol. 2018, 223, 122–134. [Google Scholar] [CrossRef]
- Cruz, M.B.; Sousa, D.F.; Almeida Oliveira, L.; França, J.P.; França, L.P.; Conceição, A.O.; Fonseca Chaves, A.L. Interference of aqueous and ethanolic solutions of Adiantum latifolium Lam. (Pteridaceae) leaves on in vitro Ceratocystis cacaofunesta mycelial growth. Arq. Inst. Biol. 2019, 86, e0192019. [Google Scholar] [CrossRef]
- De Oliveira, M.L.M.; Nunes-Pinheiro, D.C.S.; Tomé, A.R.; Mota, É.F.; Lima-Verde, I.A.; Pinheiro, F.G.d.M.; Campello, C.C.; De Morais, S.M. In vivo topical anti-inflammatory and wound healing activities of the fixed oil of Caryocar coriaceum Wittm. seeds. J. Ethnopharmacol. 2010, 129, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.d.M.; de Morais, S.A.; Martins, M.M.; Cunha, L.; da Silva, C.V.; Martins, C.H.; Leandro, L.F.; de Oliveira, A.; de Aquino, F.J.; do Nascimento, E.A. Chemical composition, antifungal, and cytotoxicity activities of Inga laurina (Sw.) Willd leaves. Sci. World J. 2019, 2019, 9423658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef]
- Sultan, I.; Rahman, S.; Jan, A.T.; Siddiqui, M.T.; Mondal, A.H.; Haq, Q.M.R. Antibiotics, Resistome and Resistance Mechanisms: A Bacterial Perspective. Front. Microbiol. 2018, 9, 2066. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.-I.; Apostolidis, E.; Labbe, R.G.; Shetty, K. Inhibition of Staphylococcus aureus by Phenolic Phytochemicals of Selected Clonal Herbs Species of Lamiaceae Family and Likely Mode of Action through Proline Oxidation. Food Biotechnol. 2007, 21, 71–89. [Google Scholar] [CrossRef]
- Garcia, J.A.A.; Corrêa, R.C.G.; Barros, L.; Pereira, C.; Abreu, R.M.V.; Alves, M.J.; Calhelha, R.C.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Phytochemical profile and biological activities of “Ora-pro-nobis” leaves (Pereskia aculeata Miller), an underexploited superfood from the Brazilian Atlantic Forest. Food Chem. 2019, 294, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Inácio, M.C.; Paz, T.A.; Pereira, A.M.S.; Furlan, M. Maytenin Plays a Special Role in the Regulation of the Endophytic Bacillus megaterium in Peritassa campestris Adventitious Roots. J. Chem. Ecol. 2019, 45, 789–797. [Google Scholar] [CrossRef]
- Costa, W.K.; Gomes, N.O.d.C.; Souza dos Santos, B.; Bezerra Filho, C.M.; de Oliveira, A.M.; da Silva, G.C.; de Veras, B.O.; Oliveira, F.G.d.S.; Aguiar, J.C.R.d.O.F.d.; Navarro, D.M.d.A.F.; et al. First report on the chemical composition of leaf essential oil of Myrciaria pilosa Sobral & Couto and its antimicrobial and antivirulence activities against Staphylococcus aureus. Nat. Prod. Res. 2020, 36, 2429–2433. [Google Scholar] [CrossRef]
- Simão, M.J.; Barboza, T.J.S.; Vianna, M.G.; Garcia, R.; Mansur, E.; Ignacio, A.C.P.R.; Pacheco, G. A comparative study of phytoconstituents and antibacterial activity of in vitro derived materials of four Passiflora species. An. Acad. Bras. Ciênc. 2018, 90, 2805–2813. [Google Scholar] [CrossRef] [Green Version]
- Amaral, G.P.; Mizdal, C.R.; Stefanello, S.T.; Mendez, A.S.L.; Puntel, R.L.; de Campos, M.M.A.; Soares, F.A.A.; Fachinetto, R. Antibacterial and antioxidant effects of Rosmarinus officinalis L. extract and its fractions. J. Tradit. Complement. Med. 2019, 9, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.C.R.; Silva, B.B.; Alves, C.C.F.; Vieira, T.M.; Crotti, A.E.M.; Souza, J.M.; Martins, C.H.G.; Ribeiro, A.B.; Squarisi, I.S.; Tavares, D.C.; et al. Biological properties and chemical composition of essential oil from Nectandra megapotamica (Spreng.) Mez. leaves (Lauraceae). Nat. Prod. Res. 2020, 34, 3149–3153. [Google Scholar] [CrossRef]
- Carvalho, R.S.; Carollo, C.A.; de Magalhães, J.C.; Palumbo, J.M.C.; Boaretto, A.G.; Nunes e Sá, I.C.; Ferraz, A.C.; Lima, W.G.; de Siqueira, J.M.; Ferreira, J.M.S. Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (Mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tannin and gallic acid. S. Afr. J. Bot. 2018, 114, 181–187. [Google Scholar] [CrossRef]
- Carneiro, N.S.; Alves, C.C.F.; Alves, J.M.; Egea, M.B.; Martins, C.H.G.; Silva, T.S.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; et al. Chemical composition, antioxidant and antibacterial activities of essential oils from leaves and flowers of Eugenia klotzschiana Berg (Myrtaceae). An. Acad. Bras. Ciênc. 2017, 89, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.M.; Dantas, M.C.S.M.; e Silva, L.S.; Aona, L.Y.S.; Tavares, I.F.; de Souza-Neta, L.C. Antibacterial activity and chemical composition of the essential oil of Croton heliotropiifolius Kunth from Amargosa, Bahia, Brazil. Ind. Crops Prod. 2017, 105, 203–206. [Google Scholar] [CrossRef]
- Leandro, L.F.; Moraes, T.d.S.; de Oliveira, P.F.; Alves, J.M.; Senedese, J.M.; Ozelin, S.D.; Resende, F.A.; De Grandis, R.A.; Varanda, E.A.; Bastos, J.K.; et al. Assessment of the antibacterial, cytotoxic and mutagenic potential of the phenolic-rich hydroalcoholic extract from Copaifera trapezifolia Hayne leaves. J. Med. Microbiol. 2016, 65, 937–950. [Google Scholar] [CrossRef]
- De Souza Silva, L.T.; Pereira, U.d.P.; de Oliveira, H.M.; Brasil, E.M.; Pereira, S.A.; Chagas, E.C.; Jesus, G.F.A.; Cardoso, L.; Mouriño, J.L.P.; Martins, M.L. Hemato-immunological and zootechnical parameters of Nile tilapia fed essential oil of Mentha piperita after challenge with Streptococcus agalactiae. Aquaculture 2019, 506, 205–211. [Google Scholar] [CrossRef]
- Ribeiro, I.C.d.O.; Mariano, E.G.A.; Careli, R.T.; Morais-Costa, F.; de Sant’Anna, F.M.; Pinto, M.S.; de Souza, M.R.; Duarte, E.R. Plants of the Cerrado with antimicrobial effects against Staphylococcus spp. and Escherichia coli from cattle. BMC Vet. Res. 2018, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Baldim, J.L.; Fernandes Silveira, J.G.; Almeida, A.P.; Carvalho, P.L.N.; Rosa, W.; Schripsema, J.; Chagas-Paula, D.A.; Soares, M.G.; Luiz, J.H.H. The synergistic effects of volatile constituents of Ocimum basilicum against foodborne pathogens. Ind. Crops Prod. 2018, 112, 821–829. [Google Scholar] [CrossRef]
- de Lima-Saraiva, S.R.G.; Oliveira, F.G.d.S.; Junior, R.G.d.O.; Araújo, C.d.S.; de Oliveira, A.P.; Pacheco, A.G.M.; Rolim, L.A.; de Amorim, E.L.C.; César, F.C.S.; Almeida, J.R.G.d.S. Chemical Analysis and Evaluation of Antioxidant, Antimicrobial, and Photoprotective Activities of Schinopsis brasiliensis Engl. (Anacardiaceae). Sci. World J. 2017, 2017, 1713921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández Tasco, A.J.; Ramírez Rueda, R.Y.; Alvarez, C.J.; Sartori, F.T.; Sacilotto, A.C.B.C.; Ito, I.Y.; Vichnewski, W.; Salvador, M.J. Antibacterial and antifungal properties of crude extracts and isolated compounds from Lychnophora markgravii. Nat. Prod. Res. 2020, 34, 863–867. [Google Scholar] [CrossRef]
- Dos Santos, C.; Galaverna, R.; Angolini, C.; Nunes, V.; De Almeida, L.; Ruiz, A.; de Carvalho, J.; Duarte, R.; Duarte, M.; Eberlin, M. Antioxidative, Antiproliferative and Antimicrobial Activities of Phenolic Compounds from Three Myrcia Species. Molecules 2018, 23, 986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, L.D.; Scur, M.C.; Souza, J.G.d.L.; Toledo, A.G.; Pinto, F.G.d.S. Antimicrobial activity and phytochemical prospection of vegetal extracts of Ocotea silvestris Vattimo-Gil and Ocotea diospyrifolia (Meisn.) against serotypes of Salmonella of poultry origin. Rev. Bras. Saúde Prod. Anim. 2018, 19, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Silva Junior, I.F.; Balogun, S.O.; de Oliveira, R.G.; Damazo, A.S.; Martins, D.T.d.O. Piper umbellatum L.: A medicinal plant with gastric-ulcer protective and ulcer healing effects in experimental rodent models. J. Ethnopharmacol. 2016, 192, 123–131. [Google Scholar] [CrossRef]
- Kildea, S.; Ransbotyn, V.; Khan, M.R.; Fagan, B.; Leonard, G.; Mullins, E.; Doohan, F.M. Bacillus megaterium shows potential for the biocontrol of septoria tritici blotch of wheat. Biol. Control 2008, 47, 37–45. [Google Scholar] [CrossRef]
- Da Silva, V.D.; Almeida-Souza, F.; Teles, A.M.; Neto, P.A.; Mondego-Oliveira, R.; Mendes Filho, N.E.; Taniwaki, N.N.; Abreu-Silva, A.L.; Calabrese, K.d.S.; Mouchrek Filho, V.E. Chemical composition of Ocimum canum Sims. essential oil and the antimicrobial, antiprotozoal and ultrastructural alterations it induces in Leishmania amazonensis promastigotes. Ind. Crops Prod. 2018, 119, 201–208. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Médicale 2017, 27, 303–311. [Google Scholar] [CrossRef]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Sarpietro, M.G.; Di Sotto, A.; Accolla, M.L.; Castelli, F. Interaction of β-caryophyllene and β-caryophyllene oxide with phospholipid bilayers: Differential scanning calorimetry study. Thermochim. Acta 2015, 600, 28–34. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. d-Alanine: d-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Programa REFLORA. Available online: floradobrasil.jbrj.gov.br (accessed on 20 July 2021).



| Species and Family | Origin | Distribution—Phytogeographic Domains | |||||
|---|---|---|---|---|---|---|---|
| Amazon | Caatinga | Cerrado | Atlantic Forest | Pampa | Pantanal | ||
| Amburana cearensis (Allemão) A.C.Sm. (Fabaceae) | Native | X | X | X | X | ||
| Acrocomia aculeata (Jacq.) Lodd. Ex Mart. (Arecaceae) | Native | X | X | ||||
| Adiantum latifolium Lam. (Pteridaceae) | Native | X | X | ||||
| Annona coriacea Mart. (Annonaceae) | Native | X | X | X | X | ||
| Annona crassiflora Mart. (Annonaceae) | Native | X | X | X | |||
| Arrabidaea brachypoda (DC.) bureau (Bignoniaceae) | Native | X | X | X | X | X | |
| Artocarpus heterophyllus Lam. (Moraceae) | Naturalized | X | X | X | |||
| Aspidosperma subincanum Mart. (Apocynaceae) | Native | X | X | X | |||
| Banisteriopsis argyrophylla (A.Juss.) B.Gates (Malpighiaceae) | Native | X | |||||
| Bauhinia rufa (Bong.) Steud. (Fabaceae) | Native | X | |||||
| Brosimum gaudichaudii Trécul. (Moraceae) | Native | X | X | X | X | ||
| Byrsonima crassifolia (L.) Kunth (Malpighiaceae) | Native | X | X | X | X | X | |
| Campomanesia adamantium (Cambess.) O. Berg. (Myrtaceae) | Native | X | X | ||||
| Campomanesia sessiliflora (O. Berg) Mattos (Myrtaceae) | Native | X | X | X | |||
| Capsicum annuum L. (Solanaceae) | Cultivated | X | |||||
| Capsicum baccatum L. (Solanaceae) | Native | X | X | X | X | X | X |
| Capsicum chinense Jacq. (Solanaceae) | Naturalized | X | |||||
| Capsicum frutescens L. (Solanaceae) | Naturalized | X | X | ||||
| Caryocar brasiliense Cambess. (Caryocaraceae) | Native | X | X | X | X | ||
| Casearia sylvestris Sw. (Salicaceae) | Native | X | X | X | X | X | X |
| Chromolaena squalida (DC.) R.M.King & H.Rob. (Asteraceae) | Native | X | X | X | X | ||
| Cochlospermum regium (Mart. Ex Schrank) Pilg. (Bixaceae) | Native | X | X | X | X | ||
| Copaifera langsdorffii Desf. (Fabaceae) | Native | X | X | X | X | ||
| Copaifera trapezifolia Hayne (Fabaceae) | Native | X | |||||
| Croton heliotropiifolius Kunth (Euphorbiaceae) | Native | X | X | X | |||
| Davilla kunthii A.St.-Hil. (Dilleniaceae) | Native | X | X | X | |||
| Dipteryx alata Vogel (Fabaceae) | Native | X | |||||
| Dypsis decaryi (Jum.) Beentje & J.Dransf. (Arecaceae) | Cultivated | No phytogeographic domain—Confirmed occurrence in the Brazilian southeast | |||||
| Endlicheria paniculata (Spreng.) J.F.Macbr. (Lauraceae) | Native | X | X | X | X | X | X |
| Erythroxylum daphnite daphnites Mart. (Erythroxylaceae) | Native | X | X | X | X | ||
| Eucalyptus citriodora Hook. (Myrtaceae) | Cultivated | Occurrence: North, Northeast, Central-West, Southeast, and South | |||||
| Eucalyptus globulus Labill. (Myrtaceae) | Cultivated | Occurrence: North, Northeast, Central-West, Southeast, and South | |||||
| Eugenia klotzschiana O.Berg (Myrtaceae) | Native | X | X | ||||
| Genipa americana L. (Rubiaceae) | Native | X | X | X | X | X | |
| Guatteria blepharophylla Mart. (Annonaceae) | Native | X | |||||
| Hedychium coronarium J.Koenig (Zingiberaceae) | Naturalized | X | X | X | X | X | X |
| Inga laurina (Sw.) Willd. (Fabaceae) | Native | X | X | X | X | ||
| Iryanthera polyneura Ducke (Myristicaceae) | Native | X | |||||
| Jatropha multifida L. (Euphorbiaceae) | Cultivated | Occurrence: North, Northeast, Southeast, and South | |||||
| Matayba guianensis Aubl. (Sapindaceae) | Native | X | X | X | X | ||
| Mauritia flexuosa L.f. (Arecaceae) | Native | X | X | X | |||
| Melissa officinalis L. (Lamiaceae) | Cultivated | Occurrence: North, Northeast, Central-West, Southeast, and South | |||||
| Mentha piperita L. (Lamiaceae) | Cultivated | No information found | |||||
| Myrcia bella Cambess. (Myrtaceae) | Native | X | |||||
| Myrcia fallax (Rich.) DC. (Myrtaceae) | Native | X | X | X | X | X | |
| Myrcia guianensis (Aubl.) DC. (Myrtaceae) | Native | X | X | X | X | X | |
| Myrsine guianensis (Aubl.) Kuntze (Primulaceae) | Native | X | X | X | X | X | |
| Nectandra megapotamica (Spreng.) Mez (Lauraceae) | Native | X | X | X | X | X | X |
| Ocimum basilicum L. (Lamiaceae) | Cultivated | Occurrence: North, Northeast, Central-West, Southeast, and South | |||||
| Ocimum canum Sims (Lamiaceae) | Naturalized | X | X | X | X | ||
| Ocotea diospyrifolia (Meisn.) Mez (Lauraceae) | Native | X | X | X | |||
| Passiflora foetida L. (Passifloraceae) | Native | X | X | X | X | X | X |
| Passiflora pohlii Mast. (Passifloraceae) | Native | X | X | X | |||
| Passiflora suberosa L. (Passifloraceae) | Native | X | X | X | X | ||
| Pentaclethra macroloba (Willd.) Kuntze (Fabaceae) | Native | X | |||||
| Pereskia aculeata Mill. (Cactaceae) | Native | X | X | X | X | ||
| Peritassa campestris Cambess.) A.C. Sm. (Celastraceae) | Native | X | X | ||||
| Persea americana Mill. Var. americana (Lauraceae) | Naturalized | X | |||||
| Peumus boldus Molina (Monimiaceae) | Naturalized | X | X | X | X | X | |
| Piper augustum Rudge (Piperaceae) | Native | X | |||||
| Piper umbellatum L. (Piperaceae) | Native | X | X | X | |||
| Piptadenia viridiflora (Kunth) Benth. (Fabaceae) | Native | X | X | X | |||
| Poincianella pyramidalis (Tul.) L.P.Queiroz (Fabaceae) | Native | X | |||||
| Porophyllum obscurum (Spreng.) DC. (Asteraceae) | Native | X | X | X | |||
| Portulaca elatior Mart. Ex Rohrb. (Portulacaceae) | Native | X | X | X | |||
| Pouteria ramiflora (Mart.) Radlk. (Sapotaceae) | Native | X | X | X | X | ||
| Pouteria torta (Mart.) Radlk. (Sapotaceae) | Native | X | X | X | X | ||
| Psidium guajava L. (Myrtaceae) | Naturalized | X | X | X | X | X | |
| Schinopsis brasiliensis Engl. (Anacardiaceae) | Native | X | X | ||||
| Rosmarinus officinalis L. (Lamiaceae) | Cultived | Occurrence: North, Northeast, Central-West, Southeast, and South | |||||
| Senna rugosa (G.Don) H.S.Irwin & Barneby (Fabaceae) | Native | X | X | X | X | ||
| Serjania lethalis A.St.-Hil. (Sapindaceae) | Native | X | X | X | X | X | |
| Sideroxylon obtusifolium (Roem. & Schult.) T.D.Penn. (Sapotaceae) | Native | X | X | X | X | ||
| Syzygium cumini (L.) Skeels (Myrtaceae) | Naturalized | X | X | X | X | ||
| Vernonia polysphaera Baker (Asteraceae) | Native | X | X | ||||
| Vitex cymosa Bertero ex Spreng. (Lamiaceae) | Native | X | X | X | X | X | |
| Vochysia divergens Pohl (Vochysiaceae) | Native | X | X | X | |||
| Ximenia americana L. (Ximeniaceae) | Native | X | X | X | X | ||
| Attalea speciosa * Mart. Ex Spreng. (Arecaceae) | Native | X | X | ||||
| Buchenavia tetraphylla * (Aubl.) R.A.Howard (Combretaceae) | Native | X | X | X | |||
| Caryocar coriaceum * Wittm. (Caryocaraceae) | Native | X | |||||
| Erythroxylum subrotundum * A.St.-Hil. (Erythroxylaceae) | Native | X | X | X | |||
| Eugenia dysenterica * (Mart.) DC (Myrtaceae) | Native | X | X | X | |||
| Harrisia adscendens * (Gürke) Britton & Rose (Cactaceae) | Native | X | |||||
| Lychnophora markgravii * G.M. Barroso (Asteraceae) | Native | X | X | ||||
| Miconia latecrenata * (DC.) Naudin (Melastomataceae) | Native | X | |||||
| Miconia willdenowii * Klotzsch ex Naudin (Melastomataceae) | Native | X | |||||
| Myrciaria pilosa * Sobral & Couto (Myrtaceae) | Native | X | X | ||||
| Ocotea minarum * (Nees & Mart.) Mez (Lauraceae) | Native | X | X | ||||
| Ocotea silvestris * Vattimo-Gil (Lauraceae) | Native | X | X | ||||
| Passiflora alata * Curtis (Passifloraceae) | Native | X | X | X | X | ||
| Poincianella pyramidalis * (Tul.) L.P.Queiroz (Fabaceae) | Native | X | |||||
| Psidium cattleianum * Sabine (Myrtaceae) | Native | X | X | X | |||
| Simaba ferruginea * A.St.-Hil. (Simaroubaceae) | Native | X | |||||
| Siparuna guianensis * Aubl. (Siparunaceae) | Native | X | X | X | X | X | |
| Spondias tuberose * Arruda (Anacardiaceae) | Native | X | X | ||||
| Stryphnodendron adstringens * (Mart.) Coville (Fabaceae) | Native | X | X | ||||
| Vanillosmopsis arborea * (Gardner) Baker (Asteraceae) | Native | X | X | ||||
| Total | 52 | 54 | 71 | 61 | 13 | 25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Queiroz, J.C.E.; Leite, J.R.S.A.; Vasconcelos, A.G. Prospecting Plant Extracts and Bioactive Molecules with Antimicrobial Activity in Brazilian Biomes: A Review. Antibiotics 2023, 12, 427. https://doi.org/10.3390/antibiotics12030427
de Queiroz JCE, Leite JRSA, Vasconcelos AG. Prospecting Plant Extracts and Bioactive Molecules with Antimicrobial Activity in Brazilian Biomes: A Review. Antibiotics. 2023; 12(3):427. https://doi.org/10.3390/antibiotics12030427
Chicago/Turabian Stylede Queiroz, José Carlos Eloi, José Roberto S. A. Leite, and Andreanne Gomes Vasconcelos. 2023. "Prospecting Plant Extracts and Bioactive Molecules with Antimicrobial Activity in Brazilian Biomes: A Review" Antibiotics 12, no. 3: 427. https://doi.org/10.3390/antibiotics12030427
APA Stylede Queiroz, J. C. E., Leite, J. R. S. A., & Vasconcelos, A. G. (2023). Prospecting Plant Extracts and Bioactive Molecules with Antimicrobial Activity in Brazilian Biomes: A Review. Antibiotics, 12(3), 427. https://doi.org/10.3390/antibiotics12030427