Testing the Antimicrobial Characteristics of Wood Materials: A Review of Methods
Abstract
:1. Introduction
2. Literature Search Method
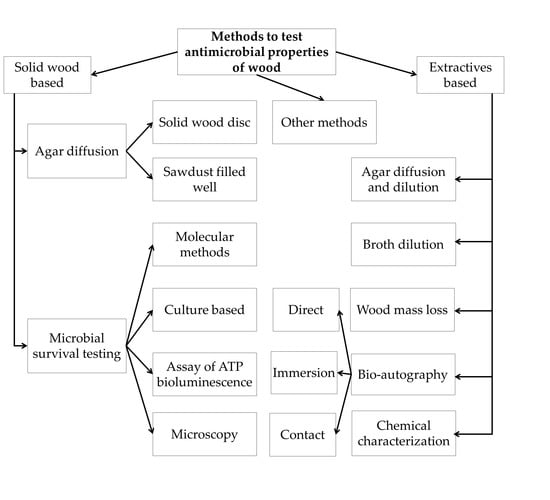
3. Results and discussion
3.1. Direct Methods
3.1.1. Agar Diffusion Method
Direct Wood Disc Agar Diffusion Method (Antiboisgram)
Sawdust-Filled Well Diffusion Method
3.1.2. Evaluation of Microbial Survival on Wood Surfaces
Microbial Recovery
Culture-Based Methods
Molecular Biology Methods
ATP Bioluminescence Assay
Microscopy of Microbes on Wood
3.2. Methods to Study the Antimicrobial Properties of Wood Extractives
3.2.1. Agar Diffusion and Dilution Methods
3.2.2. Broth Dilution Methods
3.2.3. Measurement of Wood Mass Loss to Decaying
3.2.4. Bioautography
Direct Bioautography
Contact Bioautography
Immersion (Agar-Overlay) Bioautography
3.2.5. Active Antimicrobial Ingredient Identification
3.3. Other Methods
3.4. Pros and Cons of Mthods Used to Study Antimicrobial Behavior of Wood Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, C.A.S.; Dibdiakova, J. The environmental impact of wood compared to other building materials. Int. Wood Prod. J. 2016, 7, 215–219. [Google Scholar] [CrossRef]
- Kotradyova, V.; Vavrinsky, E.; Kalinakova, B.; Petro, D.; Jansakova, K.; Boles, M.; Svobodova, H. Wood and its impact on humans and environment quality in health care facilities. Int. J. Environ. Res. Public Health 2019, 16, 3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviat, F.; Gerhards, C.; Rodriguez-Jerez, J.; Michel, V.; Bayon, I.L.; Ismail, R.; Federighi, M. Microbial Safety of Wood in Contact with Food: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 491–505. [Google Scholar] [CrossRef]
- Munir, M.T.; Belloncle, C.; Irle, M.; Federighi, M. Wood-based bedding in poultry production: A review. World’s Poult. Sci. J. 2019, 75, 5–16. [Google Scholar] [CrossRef]
- Munir, M.T.; Irle, M.; Belloncle, C.; Federighi, M. Wood Based Bedding Material in Animal Production: A Minireview. APDV 2019, 6, 1–7. [Google Scholar]
- Pailhories, H.; Munir, M.T.; Aviat, F.; Federighi, M.; Belloncle, C.; Eveillard, M. Oak in Hospitals, the Worst Enemy of Staphylococcus aureus. Infect. Cont. Amp. Hosp. Epidemiol. 2017, 38, 382–384. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.T.; Aviat, F.; Pailhories, H.; Eveillard, M.; Irle, M.; Federighi, M.; Belloncle, C. Direct screening method to assess antimicrobial behavior of untreated wood. Eur. J. Wood Wood Prod. 2019, 77, 319–322. [Google Scholar] [CrossRef]
- Laireiter, C.M.; Schnabel, T.; Köck, A.; Stalzer, P.; Petutschnigg, A.; Oostingh, G.J.; Hell, M. Active Anti-Microbial Effects of Larch and Pine Wood on Four Bacterial Strains. BioResources 2013, 9, 273–281. [Google Scholar] [CrossRef]
- Munir, M.T.; Pailhories, H.; Eveillard, M.; Aviat, F.; Lepelletier, D.; Belloncle, C.; Federighi, M. Antimicrobial Characteristics of Untreated Wood: Towards a Hygienic Environment. Health 2019, 11, 152–170. [Google Scholar] [CrossRef] [Green Version]
- Ismail, R.; Aviat, F.; Gay-Perret, P.; Le Bayon, I.; Federighi, M.; Michel, V. An assessment of L. monocytogenes transfer from wooden ripening shelves to cheeses: Comparison with glass and plastic surfaces. Food Control. 2017, 73, 273–280. [Google Scholar] [CrossRef]
- Filip, S.; Fink, R.; Oder, M.; Jevšnik, M. Hygienic acceptance of wood in food industry. Wood Sci. Tech. 2012, 46, 657–665. [Google Scholar] [CrossRef]
- Abedini, A.; Colin, M.; Hubert, J.; Charpentier, E.; Angelis, A.; Bounasri, H.; Bertaux, B.; Kotland, A.; Reffuveille, F.; Nuzillard, J.-M.; et al. Abundant Extractable Metabolites from Temperate Tree Barks: The Specific Antimicrobial Activity of Prunus Avium Extracts. Antibiotics 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.-E.; Eklund, P.; Willför, S.; Alakomi, H.-L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microb. 2013, 164, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Valgas, C.; de Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Brazi. J. Microb. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Smailagić, A.; Ristivojević, P.; Dimkić, I.; Pavlović, T.; Dabić Zagorac, D.; Veljović, S.; Fotirić Akšić, M.; Meland, M.; Natić, M. Radical Scavenging and Antimicrobial Properties of Polyphenol Rich Waste Wood Extracts. Foods 2020, 9, 319. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Li, D.; Zhang, M.; Ge, S.; Mo, B.; Li, S.; Ohkoshi, M. Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi J. Biol. Sci. 2017, 24, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Soares, V.M.; Pereira, J.G.; Viana, C.; Izidoro, T.B.; Bersot, L. dos S.; Pinto, J.P. de A.N. Transfer of Salmonella Enteritidis to four types of surfaces after cleaning procedures and cross-contamination to tomatoes. Food Microb. 2012, 30, 453–456. [Google Scholar] [CrossRef] [Green Version]
- Zangerl, P.; Matlschweiger, C.; Dillinger, K.; Eliskases-Lechner, F. Survival of Listeria monocytogenes after cleaning and sanitation of wooden shelves used for cheese ripening. Eur. J. Wood Wood Prod. 2010, 68, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.Z.M.; Zidan, Y.E.; Mansour, M.M.A.; El Hadidi, N.M.N.; Abo Elgat, W.A.A. Antifungal activities of two essential oils used in the treatment of three commercial woods deteriorated by five common mold fungi. Int. Biodeterior. Biodegrad. 2016, 106, 88–96. [Google Scholar] [CrossRef]
- Elserogy, A.; Kanan, G.; Hussein, E.; Khreis, S.A. Isolation, characterization and treatment of microbial agents responsible for the deterioration of archaeological objects in three jordanian museums. Mediter. Archaeol. Archaeom. 2016, 16, 117–126. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Rautkari, L.; Nordström, K.; Närhi, M.; Natri, O.; Kairi, M. Effect of extractives and thermal modification on antibacterial properties of Scots pine and Norway spruce. Int. Wood. Prod. J. 2013, 4, 248–252. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Kyyhkynen, A.; Rautkari, L.; Siitonen, A. Antibacterial Effects of Extracts of Pinus sylvestris and Picea abies against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Streptococcus pneumoniae. BioResources 2015, 10, 7763–7771. [Google Scholar] [CrossRef]
- Buchner, J.; Irle, M.; Belloncle, C.; Michaud, F.; Macchioni, N. Fungal and bacterial colonies growing on weathered wood surfaces. Wood Mat. Sci. Eng. 2019, 14, 33–41. [Google Scholar] [CrossRef]
- Lane, K. The Efficacy of ATP Monitoring Devices at Measuring Organic Matter on Postharvest Surfaces. Master‘s Thesis, University of Massachusetts Amherst, Amherst, MA, USA, July 2019. [Google Scholar]
- Shimoda, T.; Yano, R.; Nakamura, S.; Yoshida, M.; Matsuo, J.; Yoshimura, S.; Yamaguchi, H. ATP bioluminescence values are significantly different depending upon material surface properties of the sampling location in hospitals. BMC Res. Notes 2015, 8, 807. [Google Scholar] [CrossRef] [Green Version]
- Moricz, A.M.; Ott, P.G. Screening and Characterization of Antimicrobial Components of Natural Products Using Planar Chromatography Coupled with Direct Bioautography, Spectroscopy and Mass Spectrometry: A Review. Curr. Org. Chem. 2017, 21, 1861–1874. [Google Scholar] [CrossRef]
- Moricz, Á.M.; Häbe, T.T.; Böszörményi, A.; Ott, P.G.; Morlock, G.E. Tracking and identification of antibacterial components in the essential oil of Tanacetum vulgare L. by the combination of high-performance thin-layer chromatography with direct bioautography and mass spectrometry. J. Chromatogr. A 2015, 1422, 310–317. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharmaceuti. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Ncube, N.S.; Afolayan, A.J.; Okoh, A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr. J. Biotech. 2008, 7. [Google Scholar]
- Rios, J.L.; Recio, M.C.; Villar, A. Screening methods for natural products with antimicrobial activity: A review of the literature. J. Ethnopharmacol. 1988, 23, 127–149. [Google Scholar] [CrossRef]
- Abdul-Mutalib, N.-A.; Amin Nordin, S.; Osman, M.; Ishida, N.; Tashiro, K.; Sakai, K.; Tashiro, Y.; Maeda, T.; Shirai, Y. Pyrosequencing analysis of microbial community and food-borne bacteria on restaurant cutting boards collected in Seri Kembangan, Malaysia, and their correlation with grades of food premises. Int. J. Food Microb. 2015, 200, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Mutalib, N.-A.; Nordin, S.A.; Osman, M.; Roslan, A.M.; Ishida, N.; Sakai, K.; Tashiro, Y.; Tashiro, K.; Maeda, T.; Shirai, Y. The prevalence of foodborne pathogenic bacteria on cutting boards and their ecological correlation with background biota. Microbiology 2016, 2, 138–151. [Google Scholar] [CrossRef]
- Ahnrud, G.P.; Mendoza, A.J.; Hurley, M.J.; Marek, P.J. Efficacy of a Sonicating Swab for Removal and Capture of Microorganisms from Experimental and Natural Contaminated Surfaces. Appl. Environ. Microbiol. 2018, 84, e00208-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttner, M.P.; Cruz, P.; Stetzenbach, L.D.; Cronin, T. Evaluation of Two Surface Sampling Methods for Detection of Erwinia herbicola on a Variety of Materials by Culture and Quantitative PCR. Appl. Environ. Microbiol. 2007, 73, 3505–3510. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Lv, H.; Cao, C.; Zhang, L.; Cao, R.; Xu, B. Evaluation of antimicrobial activity of Pterocarpus extracts. Ind. Crops Prod. 2019, 140, 111668. [Google Scholar] [CrossRef]
- Chiu, T.-H.; Duan, J.; Liu, C.; Su, Y.-C. Efficacy of electrolysed oxidizing water in inactivating Vibrio parahaemolyticus on kitchen cutting boards and food contact surfaces. Lett. Appl. Microb. 2006, 43, 666–672. [Google Scholar] [CrossRef]
- Coughenour, C.; Stevens, V.; Stetzenbach, L.D. An Evaluation of Methicillin-Resistant Staphylococcus aureus Survival on Five Environmental Surfaces. Microb. Drug Resist. 2011, 17, 457–461. [Google Scholar] [CrossRef]
- Cruciata, M.; Gaglio, R.; Scatassa, M.L.; Sala, G.; Cardamone, C.; Palmeri, M.; Moschetti, G.; Mantia, T.L.; Settanni, L. Formation and Characterization of Early Bacterial Biofilms on Different Wood Typologies Applied in Dairy Production. Appl. Environ. Microbiol. 2018, 84, e02107-17. [Google Scholar] [CrossRef] [Green Version]
- DeVere, E.; Purchase, D. Effectiveness of domestic antibacterial products in decontaminating food contact surfaces. Food Microb. 2007, 24, 425–430. [Google Scholar] [CrossRef]
- Deza, M.A.; Araujo, M.; Garrido, M.J. Efficacy of Neutral Electrolyzed Water To Inactivate Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, and Staphylococcus aureus on Plastic and Wooden Kitchen Cutting Boards. J. Food Protect. 2007, 70, 102–108. [Google Scholar] [CrossRef]
- Dubreil, L.; Aviat, F.; Anthoine, V.; Ismail, R.; Rossero, A.; Federighi, M. Confocal spectral microscopy—an innovative tool for tracking of pathogen agents on contaminated wooden surfaces. Eur. J. Wood Wood Prod. 2018, 76, 1083–1085. [Google Scholar] [CrossRef]
- El-Hefny, M.; Salem, M.Z.M.; Behiry, S.I.; Ali, H.M. The Potential Antibacterial and Antifungal Activities of Wood Treated with Withania somnifera Fruit Extract, and the Phenolic, Caffeine, and Flavonoid Composition of the Extract According to HPLC. Processes 2020, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Elom, M.O.; Ugah, U.I.; Omote, V. Microbial Contaminants of Wooden Toothpicks in Abakaliki Metropolis, Ebonyi State, Nigeria. World J. Life Sci. Med. Res. 2014, 3, 101. [Google Scholar]
- Fernández-Agulló, A.; Freire, M.S.; González-Álvarez, J. Effect of the extraction technique on the recovery of bioactive compounds from eucalyptus (Eucalyptus globulus) wood industrial wastes. Ind. Crops Prod. 2015, 64, 105–113. [Google Scholar] [CrossRef]
- Frontino, G. Comparison of Methods for Detection of Listeria on Wooden Shelves used for Cheese Aging: Challenges Associated with Sampling Porous Surfaces. Master’s Thesis, The University of Vermont, Burlington, VT, USA, July 2019. [Google Scholar]
- Goh, S.G.; Leili, A.-H.; Kuan, C.H.; Loo, Y.Y.; Lye, Y.L.; Chang, W.S.; Soopna, P.; Najwa, M.S.; Tang, J.Y.H.; Yaya, R.; et al. Transmission of Listeria monocytogenes from raw chicken meat to cooked chicken meat through cutting boards. Food Control. 2014, 37, 51–55. [Google Scholar] [CrossRef]
- Gonçalves, F.; Correia, P.; Silva, S.P.; Almeida-Aguiar, C. Evaluation of antimicrobial properties of cork. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graikou, K.; Gortzi, O.; Mantanis, G.; Chinou, I. Chemical composition and biological activity of the essential oil from the wood of Pinus heldreichii Christ. var. leucodermis. Eur. J. Wood Prod. 2012, 70, 615–620. [Google Scholar] [CrossRef]
- Gupta, M.; Bisesi, M.; Lee, J. Comparison of survivability of Staphylococcus aureus and spores of Aspergillus niger on commonly used floor materials. Am. J. Infect. Control 2017, 45, 717–722. [Google Scholar] [CrossRef]
- Imhof, R.; Schwendimann, L.; Scettrini, P.R. Sanitising wooden boards used for cheese maturation by means of a steam-mediated heating process. J. Consum. Prot. Food Saf. 2017, 12, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Ismail, R.; Bayon, I.L.; Michel, V.; Jequel, M.; Kutnik, M.; Aviat, F.; Fédérighi, M. Comparative Study of Three Methods for Recovering Microorganisms from Wooden Surfaces in the Food Industry. Food Anal. Methods 2015, 8, 1238–1247. [Google Scholar] [CrossRef]
- Kavian-Jahromi, N.; Schagerl, L.; Dürschmied, B.; Enzinger, S.; Schnabl, C.; Schnabel, T.; Petutschnigg, A. Comparison of the antibacterial effects of sapwood and heartwood of the larch tree focusing on the use in hygiene sensitive areas. Eur. J. Wood Prod. 2015, 73, 841–844. [Google Scholar] [CrossRef]
- Khurram, M. Evaluation of anticandidal potential of Quercus baloot Griff. using contact bioautography technique. Afr. J. Pharm. Pharmacol. 2011, 5, 1538–1542. [Google Scholar] [CrossRef] [Green Version]
- Lucke, F.-K.; Skowyrska, A. Hygienic aspects of using wooden and plastic cutting boards, assessed in laboratory and small gastronomy units. J. Verbr. Lebensm. 2015, 10, 317–322. [Google Scholar] [CrossRef]
- Milling, A.; Kehr, R.; Wulf, A.; Smalla, K. Survival of bacteria on wood and plastic particles: Dependence on wood species and environmental conditions. Holzforschung 2005, 59, 72–81. [Google Scholar] [CrossRef]
- Milling, A.; Smalla, K.; Kehr, R.; Wulf, A. Wulf The use of wood in practice–A hygienic risk? Holz Roh Werkst. 2005, 63, 463–472. [Google Scholar] [CrossRef]
- Miranda, R.C.; Schaffner, D.W. Longer Contact Times Increase Cross-Contamination of Enterobacter aerogenes from Surfaces to Food. Appl. Environ. Microbiol. 2016, 82, 6490–6496. [Google Scholar] [CrossRef] [Green Version]
- Montibus, M.; Ismaïl, R.; Michel, V.; Federighi, M.; Aviat, F.; Le Bayon, I. Assessment of Penicillium expansum and Escherichia coli transfer from poplar crates to apples. Food Control. 2016, 60, 95–102. [Google Scholar] [CrossRef]
- Moore, G.; Blair, I.S.; McDowell, D.A. Recovery and transfer of Salmonella Typhimurium from four different domestic food contact surfaces. J. Food Prot. 2007, 70, 2273–2280. [Google Scholar] [CrossRef]
- Revol-Junelles, A.-M.; Miguindou-Mabiala, R.; Roger-Maigné, D.; Millière, J.-B. Behavior of Escherichia coli cells and Bacillus cereus spores on poplar wood crates by impedance measurements. J. Food Prot. 2005, 68, 80–84. [Google Scholar] [CrossRef]
- Ripolles-Avila, C.; Hascoët, A.S.; Ríos-Castillo, A.G.; Rodríguez-Jerez, J.J. Hygienic properties exhibited by single-use wood and plastic packaging on the microbial stability for fish. LWT 2019, 113, 108309. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mansour, M.M.A.; Elansary, H.O. Evaluation of the effect of inner and outer bark extracts of sugar maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J. Wood Chem. Tech. 2019, 39, 136–147. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Elansary, H.O.; Elkelish, A.A.; Zeidler, A.; Ali, H.M.; EL-Hefny, M.; Yessoufou, K. In vitro Bioactivity and Antimicrobial Activity of Picea abies and Larix decidua Wood and Bark Extracts. BioResources 2016, 11, 9421–9437. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, R.; Farooq, U.; Naz, S.; Riaz, N.; Majid Bukhari, S.; Rauf, A.; Mabkhot, Y.N.; Al-Showiman, S.S. Isolation and Characterization of Two New Antimicrobial Acids from Quercus incana (Bluejack Oak). BioMed Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subhashini, S.; Begum, S.M.; Rajesh, G. Antimicrobial characterisation combining spectrophotometric analysis of different oak species. Int. J. Herb. Med. 2016, 4, 32–35. [Google Scholar]
- Tang, J.Y.H.; Nishibuchi, M.; Nakaguchi, Y.; Ghazali, F.M.; Saleha, A.A.; Son, R. Transfer of Campylobacter jejuni from raw to cooked chicken via wood and plastic cutting boards. Lett. Appl. Microb. 2011, 52, 581–588. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Kyyhkynen, A.; Viitaniemi, P.; Siitonen, A. Pine heartwood and glass surfaces: Easy method to test the fate of bacterial contamination. Eur. J. Wood Wood Prod. 2011, 69, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Vainio-Kaila, T.; Zhang, X.; Hänninen, T.; Kyyhkynen, A.; Johansson, L.-S.; Willför, S.; Österberg, M.; Siitonen, A.; Rautkari, L. Antibacterial Effects of Wood Structural Components and Extractives from Pinus sylvestris and Picea abies on Methicillin-Resistant Staphylococcus aureus and Escherichia coli O157:H7. BioResources 2017, 12, 7601–7614. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Hänninen, T.; Kyyhkynen, A.; Ohlmeyer, M.; Siitonen, A.; Rautkari, L. Effect of volatile organic compounds from Pinus sylvestris and Picea abies on Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae and Salmonella enterica serovar Typhimurium. Holzforschung 2017, 71, 905–912. [Google Scholar] [CrossRef]
- Valimaa, A.-L.; Honkalampi-Hämäläinen, U.; Pietarinen, S.; Willför, S.; Holmbom, B.; von Wright, A. Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int. J. Food Microb. 2007, 115, 235–243. [Google Scholar] [CrossRef]
- Vek, V.; Oven, P.; Humar, M. Phenolic extractives of wound-associated wood of beech and their fungicidal effect. Int. Biodeteriorat. Biodegradat. 2013, 77, 91–97. [Google Scholar] [CrossRef]
- Welker, C.; Faiola, N.; Davis, S.; Maffatore, I.; Batt, C.A. Bacterial Retention and Cleanability of Plastic and Wood Cutting Boards with Commercial Food Service Maintenance Practices. J. Food Prot. 1997, 60, 407–413. [Google Scholar] [CrossRef]
- Nikiforuk, A.M.; Cutts, T.A.; Theriault, S.S.; Cook, B.W.M. Challenge of Liquid Stressed Protective Materials and Environmental Persistence of Ebola Virus. Sci. Rep. 2017, 7, 4388. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, R.; Kahlmeter, G.; Leclercq, R. Antimicrobial susceptibility testing. In European Manual of Clinical Microbiology; Cornaglia, G., Courcol, R., Herrmann, J.-L., Eds.; European Society for Clinical Microbiology and Infections Diseases (Société française de microbiologie): Basel, Switzerland, 2012; pp. 77–85. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed. Available online: https://clsi.org/standards/products/microbiology/documents/m02/ (accessed on 12 March 2020).
- EUCAST. EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing, Version 8.0. Available online: https://www.eucast.org/documents/publications_in_journals/ (accessed on 12 March 2020).
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Ngassapa, O.; Matee, M.I.N. Antimicrobial Activity of Tanzanian Chewing Sticks Against Oral Pathogenic Microbes. Pharm. Biol. 2000, 38, 235–240. [Google Scholar] [CrossRef]
- Nakmee, P.S.; Khuntong, S.; Nuengchamnong, N. Phytochemical Constituents with Antimicrobial and Antioxidant Activities from Xylia xylocarpa (Roxb.) Taub. Sawdust Extracts. Chiang Mai J. Sci. 2016, 43, 11–21. [Google Scholar]
- Kim, S.W.; Kim, K.S.; Lamsal, K.; Kim, Y.-J.; Kim, S.B.; Jung, M.; Sim, S.-J.; Kim, H.-S.; Chang, S.-J.; Kim, J.K.; et al. An in vitro study of the antifungal effect of silver nanoparticles on oak wilt pathogen Raffaelea sp. J. Microbiol. Biotechnol. 2009, 19, 760–764. [Google Scholar]
- Das, K.; Tiwari, R.K.S.; Shrivastava, D.K. Techniques for evaluation of medicinal plant products as antimicrobial agents: Current methods and future trends. JMPR 2010, 4, 104–111. [Google Scholar] [CrossRef]
- Mansour, M.M.A.; Salem, M.Z.M. Evaluation of wood treated with some natural extracts and Paraloid B-72 against the fungus Trichoderma harzianum: Wood elemental composition, in-vitro and application evidence. Int. Biodeterior. Biodegrad. 2015, 100, 62–69. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Zidan, Y.E.; El Hadidi, N.M.N.; Mansour, M.M.A.; Abo Elgat, W.A.A. Evaluation of usage three natural extracts applied to three commercial wood species against five common molds. Int. Biodeterior. Biodegrad. 2016, 110, 206–226. [Google Scholar] [CrossRef]
- Lu, H.; Ip, E.; Scott, J.; Foster, P.; Vickers, M.; Baxter, L.L. Effects of particle shape and size on devolatilization of biomass particle. Fuel 2010, 89, 1156–1168. [Google Scholar] [CrossRef]
- Ismail, R.; Aviat, F.; Michel, V.; Le Bayon, I.; Gay-Perret, P.; Kutnik, M.; Fédérighi, M. Methods for Recovering Microorganisms from Solid Surfaces Used in the Food Industry: A Review of the Literature. Int. J. Environ. Res. Public Health 2013, 10, 6169–6183. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, S.; Ciric, L.; Cloutman-Green, E. How to carry out microbiological sampling of healthcare environment surfaces? A review of current evidence. J. Hosp. Infec. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plötze, M.; Niemz, P. Porosity and pore size distribution of different wood types as determined by mercury intrusion porosimetry. Eur. J. Wood Prod. 2011, 69, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Cliver, D.O. Cutting boards in Salmonella cross-contamination. J. AOAC Int. 2006, 89, 538–542. [Google Scholar] [PubMed]
- Schönwälder, A.; Kehr, R.; Wulf, A.; Smalla, K. Wooden boards affecting the survival of bacteria? Holz als Roh-und Werkstoff 2002, 60, 249–257. [Google Scholar] [CrossRef]
- Barnes, B.I.; Cassar, C.A.; Halablab, M.A.; Parkinson, N.H.; Miles, R.J. An in situ method for determining bacterial survival on food preparation surfaces using a redox dye. Lett. Appl. Microb. 1996, 23, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M. Characterization of Microbial Contaminants Associated with Floor Material Types. Available online: https://www.semanticscholar.org/paper/Characterization-of-Microbial-Contaminants-with-Gupta/34640ec23e17fd96c6dab8a55afee6d5b154574e (accessed on 12 March 2020).
- Chai, J.; Donnelly, T.; Wong, T.; Bryce, E. Environmental sampling of hospital surfaces: Assessing methodological quality. Canad. J. Infect. Control. 2018, 33, 138–145. [Google Scholar]
- Williams, A.P.; Avery, L.M.; Killham, K.; Jones, D.L. Persistence of Escherichia coli O157 on farm surfaces under different environmental conditions. J. Appl. Microb. 2005, 98, 1075–1083. [Google Scholar] [CrossRef]
- Coughenour, C. An evaluation of methicillin resistant Staphylococcus aureus survival on five environmental surfaces under two different humidities, with and without the addition of bovine serum albumin. Master’s Thesis, University of Navada, Las Vegas, NV, USA, July 2009. [Google Scholar]
- Exum, N.G.; Kosek, M.N.; Davis, M.F.; Schwab, K.J. Surface sampling collection and culture methods for Escherichia coli in household environments with high fecal contamination. Int. J. Environ. Res. Public Health 2017, 14, 947. [Google Scholar] [CrossRef] [Green Version]
- Lortal, S.; Di Blasi, A.; Madec, M.-N.; Pediliggieri, C.; Tuminello, L.; Tanguy, G.; Fauquant, J.; Lecuona, Y.; Campo, P.; Carpino, S.; et al. Tina wooden vat biofilm: A safe and highly efficient lactic acid bacteria delivering system in PDO Ragusano cheese making. Int. J. Food Microb. 2009, 132, 1–8. [Google Scholar] [CrossRef]
- Miller, A.J.; Brown, T.; Call, J.E. Comparison of wooden and polyethylene cutting boards: Potential for the attachment and removal of bacteria from ground beef. J. Food Prot. 1996, 59, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Lee, J.-Y.; Suk, H.-J.; Lee, S.; Lee, H.; Lee, S.; Yoon, Y. Modeling To Predict Growth/No Growth Boundaries and Kinetic Behavior of Salmonella on Cutting Board Surfaces. J. Food Prot. 2012, 75, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Copes, J.; Pellicer, K.; Malvestiti, L.; Stanchi, N.O. Sobrevivencia en tablas de cocina de madera y plástico inoculadas experimentalmente con Listeria monocytogenes. Survival of Listeria monocytogenes in cutting boards of plastic and wood experimentally contaminated. Available online: http://sedici.unlp.edu.ar/handle/10915/11119 (accessed on 12 March 2020).
- Boucher, S.N.; Chamberlain, A.H.L.; Adams, M.R. Enhanced survival of Campylobacter jejuni in association with wood. J. Food Prot. 1998, 61, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Prechter, S.; Betz, M.; Cerny, G.; Wegener, G.; Windeisen, E. Hygienische Aspekte von Schneidebrettern aus Holz bzw. Kunststoff. Holz als Roh-und Werkstoff 2002, 60, 239–248. [Google Scholar] [CrossRef]
- Baymiev, A.K.; Kuluev, B.R.; Shvets, K.Y.; Yamidanov, R.S.; Matniyazov, R.T.; Chemeris, D.A.; Zubov, V.V.; Alekseev, Y.I.; Mavzyutov, A.R. Modern Approaches to Differentiation of Live and Dead Bacteria Using Selective Amplification of Nucleic Acids. Microbiology 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Rozman, U.; Turk, S.Š. PCR Technique for the Microbial Analysis of Inanimate Hospital Environment. Polym. Chain React. Biomed. Appl. 2016, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Bae, S. Molecular viability testing of viable but non-culturable bacteria induced by antibiotic exposure. Microb. Biotech. 2018, 11, 1008–1016. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, S.G.; Sayles, H.; Colbert, E.M.; Hewlett, A.; Chaika, O.; Smith, P.W. Evaluation of the Relationship between the Adenosine Triphosphate (ATP) Bioluminescence Assay and the Presence of Bacillus anthracis Spores and Vegetative Cells. Int. J. Environ. Res. Public Health 2014, 11, 5708–5719. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kim, H.R.; Jung, J.H.; Lee, K.-B.; Kim, B.C. The development of paper discs immobilized with luciferase/D-luciferin for the detection of ATP from airborne bacteria. Sens. Actuat. B Chem. 2018, 260, 274–281. [Google Scholar] [CrossRef]
- Raia, D.D.; Cannova, L.; Provenzano, S.; Santangelo, O.E.; Piazza, D.; Alagna, E.; Bonanno, V.; Aprea, L.; Firenze, A. Comparison between adenosine triphosphate bioluminescence and aerobic colony count to assess surface sanitation in the hospital environment. Epidemiol. Biostat. Public Health 2018, 15, e12710. [Google Scholar] [CrossRef]
- Bang, J.; Hong, A.; Kim, H.; Beuchat, L.R.; Rhee, M.S.; Kim, Y.; Ryu, J.-H. Inactivation of Escherichia coli O157:H7 in biofilm on food-contact surfaces by sequential treatments of aqueous chlorine dioxide and drying. Int. J. Food Microb. 2014, 191, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Didienne, R.; Defargues, C.; Callon, C.; Meylheuc, T.; Hulin, S.; Montel, M.-C. Characteristics of microbial biofilm on wooden vats (‘gerles’) in PDO Salers cheese. Int. J. Food Microb. 2012, 156, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Cruciata, M.; Gerlando, R.D.; Scatassa, M.L.; Cardamone, C.; Mancuso, I.; Sardina, M.T.; Moschetti, G.; Portolano, B.; Settanni, L. Microbial activation of wooden vats used for traditional cheese production and evolution of the neo-formed biofilms. Appl. Environ. Microbiol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Guillier, L.; Stahl, V.; Hezard, B.; Notz, E.; Briandet, R. Modelling the competitive growth between Listeria monocytogenes and biofilm microflora of smear cheese wooden shelves. Int. J. Food Microb. 2008, 128, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mariani, C.; Briandet, R.; Chamba, J.-F.; Notz, E.; Carnet-Pantiez, A.; Eyoug, R.N.; Oulahal, N. Biofilm Ecology of Wooden Shelves Used in Ripening the French Raw Milk Smear Cheese Reblochon de Savoie. J. Dairy Sci. 2007, 90, 1653–1661. [Google Scholar] [CrossRef]
- Scatassa, M.L.; Gaglio, R.; Macaluso, G.; Francesca, N.; Randazzo, W.; Cardamone, C.; Di Grigoli, A.; Moschetti, G.; Settanni, L. Transfer, composition and technological characterization of the lactic acid bacterial populations of the wooden vats used to produce traditional stretched cheeses. Food Microb. 2015, 52, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Stührk, C.; Fuhr, M.J.; Schwarze, F.W.M.R. Imaging hyphal growth of Physisporinus vitreus in Norway spruce wood by means of confocal laser scanning microscopy (CLSM). Holzforschung 2014, 68, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Wakeling, R.N.; Singh, A.P. Use of confocal microscopy in examining fungi and bacteria in wood. Biofouling 2000, 15, 231–239. [Google Scholar] [CrossRef]
- Robson, A.-L.; Dastoor, P.C.; Flynn, J.; Palmer, W.; Martin, A.; Smith, D.W.; Woldu, A.; Hua, S. Advantages and Limitations of Current Imaging Techniques for Characterizing Liposome Morphology. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Agulló, A.; Freire, M.S.; Ramírez-López, C.; Fernández-Moya, J.; González-Álvarez, J. Valorization of residual walnut biomass from forest management and wood processing for the production of bioactive compounds. Biomass Conv. Bioref. 2020. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hammud, K.K.; Neema, R.R.; Ali, S.G.; Hamza, I.S. Direct Solid Disc as a Novel antibacterial testing method. Int. J. Adv. Pharm. Biol. Chem. 2015, 4, 844–851. [Google Scholar]
- Jain, P.; Shekhar, N.; Gaurav, K. Antimicrobial activity and phytochemical analysis of Eucalyptus tereticornis bark and leaf methanolic extracts. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 126–128. [Google Scholar]
- Nostro, A.; Germanò, M.P.; D’Angelo, V.; Marino, A.; Cannatelli, M.A. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microb. 2000, 30, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.Z.M.; Zayed, M.Z.; Ali, H.M.; El-Kareem, M.S.M.A. Chemical composition, antioxidant and antibacterial activities of extracts from wood branch growing in Egypt. J. Wood Sci. 2016, 62, 548–561. [Google Scholar] [CrossRef] [Green Version]
- Fentahun, M.; Yilkal, B.A.; Amsalu, N.; Alemayehu, A.; Amsalu, G. Antibacterial Evaluation and Phytochemical Analysis of Selected Medicinal Plants against Some Pathogenic Enteric Bacteria in Gozamin District, Ethiopia. J. Pharmacovigil. 2017, 5, 244. [Google Scholar] [CrossRef]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The agar microdilution method-a new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef]
- Dasgupta, A.; Krasowski, M.D. Chapter 10-Therapeutic drug monitoring of antimicrobial, antifungal and antiviral agents. In Therapeutic Drug Monitoring Data (Fourth Edition); Dasgupta, A., Krasowski, M.D., Eds.; Academic Press: Oxford, UK, 2020; pp. 159–197. [Google Scholar]
- Dewanjee, S.; Gangopadhyay, M.; Bhattacharya, N.; Khanra, R.; Dua, T.K. Bioautography and its scope in the field of natural product chemistry. J. Pharm. Anal. 2015, 5, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Choma, I.; Jesionek, W. TLC-Direct Bioautography as a High Throughput Method for Detection of Antimicrobials in Plants. Chromatography 2015, 2, 225–238. [Google Scholar] [CrossRef]
- Masoko, P.; Masiphephethu, M.V. Phytochemical Investigation, Antioxidant and Antimycobacterial Activities of Schkuhria pinnata (Lam) Thell Extracts Against Mycobacterium smegmatis. J. Evid.-Based Complement. Altern. Med. 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Suleimana, M.; McGaw, L.; Naidoo, V.; Eloff, J. Detection of Antimicrobial Compounds by Bioautography of Different Extracts of Leaves of Selected South African Tree Species. Afr. J. Tradit. Complet. Altern. Med. 2009, 7, 64–78. [Google Scholar] [CrossRef]
- Kovács, J.K.; Horváth, G.; Kerényi, M.; Kocsis, B.; Emődy, L.; Schneider, G. A modified bioautographic method for antibacterial component screening against anaerobic and microaerophilic bacteria. J. Microb. Meth. 2016, 123, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Choma, I.M.; Grzelak, E.M. Bioautography detection in thin-layer chromatography. J. Chromatol. A 2011, 1218, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.-Y.; Levasseur, M.; Buisson, D.; Touboul, D.; Eparvier, V. Identification of Antimicrobial Compounds from Sandwithia guyanensis-Associated Endophyte Using Molecular Network Approach. Plants 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukai, A.; Takahashi, K.; Kofujita, H.; Ashitani, T. Antitermite and antifungal activities of thujopsene natural autoxidation products. Eur. J. Wood Prod. 2019, 77, 311–317. [Google Scholar] [CrossRef]











| Material | Microorganism | Objective of the Study | Methods | Main Findings | Reference |
|---|---|---|---|---|---|
| Oak and pine | Staphylococcus aureus, Salmonella enteritidis | Survival of pathogens on wooden surfaces in healthcare facilities | Swabbing, planning, and plate count | Wood surfaces showed antimicrobial properties | [2] |
| Oak wood | Isolates of S. aureus | Oak in hospitals, the worst enemy of Staphylococcus aureus | Direct disc diffusion method | The method was efficient to show the antimicrobial properties of wood | [6] |
| Pine and spruce wood-associated polyphenols | Salmonella, Listeria monocytogenes, S. epidermidis, S. aureus, Candida tropicalis, Saccharomyces cerevisiae | The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms | Microbial cell wall permeability and membrane damage | Several stilbenes showed antimicrobial activities against food pathogens and spoilage organisms | [13] |
| Populus lasiocarpa, P. tomentosa | N/A | Characteristics of antibacterial molecular activities in poplar wood extractives | GC/MS | The molecules were identified that are known to have antimicrobial properties | [16] |
| Abies alba, Q. rubra, European oak, Fagus sylvatica | S.aureus, E. coli, P. aeruginosa, E. faecalis | Direct screening method to assess antimicrobial behavior of untreated wood | Direct disc diffusion method | The method was efficient to show the antimicrobial properties of wood | [7] |
| Larch (Larix decidua Mill.) and Pine (Pinus sylvestris L.) | Bacillus subtilis, S. aureus, Enterococccus faecium, Pseudomonas aeruginosa | Testing the antimicrobial activities of different wood and their parts against different bacteria | Direct disc diffusion, paper disc diffusion | Antimicrobial activities depended upon the type of wood, part of tree, and type of bacteria | [8] |
| Spruce wood (P. abies), glass, polypropylene | L. monocytogenes | An assessment of bacterial transfer from wooden ripening shelves to cheeses | Food contact with surface | Wooden shelves had the lowest transfer rate of bacteria compared to other surfaces | [10] |
| Wood and other cutting boards | S. Enteritidis | Transfer of bacteria to food after cleaning the surfaces | Swabbing and contact press | Efficacy of cleaning methods was tested | [17] |
| Spruce wood shelves | L. monocytogenes | Survival of bacteria after the cleaning and sanitation of cheese preparation boards | Surface contact/blot planning and blending | Bacteria could not be cleaned by brushing and rubbing | [18] |
| Wood and other archeological objects | Variety of microbes | Isolation, characterization, and treatment of microbial agents responsible for the deterioration of archaeological objects | Swabbing | All samples were contaminated with various types of surface degrading microbes | [20] |
| P. sylvestris, Picea abies | E.coli | Effect of extractives and thermal modification on antibacterial properties | Plate count method | Thermal treatments and extraction influence on the antimicrobial properties of wood | [21] |
| P. sylvestris, P. abies | S. aureus, E. faecalis, E. coli, Streptococcus pneumoniae | Antibacterial properties of wooden extracts | Direct (extractive) agar diffusion method | Extractive showed antimicrobial properties | [22] |
| Oak and Douglas fir wood | Wood degrading microbes | Interaction of bacteria and fungi on wooden surfaces | Scanning electron microscopy and plate contact test | Environmental factors’ influence on the microbial interaction on wooden surfaces | [23] |
| Melamine, vinyl chloride, stainless steel, wood, and acrylonitrilebutadiene styrene | Total microbial count | ATP bioluminescence values are significantly different depending upon the material surface properties of the sampling location in hospitals | ATP bioluminescence, SEM, agar stamp/blotting | ATP and colony-forming unit (CFU) were different for wooden surfaces | [25] |
| Wood and plastic | Foodborne bacteria | Analysis of microbial community and food-borne bacteria on restaurant cutting boards | Pyrosequencing | Distribution of 32 genera was identified | [32,33] |
| Wood, plastic, vinyl, quarry clay tile | L. monocytogenes | Efficacy of sonicating swabs to recover microbes from surfaces | Sonicating swab compared to cotton, sponge, and foam swab | Sonicating swabs recovered significantly higher number of microbes | [34] |
| Contact surfaces including wood | Erwinia herbicola | Evaluation of two surface sampling methods for microbial detection on materials by culture and qPCR | Sponge and swabbing used for sample collection and tested by qPCR and plate count | qPCR is more sensitive than culturing, and swabbing was more efficient than sponge | [35] |
| Pterocarpus spp. and poplar wood | White and brown rot fungus | Evaluation of antimicrobial activity of ethanol and aqueous extracts | Wood mass loss calculation and gas chromatography-mass spectrometry | The wood extracts provided protection against degradation owing to antimicrobial properties | [36] |
| Wood and bamboo cutting boards | Vibrio parahaemolyticus | Efficacy of disinfectant to clean the cutting boards | Stirring method for microbial recovery | More microbes were recovered from plastic as compared to wood and bamboo | [37] |
| Wood cutting board and other surfaces | Methicillin-resistant Staphylococcus aureus (MRSA) | Microbial survival on five environmental surfaces | Swabbing | Survival and recovery of microbes depends upon the type of surfaces and moisture conditions | [38] |
| Calabrian and Sicilian chestnut, cedar, cherry, ash, walnut, black pine, poplar | Salmonella, Listeria, E.cli, S. aureus, Lactic acid bacteria (LAB) | Formation and characterization of early bacterial biofilms on different wood typologies | SEM for biofilm observation and paper disc method to determine antimicrobial activities | LAB represent efficient barriers to the adhesion of the main dairy, pathogens, probably due to their acidity and bacteriocin generation | [39] |
| Rubber wood cutting boards, plastic, glass | E. coli, S. aureus | Effectiveness of domestic antibacterial products in decontaminating food contact surfaces | Agar overlay method for microbial recovery | This method gave good results for testing the cleanability of surfaces | [40] |
| Pine and plastic | E. coli, P. aeruginosa, S. aureus, L. monocytogenes | Efficacy of electrolyzed water to inactivate different bacteria on cutting boards | Swabbing | Treatment was efficient for reducing microbial contamination | [41] |
| Poplar wood | E.coli | Confocal spectral microscopy—An innovative tool for the tracking of pathogen agents on contaminated wooden surfaces | Confocal spectral laser microscopy | The microbes could be located for their distribution by this method | [42] |
| Melia azedarach wood | Agrobacterium tumefaciens, Dickeya solani, Erwinia amylovora, P. cichorii, Serratia pylumthica, Fusarium culmorum, Rhizoctonia solani | Wood preservation potential of extracts | Direct diffusion method | Antimicrobial properties were observed using the disc diffusion method | [43] |
| Wooden toothpicks | Variety of microbes | Determination of microbial contamination of wood | Wet preparation techniques, concentration techniques, culture, biochemical tests | Wooden samples were found contaminated with a wide range of microorganisms | [44] |
| Eucalyptus globulus wood | B. subtilis, S. aureus, S. epidermis, E. coli, C. krusei, P. aeruginosa C. parapsilosis, C. glabrata, C. albicans, Saccharomyces cerevisiae | Extraction of bioactive compounds from biomass of forest management and wood processing | Well diffusion method | Antimicrobial compounds were identified | [45] |
| Spruce wood | L. monocytogenes, L. innocua | Comparison of methods for the detection of listeria on porous surfaces | Sponge swabbing | Porosity influences the recovery of microbes | [46] |
| Rubber wood and plastic | L. monocytogenes | Transmission of bacteria from raw chicken meat to cooked chicken meat through cutting boards | Rinsing with normal saline to remove bacteria and meat contact to study transmission | Surfaces play role in transmission of bacteria | [47] |
| Cork wood | S. aureus and E. coli | Evaluation of antimicrobial properties of cork | Agar dilution method | Cork has antimicrobial properties | [48] |
| Wood of P. heldreichii Christ. var. leucodermis | S. aureus, S.epidermidis, E. coli, Enterobacter cloacae, Klebsiella pneumoniae, P. aeruginosa, C. albicans, C. tropicalis, C. glabrata | Chemical composition and biological activity of the essential oil from pine wood | GC and GC/MS and Agar dilution method | Antimicrobial activities of pine wood were identified and characterized | [49] |
| Hardwood, carpets, vinyl and porcelain tiles | S. aureus, Aspergillus niger | Microbial survival on floor materials | Bulk rinsate, agar plate contact, vacuum suction | Microbial survival depends on the recovery method and surface type in hospitals (vet and human) and office buildings | [50] |
| Spruce fir boards (P. abies) | L. monocytogenes, L. innocua | Sanitizing wooden boards used for cheese maturation by means of a steam-mediated heating process | Planning and cotton swabbing and then stomacher | Both recovery methods showed identical results | [51] |
| Pine, poplar, spruce | E. coli, L. monocytogenes, P. expansum | Comparative study of 3 methods for recovering microorganisms from wooden surfaces in the food industry | Planning, grinding and brushing | Humidity, type of wood and microbe, and recovery method influenced the recovery rates | [52] |
| Sapwood and heartwood of the larch | K. pneumoniae,MRSA | Antimicrobial properties of wood against hygienic microbes | Blotting and vibration | Microbial quantities decreased after contact with wood | [53] |
| Quercus baloot | C. albicans | Evaluation of anticandidal potential of wood | Thin-layer chromatography, contact bioautography, disc diffusion method, broth microdilution | Chemical constituents were identified and antimicrobial activities were reported | [54] |
| Maple and Beech | Aerobic mesophilic microorganisms Enterobacteriaceae, Pseudomonas spp. | Hygienic aspects of using wooden and plastic cutting boards | Swabbing | Survival of microbes on different cutting boards before and after cleaning | [55] |
| Pine, larch, spruce, beech, maple, poplar, oak, polyethylene | E.coli, E. faecium | Studying the survival of pathogenic organisms in contact with wood material | PCR and culture-based recovery methods | Wood material has antimicrobial properties | [56,57] |
| Maple wood, steel, ceramic and carpet | Enterobacter aerogenes | Longer contact times increase cross-contamination of Enterobacter aerogenes from surfaces to food | Vortex for microbial recovery plate count method for enumeration | Contact time, food, and surface type all had highly significant effects on the log percent transfer of bacteria | [58] |
| Poplar | E. coli, P. expansum | Assessment of Penicillium expansum and Escherichia coli transfer from poplar crates to apples | Grinding/blending | There is a low transmission of microbes from wood to food (apple) as compared to glass and plastic | [59] |
| Wood, stainless steel, Formica, polypropylene | Salmonella Typhimurium | Recovery and transfer of Salmonella Typhimurium from four different domestic food contact surfaces | Swabbing (vortexting), contact pressing (635 g) and food contact | Number of microbes recovered and their transfer from wood to food was lowest compared to other surfaces | [60] |
| Poplar | B. cereus spores, E. coli cells | Behavior of bacteria on poplar wood crates by impedance measurements | Direct contact (wood in broth) | Microbes in contact with wood present in broth showed decrease in CFU | [61] |
| Poplar and pine | Total microbial counts, S. aureus | Hygienic properties exhibited by single-use wood and plastic packaging on the microbial stability for fish | Vortexing to recover microbes and enumerated by the TEMPO® system | Microbes decreased fastest on wood | [62] |
| Leucaena leucocephala | Trichoderma viride, Fusarium subglutinans, A. niger | Antimicrobial properties of wood treated with natural extracts | GC-MS, direct diffusion method | Antifungal properties were observed | [63] |
| P. abies, Larix decidua | P. funiculosum, P. ochrochloron, A. niger, C.albicans, A. flavus, A. ochraceus, E.coli, S. aureus, Micrococcus flavus, B. cereus, L. monocytogenes, P. aeruginosa, Pectobacterium atrosepticum, Pec. carotovorum, Dickeya solani | Antimicrobial properties of bark and wood extracts | GC-MS, microdilution method | The extracts showed antimicrobial properties, minimum inhibitory concentration (MIC) was determined | [64] |
| Quercus incana | S.aureus, Micrococcus luteus, B. subtilis, E. coli, Ps. pickettii, Shigella flexneri, A. niger, A flavus | Identification, isolation, and characterization of novel antimicrobial compounds | Disc diffusion method, well diffusion method | Two new compounds were identified with their antimicrobial properties | [65] |
| Q. suber, Q. macrocarpa, Q. montana, Q. griffithii, Q. serrata | B. subtilis, S. pneumonia, E. coli, S. aureus, A. niger, Penicillium spp., Fusarium oxysporum | Antimicrobial characterization combining spectrophotometric analysis of different oak species | Paper disc diffusion method and UV spectrophotometric analysis | Antimicrobial properties and active compounds were identified | [66] |
| Rubber wood | Campylobacter jejuni | Transfer of Campylobacter jejuni from raw to cooked chicken via wood and plastic cutting boards | Rinsing with normal saline and then counting CFU by combined most-probable-number (MPN)-PCR | Transfer during uncooked/cooked meat chopping on unscored and scored cutting boards | [67] |
| Heartwood of Scots pine (P. sylvestris) | L. monocytogenes, E. coli | Pine heartwood and glass surfaces: easy method to test the fate of bacterial contamination | Plate count and broth turbidity test | Wood does not allow the survival of microbes | [68] |
| P. sylvestris and P. abies | MRSA, E.coli O157:H7 | Microbial survival on extractive-treated glass cylinders was studied | Vortexting and plate count method | Extractive showed antimicrobial properties | [69] |
| P. sylvestris and P. abies | S. aureus, E. coli, S. pneumoniae, S. enterica Typhimurium | Antimicrobial properties of volatile organic compounds (VOCs) of wood | Glass chamber and plate count method | VOCs reduced the microbial survival | [70] |
| 30 species of trees | B. cereus, S. aureus, L. monocytogenes, Lactobacillus plantarum, E. coli, Salmonella infantis, P. fluorescens, C albicans, Saccharomyces cerevisiae, A. fumigatus, Penicillium brevicompactum | Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms | Broth dilution and agar well dilution methods | Antimicrobial properties were observed | [71] |
| Beech wood (F. sylvatica L.) | Gloeophyllum trabeum, Trametes versicolor | Phenolic extractives of wound-associated wood of beech and their fungicidal effect | Spectrophotometrically analyzed and a paper disc screening test | Wood wounds have defensive chemicals to counter fungal invasion | [72] |
| Hard maple and plastic cutting boards | E. coli | Bacterial retention and cleanability of cutting boards with commercial food-service maintenance practices | Wet sponge swabbing | Microbial recovery was 0.25% and 0.1% from plastic and wood respectively in dry conditions and was similar in wet conditions | [73] |
| Method Name | Procedure | Advantage | Disadvantage | |
|---|---|---|---|---|
| Direct methods | Direct diffusion method (Well and disc) | The wood material is directly placed on microbe-inoculated agar or in a well and incubated for recommended time Presence of the zone of inhibition is considered a positive result | 1. Rapid and time saving 2. Applicable for low amount of material 3. Adapted for screening | 1. Disc preparation time 2. High variability for quantitative applications 3. Studies only the effect of agar-diffused chemicals 4. May require the sterilization of wood samples |
| Culture-based microbial survival test | Initial microbial quantity is inoculated on wood samples and after the incubation time, the microbes are recovered, cultured, and viable cells are counted | 1. Can study the structural and chemical role of wood components 2. Qualitative and quantitative results 3. Applicable for low amount of material | 1. Difficulty in recovering all microbes present in pores 2. Microbial quantification is an extra step needed 3. Only viable cells are identified, while there can be still non-viable infectious cells present | |
| Microscopy | The behavior and distribution of inoculated microbes on wooden structures is observed via microscopy | 1. Rapid and time saving 2. Applicable for low amount of material 3. Adapted for screening | 1. May require the fixation of samples 2. Difficult to differentiate microbial structures from wooden structures 3. May require competencies of image analysis | |
| ATP luminescence | The ATP of microbes on wood is measured | 1. Rapid and easy 2. Applicable for low amount of material 3. Adapted for screening | 1. Difficult to differentiate the microbial ATP from other organic debris 2. Adapted only for solid surfaces | |
| Molecular biology methods | The quantity and viability of microbes is tested via nucleic acid amplification | Accurately measures the microbial survival | 1. Expensive 2. Require sophisticated handling | |
| Extractive based methods | Extractive-based diffusion and dilution method | Extractives are placed on agar or in agar wells, or in broth, after loading on filter paper discs or directly | 1. Adapted for qualitative and quantitative antimicrobial studies 2. Specific chemicals can be extracted depending upon the solvent used | 1. Involves chemical handling Extra step of extraction 2. One solvent cannot extract all active components 3. Does not study the role of structure of wood |
| Bioautography | Extractives are loaded on a chromatographic layer, and then the diffusion of active chemicals is studied for their antimicrobial properties | 1. Adapted for qualitative antimicrobial studies 2. Specific chemicals can be extracted depending upon the solvent used and identified on the basis of their diffusion on the chromatographic layer | 1. Involves chemical handling and extraction 2. One solvent cannot extract all active components 3. Does not study the role of structure of wood 4. Not a quantitative method | |
| Mass spectrometry | The total profile of microbes is measured | 1. Applicable for a low amount of material 2. Accurately measure the content of the active ingredient | For more specific results, the identified compounds are supposed to be tested by other culture-based methods |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, M.T.; Pailhories, H.; Eveillard, M.; Irle, M.; Aviat, F.; Dubreil, L.; Federighi, M.; Belloncle, C. Testing the Antimicrobial Characteristics of Wood Materials: A Review of Methods. Antibiotics 2020, 9, 225. https://doi.org/10.3390/antibiotics9050225
Munir MT, Pailhories H, Eveillard M, Irle M, Aviat F, Dubreil L, Federighi M, Belloncle C. Testing the Antimicrobial Characteristics of Wood Materials: A Review of Methods. Antibiotics. 2020; 9(5):225. https://doi.org/10.3390/antibiotics9050225
Chicago/Turabian StyleMunir, Muhammad Tanveer, Hélène Pailhories, Matthieu Eveillard, Mark Irle, Florence Aviat, Laurence Dubreil, Michel Federighi, and Christophe Belloncle. 2020. "Testing the Antimicrobial Characteristics of Wood Materials: A Review of Methods" Antibiotics 9, no. 5: 225. https://doi.org/10.3390/antibiotics9050225
APA StyleMunir, M. T., Pailhories, H., Eveillard, M., Irle, M., Aviat, F., Dubreil, L., Federighi, M., & Belloncle, C. (2020). Testing the Antimicrobial Characteristics of Wood Materials: A Review of Methods. Antibiotics, 9(5), 225. https://doi.org/10.3390/antibiotics9050225