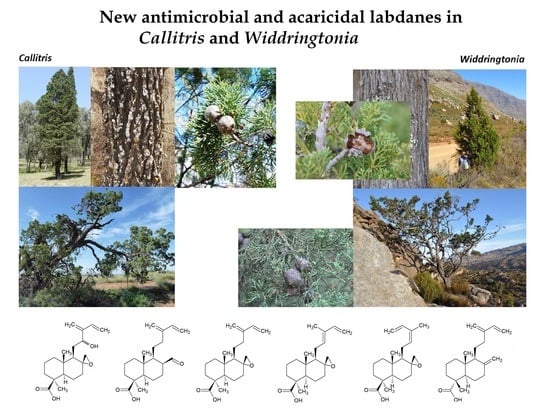
2.1. Chemistry of South African Widdringtonia
For reasons of conservation, only leaves and branches (two-inch circumference) of
Widdringtonia were extracted in the present study, as heartwood harvesting is destructive. A series of known (
1–
3) and new labdane derivatives (
4–
8) have been identified in extracts from leaves and other aerial parts (
Table 1), along with volatile terpenes consistent with those identified in earlier phytochemical studies on essential oils from
Widdringtonia (
Table 2). The overwhelmingly higher representation of diterpenes in the extracts of leaves is surprising, but consistent across all specimens and species investigated in
Widdringtonia. Since diterpenes were not isolated in the earlier studies that focused on heartwood, it is likely that the diterpenes are restricted to branching parts and sesquiterpenes are obtained in higher yield in the heartwood.
The most abundant diterpenes are already well known from Cupressaceae with sandaracopimaric acid (
1) being the dominant terpene in our extracts, which is followed by
Z-communic acid (
2) (
Table 1). The name sandaracopimaric acid has its etymology in the product ‘sandarac’, historically derived from the Moroccan species
Tetraclinis articulata (Vahl) Mast., which is another member of Cupressaceae [
24]. The communic acid isomers are evidently familiar with the fruits of
Juniperus communis L. [
25], which is popularly known for its essential oil wherein the acids are absent due to higher boiling points.
The other lesser abundant diterpenes (
4–
8) (Nuclear Magnetic Resonance (NMR) data,
Table 3 and
Table 4) are undescribed and, as far as we can tell, restricted to
Widdringtonia. The compounds were assigned as structural analogues of isocommunic and communic acids (
Figure 2), differing by oxidation at either the C8 position or C12 on the branching chain moiety. The prevailing characteristic of these new diterpenes is the spiroepoxy at C8, which is evident in all except for the aldehyde (
5).
In most plant parts, the spiroepoxy diterpenes were of low relative abundance, except for in the cones of
W. nodiflora, which were richer in the new diterpenes as compared to 1 and 2. Furthermore, the cones yielded over 1%
w/w spathulenol in flash chromatography. Spathulenol (
Table 5) is an antimicrobial sesquiterpene [
26] that demonstrates enhanced activity against skin pathogens when encapsulated (
Table 6). An encapsulation effect similar to that derived from inclusion in the chemically diverse gum that exudes from the cones. Spathulenol is also present at lower concentrations in the essential oils of other plant parts (
Table 5). The pronounced chemical difference of the cones compared to other plant parts provides the first insight into why these are chosen above other organs in therapeutic applications consistent with antimicrobial outcomes. Antimicrobial testing provided further testimony to this (
Table 6). See
Section 2.3 for more details.
Essential oils from leaves and stem pulp (
Table 5) of
W. nodiflora and
W. schwartzii were chemically similar to essential oils from aerial parts of
W. wallichii described in other studies [
10]. While the essential oils of branches displayed high chemical consistency with that reported for the heartwood, the yields obtained by us were still lower than the reported yields in heartwood.
2.2. Chemistry of Callitris
Essential oils from leaves of the two species of
Callitris sampled in the current study (
C. endlicheri and
C. columellaris) have been previously characterised [
16,
27]. Furthermore, timber essential oils and solvent extracts have also been studied [
18]. Thus, in the current study, an examination of solvent extracted (non-volatile) components from the leaves was undertaken to gratify this neglected area of research in order to make a comparison to
Widdringtonia. Unlike
Widdringtonia, interspecific differences in the Genus were pronounced. Major diterpenes in the leaves of
C. columellaris were pisiferal (
9) and pisiferic acid (
10), whereas a single diterpene was evident in
C. endlicheri leaves, being isoozic acid (
11), which is the isomer of the more widespread ozic acid in other species of
Callitris [
21]. In addition, sandaracopimaric acid (
1) was identified in both species, but at lower concentrations as compared to
Widdringtonia. The study by Simoneit et al. [
21] reported 2 and 3 extensively in most other species
of Callitris, which is similar to the pattern evident in
Widdringtonia, but these diterpenes were not identified in the two species of
Callitris in the current study. Nevertheless, mass spectral analysis can be used to verify if they occur in trace amounts. The identification of 12-hydroxycallitrisic acid in the resin of
C. baileyi C.T. White by Simoneit et al. [
21] is interesting since it only differs from pisiferic acid by the positioning of the carboxyl group.
The species sampled for the current study (
C. endlicheri &
C. columellaris) were geographically disjunct from those studied by Simoneit et al. [
21]. Within Australia,
C. columellaris is treated as three distinct species, as previously mentioned [
28]. Because Simoneit et al. [
21] did not identify the abietanes pisiferal (
9) or pisiferic acid (
10), it is likely that they sampled the actual
C. columellaris cultivated from the coastal south-east Qld genepool, which grows in the sand dunes. The specimens of
C. columellaris sampled for the current study were all taken from the inland temperate regions and represent the taxa that is widely recognized in Australia as
C. glaucophylla. In the current study, we have chosen to recognize these interior specimens as a distinct chemotype.
Nevertheless, the current study constitutes the first appearance of the abietanes pisiferal (
9) and pisiferic acid (
10) in
Callitris. Otherwise, they are familiar to Cupressaceae, first isolated from the etymologically related Japanese species
Chamaecyparis pisifera (Siebold and Zucc.) Endl. [
29]. The authors considered the discovery of pisiferic acid (
10) in
C. columellaris leaves fortuitous since it has been recognised as having significant commercial potential in the context of preservatives and topical antimicrobial therapy. It yields at 0.1%
w/w from the leaves and is sustainably harvested, which ensures that trees are able to recover without long-term negative effects. Since 10 has a carboxylic acid moiety, it can be enriched using an organic/base partition using aqueous ammonia extraction and then neutralised with HCl to create a composition of 40–50% purity.
In the interest of confirming chemical similarity of C. columellaris over a wide geographic range, the specimens were sampled from several locations in Qld and NSW, which all displayed similar yields of 10. Specimens collected from as far south as Peak Hill (central NSW) to as far north as Blackall (north Qld) all yielded 10. In contrast, C. endlicheri was considerably variable, but a full report of the chemical character is beyond the scope of this study. Variation can be explained by the disjunct nature of its distribution since it is restricted to rocky slopes and plains, gorges, and other geographical features that are not widespread. This creates barriers to gene spread and inevitably leads to chemical divergence.
2.3. Structural Characterisation of Diterpenes 4–8
Most of the new structures are derivatives (4, 6–8) or an aldehyde (5) of the two known labdane isomers,
Ε- and
Ζ-communic acids 2 and 3, and isocommunic acid [
30]. The butadiene moiety made these compounds susceptible to degradation in storage during flash chromatography using silica gel. Nevertheless, the structures were successfully assigned using 1D and 2D NMR spectra and high resolution electrospray ionization mass spectrometry (HRESIMS).
The butadiene moiety of compounds 4–6 had characteristic broad proton singlets in the olefinic region (4: δ
H 5.11, 5.21, 5.23 and 5.43 ppm) and were seen in the HSQC (heteronuclear single quantum coherence) spectrum to be part of two methylidenes. In the HMBC (heteronuclear multiple bond correlation) spectrum, these protons interacted with the neighbouring olefin (
Table 3). The overlap of
13C shifts to isocommunic acid [
30] showed strong agreement (
Supplementary Files, Table S1) after allowing for the electronic influences of the oxidised and adjacent carbons. For example, the C12-OH shift on 4 altered most of the
13C shifts in the butadiene moiety, but, on 5 and 6, the shifts from C13 to C16 had <1 ppm difference as compared to isocommunic acid.
HRESIMS of 4 gave a molecular ion peak at
m/z 357.2030 [M + Na]
+, which is consistent with a molecular formula of C
20H
30O
4. This only differs from isocommunic acid by the addition of two oxygen atoms. This molecular formula gives an index of hydrogen deficiency (IHD) of 6 with four olefinic carbons (δ
C 115.0, 115.5, 136.6 & 145.9 ppm) and a resonance in the acid region (δ
C 182.3). This indicated a tricyclic molecule. Relative to isocommunic acid, 4 does not have the C8–C17 methylidene, but instead displays two resonances consistent with an epoxide (C8 δ
C 60.3 ppm & C17 δ
C 50.4 ppm). The HSQC spectrum was used to assign the two oxirane protons at 2.82 and 2.60 ppm to C17 at δ
C 50.4 ppm. The HMBC spectrum indicated that these oxirane protons have a
1H-
13C interaction with the quaternary C8 position, and, in the COSY spectra, a
4J 1H–
1H coupling was observed between the downfield oxirane proton (δ
H 2.82 ppm) and one of the C7 protons (δ
H 1.92 ppm). Similar chemical shifts were observed in several other labdanes [
31,
32,
33,
34,
35,
36] with a spiro-epoxy group at C8. Another downfield resonance was observed at δ
C 83.8 ppm, which is consistent with an allylic alcohol, with the position established as C12 using the HMBC spectrum. A
1H-
13C interaction from H16/H16’ to C12 was observed, and the reverse interaction, H12 to the olefinic resonance C16 was also seen. These observations led to the structure of 12-hydroxy-8
R,17-spiroepoxy-isocommunic acid for 4 with the 2D NMR spectra being consistent with the proposed structure (
Table 3). The configuration of C12 could not be unambiguously determined due to free rotation in the chain. In the 2D NOESY (nuclear overhauser effect spectroscopy) spectrum, a through-space interaction from the downfield C17 oxirane proton (δ
H 2.82 ppm) to one of the C11 protons and the C20 methyl group were seen. However, this was insufficient to assign the configuration at C12. The replacement of the C8–C17 methylidene with an oxirane also presumably affects the chemical shifts throughout the spectrum of 4 relative to isocommunic acid (
Supplementary: Tables S1 and S2) [
37].
Determining the configuration of spiroepoxy groups can be challenging due to the small differences in chemical shifts and the similar position for the oxiranyl hydrogens in the two isomers. Bastard et al. [
32] have reported that the
13C shift at C17 of 8-spiroexpoxy labdanes is further downfield in the 8
R configuration (which they refer to as α-configuration) with values ranging from δ
C 50–51 ppm. This contrasts with the 8
S configuration with C17 shifts of δ
C 48–49 ppm. The oxirane protons are also affected with H17 shifting on the 8
R epimer ranging from δ
H 2.55–2.60 ppm and δ
H 2.70–2.82 ppm in contrast with δ
H 2.20–2.30 ppm and δ
H 2.42–2.70 ppm on the 8
S epimer. The difference is believed to be a consequence of equatorial vs. axial shielding effects [
32]. By comparing chemicals shifts for eight labdanes with the 8
R-configuration and five with the 8
S-configuration (
Supplementary: Tables S3–S5 and Figures S1–S3), the relationship appears to hold. While it is possible that the
13C NMR chemical shift of C17 on the 8
S epimer can be shifted due to the C11–C16 moiety [
36], the oxiranyl protons in 4 demonstrated shifts within the predicted range for the 8
R configuration.
HRESIMS of 5 detected a molecular ion peak at
m/z 341.2094 [M + Na]
+, which is consistent with a molecular formula of C
20H
30O
3 and an IHD of 6. The
13C NMR shifts in the butadiene moiety (C13–C16) very closely and coincided with those reported for isocommunic acid with Δδ < 0.6 and the compound differed from 4 due to the absence of the C12-OH. The
13C NMR spectrum of 5 lacked the oxiranyl signals and contained a C17 aldehyde with signals at δ
C 205.2 ppm and δ
H 9.56. The H17 aldehyde resonance coupled to H8 in the COSY spectrum. The configuration at C8 is also believed to be
S as the NOESY spectrum showed that H8 interacted with H9, and there was an interaction between H17 at δ
H 9.56 ppm and H11 at δ
H 1.18 ppm, which supports the cis configuration. Thus, 5 was tentatively assigned as 8
S-formyl-isocommunic acid and HMBC couplings corroborated this (
Table 3).
Similar to 5, the
13C shifts on the butadiene moiety of 6 were consistent with isocommunic acid (
Supplementary: Table S1). HRESIMS of 6 detected a molecular ion peak at
m/z 319.2258 [M + H]
+, which is consistent with a molecular formula of C
20H
30O
3 and an IHD of 6. Aside from the butadiene moiety, the
13C and
1H spectrums of 6 closely resembled that of 4 (
Table 3), which indicates a structure differing only from isocommunic acid by the spiroepoxy moiety. By examination of the HMBC couplings on 6 (
Table 3), the structure was assigned as 8
R,17-epoxy-isocommunic acid.
HRESIMS of 7 and 8 gave a molecular ion peak at
m/z 341.2081 [M + Na]
+ for both compounds, which is again consistent with a molecular formula of C
20H
30O
3 and an IHD of 6. Out of the new compounds, only 7 and 8 were demonstrated to be derivatives of the communic acid cis/trans isomers [
38]. The
1H and
13C spectra of both isomers (
Table 4) very closely matched that of 6 (
Table 3) with the exception of the butadiene chain, where the second methylidene in 6 was not observed in 7 or 8. In contrast, the olefinic carbon at C13 was substituted with a methyl group (C16), which gave a 3H singlet in the
1H NMR spectrum in the olefinic methyl region (
7: δ
H 1.68 ppm,
8: δ
H 1.77 ppm). Since the most significant differences between 7 and 8 were on the side chain moiety, it was established that they differed via isomerism at C13. A key diagnostic chemical shift at C14 can be used to distinguish between the isomers, with δ
H 6.30 indicating the
E-isomer and δ
H 6.77 ppm for the
Z-isomer [
38]. In the current study, the H14 in
7 was observed at δ 6.33 ppm, and, on 8, H14 was observed at δ 6.76 ppm (
Table 4). The
13C shifts of C14 are also diagnostic with δ
C 141.8 ppm seen for the
E-isomer and δ
C 133.9 ppm for the Z-isomer [
38], which were identical to the
13C chemical shifts for C14 on 7 and 8, respectively. Thus,
7 was assigned as 8
R-17-epoxy-
E-communic acid and 8 as 8
R-17-epoxy-
Z-communic acid.
A detailed summary of
13C NMR shifts for derivatives of labdane C4 acid esters are given by Barrero and Altarejos [
33] and labdane C4 dimethyl derivatives by Bastard et al. [
32]. However, a more comprehensive comparison to data in the current manuscript is provided in the
Supplementary Files.
2.4. Antimicrobial and Acaricidal Activity
Regrettably, due to the low yield and instability of the spiroexpoxy communic acid derivatives (4–8), acaricidal activities were not measured for these compounds. However, the major diterpenes of
Widdringtonia displayed very modest acaricidal activity using ticks as our model organism (
Table 7, IC50 = 84–482 µg/mL). Although LC
50 values within the range of 90–500 µg/mL are included in
Table 7, only values lower than 25 µg/mL were considered interesting. In this regard, values for pisiferic acid were only moderate, but the treatments with noteworthy activity included guaiol (
12) (6.9–15.1 µg/mL) and the sesquiterpene rich essential oil from
Widdringtonia timber (15.5–39.8 µg/mL).
Guaiol is one of the predominating sesquiterpenes in the timber of all
Callitris and may be considered important in the termite resistance [
39], but this is the first account of activity against Acari. Since this outcome was also evident using essential oils from timbers of
Widdringtonia, it may be feasible to correlate termite resistant timbers to resistance against Acari in general. This generates more questions about possible insect repellent activity and may translate to the ethnopharmacological context where topical applications may have alleviated ectoparasitic problems.
The antimicrobial activity of guaiol is moderate with values as low as 120–250 μg/mL against Gram-positive bacteria. The diterpenes 1 and 2 were not active at the starting concentrations used, but the spiroepoxy diterpenes were moderate to interesting, in particular 4 and 5 with MIC values ranging from 43–400 μg/mL against Gram-positive strains and 160 μg/mL against
P. aeruginosa (
Table 6). Moderate MIC values for spathulenol and guaiol were demonstrated, which ranged from 100–250 μg/mL against Gram-positives and 300 μg/mL against both Gram-negative organisms. All of these compounds are of high relative abundance in the cones of
Widdringtonia with the yield of spathulenol at 1% by mass (
Table 2). Thus, these specialised metabolites evidently provide the pharmacological basis of the traditional use of the clear, hard gum in applications consistent with antimicrobial or acaricidal activity.
The activity of 10 (pisiferic acid) was reiterated here, with results not unlike those reported previously [
40], by inhibiting Gram-positive bacteria at concentrations as low as 50 µg/mL, but not Gram-negative bacteria at the concentrations tested (
Table 6). In the current study, 10 was then screened against an MRSA strain and it was clear that the activity was consistently 50 µg/mL.