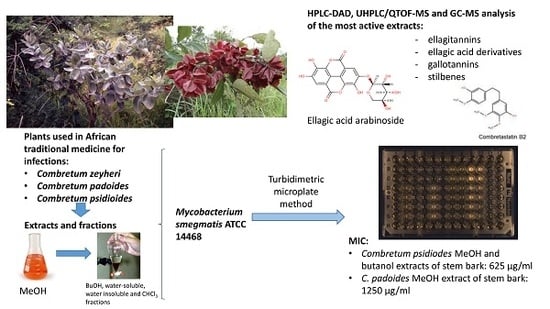
HPLC-DAD and UHPLC/QTOF-MS Analysis of Polyphenols in Extracts of the African Species Combretum padoides, C. zeyheri and C. psidioides Related to Their Antimycobacterial Activity
Abstract
:1. Introduction
2. Results
2.1. Antimycobacterial Effects of Extracts and Fractions
2.2. Phytochemistry
2.2.1. Combretum Psidioides
2.2.2. Combretum Padoides
2.2.3. Combretum Zeyheri
3. Discussion
3.1. Antimycobacterial Effects of the Extracts of the Studied Species of Combretum in Relation to Other Studies on the Antimycobacterial Effects of Combretum spp
3.2. Ellagitannins in the Species of Combretum and Their Suggested Impact on the Antimycobacterial Effects of These Species
3.3. Suggested Antimycobacterial Impact of Ellagic Acid Derivatives in the Species of Combretum Used in This Study
3.4. Stilbenes in Combretum Psidioides and Their Possible Antimycobacterial Effects
3.5. Epigallocatechin Gallate
3.6. Extraction Yield and Its Impact on the Total Antimycobacterial Activity of the Extracts of the Species of Combretum Used in This Study
4. Materials and Methods
4.1. Plant Material and Ethnopharmacological Background Data
4.2. Extraction
4.2.1. Soxhlet Extraction
4.2.2. Solvent Fractionation
4.3. Chromatography and Mass Spectrometry
4.3.1. HPLC-UV/DAD Method I
4.3.2. HPLC-UV/DAD Method II
4.3.3. UHPLC/Q-TOF MS Method
4.3.4. GC-MS Method
4.4. Assays for Testing Antimycobacterial Activity
4.4.1. Agar Disk Diffusion
4.4.2. Microplate Dilution Method
4.5. Calculation of Total Antimycobacterial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018; p. 243. [Google Scholar]
- Semenya, S.S.; Marouy, A. Medicinal Plants used for the treatment of Tuberculosis by Bapedi Traditional Healers in three districts of the Limpopo Province, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoi, S.; Friedland, G. Extensively Drug-Resistant Tuberculosis: A New Face to an Old Pathogen. Annu. Rev. Med. 2009, 60, 307–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Tuberculosis Control–Surveillance, Planning, Financing: WHO Report 2008; WHO/HTM/TB/2008.393; WHO: Geneva, Switzerland, 2008; Available online: http://www.who.int/tb/publications/2008/en/ (accessed on 31 March 2008).
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-Estimation Using Mathematical Modeling. PLoS Med. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egwaga, S.M. The impact of HIV on transmission of tuberculosis in Tanzania. Tuberculosis 2003, 83, 66–67. [Google Scholar] [CrossRef]
- Ballell, L.R.; Field, A.; Duncan, K.; Young, R.J. New small molecule synthetic antimycobacterials. Antimicrob. Agents Chemother. 2005, 49, 2153–2163. [Google Scholar] [CrossRef] [Green Version]
- Vitoria, M.; Granich, R.; Gilks, C.F.; Gunneberg, C.; Hosseini, M.; Were, W.; Raviglione, M.; De Cock, K.M. The Global Fight against HIV/AIDS, Tuberculosis and Malaria. Am. J. Clin. Pathol. 2009, 131, 844–848. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.D.; Cegielsky, J.P.; Nelson, L.J.; Laserson, K.F.; Holtz, T.H.; Finlay, A.; Castro, K.G.; Weyer, K. HIV-infection and Multi-Drug Resistant Tuberculosis- The Perfect Storm. J. Infect. Dis. 2016, 196 (Suppl. 1), S86–S107. [Google Scholar] [CrossRef] [Green Version]
- Matteelli, A.; Roggi, A.; Carvalho, A.C.C. Extensively drug-resistant tuberculosis: Epidemiology and management. Clin. Epidemiol. 2014, 6, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Connolly, L.E.; Edelstein, P.H.; Ramakrishnan, L. Why is long-term therapy required to cure tuberculosis? PLoS Med. 2007, 4, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Kandel, T.R.; Mfenyana, K.; Chandia, J.; Yogeswaran, P. The prevalence of and reasons for interruption of antituberculosis treatment by patients at Mbekweni Health Centre in the King Sabata Dalidyebo (KSD) district in the Eastern Cape Province. S. Afr. Fam. Pract. 2008, 50, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Asres, K.; Bucar, F.; Edelsbrunner, S.; Kartnig, T.; Höger, G.; Thiel, W. Investigations on Antimycobacterial Activity of Some Ethiopian Medicinal Plants. Phytother. Res. 2001, 15, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Jassal, M.; Bishai, W.R. Extensively drug-resistant tuberculosis. Lancet Infect. Dis. 2009, 9, 19–30. [Google Scholar] [CrossRef]
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenges of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Rogoza, L.N.; Salakahutdinov, N.F.; Tolstikov, G.A. Anti-tubercular activity of natural products: Recent developments. In Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry; Research Signpost: Trivandrum, India, 2011; pp. 103–120. [Google Scholar]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Copp, B.R.; Pearce, N.A. Natural product growth inhibitors of Mycobacterium tuberculosis. Nat. Prod. Rep. 2007, 24, 278–297. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, B.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Antimycobacterial, anti-inflammatory and genotoxicity evaluation of plants used for treatment of tuberculosis and related symptoms in South Africa. J. Ethnopharmacol. 2014, 153, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K.C. Tuberculosis and nature’s pharmacy of putative anti-tuberculosis agents. Acta Trop. 2016, 153, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, N.N.; Nvau, J.B.; Oladosu, P.O.; Usman, A.M.; Ibrahim, K.; Boshoff, H.I.; Dowd, C.S.; Orisadipe, A.T.; Aiyelaagbe, O.; Adesomoju, A.A.; et al. Some Nigerian Anti-Tuberculosis Ethnomedicines: A Preliminary Efficacy-Assessment. J. Ethnopharmacol. 2014, 155, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Mitscher, L.A.; Baker, W.R. A search for novel Chemotherapy against tuberculosis amongst natural products. Pure Appl. Chem. 1998, 70, 365–371. [Google Scholar] [CrossRef]
- Okunade, A.L.; Elvin-Lewis, M.P.F.; Lewis, W.H. Natural antimycobacterial metabolites: Current status. Phytochemistry 2004, 65, 1017–1032. [Google Scholar] [CrossRef]
- Iwu, M.M. Handbook of African Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1993; p. 464. [Google Scholar]
- Kuete, V.; Tabopda, T.K.; Ngameni, B.; Nana, F.; Tshikalange, T.E.; Ngadjui, B.T. Antimycobacterial, antibacterial and antifungal activities of Terminalia superba (Combretaceae). S. Afr. J. Bot. 2010, 76, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Breedenkamp, C.L. Combretaceae. In Seed Plants of Southern Africa: Families and Genera; Strelitzia; Leistner, O.A., Ed.; National Botanical Institute: Pretoria, South Africa, 2000; Volume 10, pp. 228–229. [Google Scholar]
- McGaw, L.J.; Rabe, T.; Sparg, S.G.; Jäger, A.K.; Eloff, J.N.; van Staden, J. An investigation of the biological activity of Combretum species. J. Ethnopharmacol. 2001, 75, 45–50. [Google Scholar] [CrossRef]
- Mbwambo, Z.H.; Mushi, N.F.; Nondo, R.S.O.; Kidukuli, A.W.; Mwangomo, D.T. Antibacterial, antioxidant, cytotoxic and preliminary phytochemical screening of extracts from Combretum schumannii Engl. J. Med. Plants Res. 2013, 7, 2483–2488. [Google Scholar]
- Klopper, R.R.; Catelain, C.; Bänninger, V.; Habashi, C.; Steyn, H.M.; De Wet, B.C.; Arnold, T.H.; Gautier, L.; Smith, G.F.; Spichiger, R. Checklist of the Flowering Plants of Sub-Saharan Africa. South African Botanical Diversity Network Report; No. 42. SABONET: Pretoria, South Africa, 2006. [Google Scholar]
- Matu, E.N. Combretum coccineum and Combretum padoides. In Plant Resources of Tropical Africa; Schmelzer, G.H., Gurib-Fakim, A., Arroo, R., Eds.; Medicinal Plants 2; PROTA Foundation: Wageningen, The Netherlands, 2013. [Google Scholar]
- Wickens, G.E. Flora of Tropical East Africa. Combretaceae; East African Community: Arusha, Tanzania, 1973; p. 99. [Google Scholar]
- Fyhrquist, P. Traditional Medicinal Uses and Biological Activities of Some Plant Extracts of African Combretum Loefl., Terminalia L., and Pteleopsis Engl. Species (Combretaceae). Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2007. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern; Africa. E. and S. Livingstone Ltd.: London, UK, 1962; p. 194. [Google Scholar]
- Haerdi, F. Die Eingeborenen-Heilpflanzen des Ulanga-Distriktes Tanganjikas (Ostafrika). Acta Trop. Suppl. 1964, 8, 1–278. [Google Scholar]
- Drummond, R.B.; Coates-Palgrave, K. Common Trees of the Highweld; ISBN: Rhodesia, Zimbabwe, 1973. [Google Scholar]
- Kerharo, J. La Pharmacopée Sénégalaise Traditionelle-Plantes Médicinales et Toxiques; Vigot Freres Edn.: Paris, France, 1974. [Google Scholar]
- Kokwaro, O. Medicinal Plants of East Africa; East African Literature: Nairobi, Kenya, 1976. [Google Scholar]
- Hedberg, I.; Hedberg, O.; Madati, P.; Mshigeni, K.E.; Mshiu, E.N. Inventory of plants used in traditional medicine in Tanzania. I. Plants of the families Acanthaceae-Cucurbitaceae. J. Ethnopharmacol. 1982, 6, 29–60. [Google Scholar] [CrossRef]
- Chhabra, S.C.; Mahunnah, R.L.A.; Mshiu, E.N. Plants used in traditional medicine in Eastern Tanzania. II. Angiosperms (Capparidaceae-Ebenaceae). J. Ethnopharmacol. 1989, 25, 339–359. [Google Scholar] [CrossRef] [Green Version]
- Van Wyk, B.; Van Wyk, P. Field Guide to Trees of Southern Africa; Penguin Random House South Africa: Pretoria, South Africa, 1997; 525p. [Google Scholar]
- Neuwinger, H.D. African Traditional Medicine. A Dictionary of Plant Use and Applications; Medpharm Scientific Publishers: Stuttgart, Germany, 2000; 589p. [Google Scholar]
- Asres, K.; Mazumder, A.; Bukar, F. Antibacterial and antifungal activities of extracts of Combretum molle. Ethiop. Med. J. 2006, 44, 269. [Google Scholar]
- De Morais Lima, G.D.; de Sales, I.R.F.; Caldas Filho, M.R.D.; de Jesus, N.Z.T.; de Sousa Falcão, H.; Barbosa-Filho, J.M.; Cabral, A.G.S.; Lopes Souto, A.; Tavares, J.F.; Batista, L.M. Bioactivities of the genus Combretum (Combretaceae): A Review. Molecules 2012, 17, 9142–9206. [Google Scholar] [CrossRef] [Green Version]
- Rogers, C.B.; Verotta, L. Chemistry and Biological Properties of the African Combretaceae. In Chemistry, Biological and Pharmacological Properties of African Medicinal Plants; Hostettman, K., Chinyanganga, F., Maillard, M., Wolfender, J.L., Eds.; University of Zimbabwe Publications: Harare, Zimbabwe, 1996. [Google Scholar]
- Pegel, K.A.; Rogers, C.B. The characterization of mollic acid-3β-d-xyloside and its genuine aglycone mollic acid, two novel 1α-hydroxycycloartenoids from Combretum molle. J. Chem. Soc. Perkin Trans. 1985, 1, 1711–1715. [Google Scholar] [CrossRef]
- Rogers, C.B. New mono- and bidesmosidic triterpenoids isolated from Combretum padoides leaves. J. Nat. Prod. 1989, 52, 528–533. [Google Scholar] [CrossRef]
- Carr, J.D.; Rogers, C.B. Chemosystematic studies of the genus Combretum (Combretaceae). I. A convenient method of identifying species of this genus by a comparison of the polar constituents extracted from leaf material. S. Afr. J. Bot. 1987, 53, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Kilonda, A.; Lohohola, O.; Toppet, S.; Compernolle, F. Acetylation products of pentacyclic triterpene glucosides from Combretum psidioides. Arkivoc (Iv) 2003, 4, 3–21. [Google Scholar]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Antimicrobial activity of pentacyclic triterpenes isolated from African Combretaceae. Phytochemistry 2003, 63, 81–88. [Google Scholar] [CrossRef]
- Angeh, J.E.; Huang, X.; Swan, G.E.; Möllman, U.; Sattler, I.; Eloff, J.N. Novel antibacterial triterpenoid from Combretum padoides (Combretaceae). Arkivoc (Ix) 2007, 2007, 113–120. [Google Scholar]
- Adnyana, I.K.; Tezuka, Y.; Banskota, A.H.; Xiong, Q.; Tran, K.Q.; Kadota, S. Quadranosides I-V, New Triterpene Glucosides from the Seeds of Combretum quadrangulare. J. Nat. Prod. 2000, 63, 496–500. [Google Scholar] [CrossRef]
- Adnyana, I.K.; Tezuka, Y.; Banskota, A.H.; Tran, K.Q.; Kadota, S. Three New Triterpenoids from the Seeds of Combretum quadrangulare and Their Hepatoprotective Activity. J. Nat. Prod. 2001, 64, 360–363. [Google Scholar] [CrossRef]
- Pietrovski, E.F.; Rosa, K.A.; Facundo, V.A.; Rios, K.; Marques, M.C.A.; Santos, A.R.S. Antinociceptive properties of the ethanolic extract and of the triterpene 3β,6β,16β-trihydroxylup-20(29)-ene obtained from flowers of Combretum leprosum in mice. Pharmacol. Biochem. Behav. 2006, 83, 90–99. [Google Scholar] [CrossRef]
- Runyoro, D.K.B.; Srivastava, S.K.; Darokar, M.P.; Olipa, N.D.; Cosam, C.J.; Mecky, I.N.M. Anticandidiasis agents from a Tanzanian plant, Combretum Zeyheri. Med. Chem. Res. 2013, 22, 1258–1262. [Google Scholar] [CrossRef]
- Katerere, D.R.; Gray, A.I.; Kennedy, A.R.; Nash, R.J.; Waigh, R.D. Cyclobutanes from Combretum albopunctatum. Phytochemistry 2004, 65, 433–438. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Tran, K.Q.; Tanaka, K.; Saiki, I.; Kadota, S. Thirteen Novel Cycloartane-Type Triterpenes from Combretum quadrangulare. J. Nat. Prod. 2000, 63, 57–64. [Google Scholar] [CrossRef]
- Moosophon, P.; Kanokmedhakul, S.; Kanokmedhakul, K. Diarylpropanes and an Arylpropyl Quinone from Combretum griffithii. J. Nat. Prod. 2011, 74, 2216–2218. [Google Scholar] [CrossRef]
- Moosophon, P.; Kanokmedhakul, S.; Kanokmedhakul, K.; Buayairaksa, M.; Noichan, J.; Poopasit, K. Antiplasmodial and Cytotoxic Flavans and Diarylpropanes from the Stems of Combretum griffithii. J. Nat. Prod. 2013, 76, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.D.; Katerere, D.R.P.; Eloff, J.N. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 2004, 93, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hattas, D.; Hjältén, J.; Julkunen-Tiitto, R.; Scogings, P.F.; Rooke, T. Differential phenolic profiles in six African savanna woody species in relation to antiherbivore defence. Phytochemistry 2011, 72, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Letcher, R.M.; Nhamo, L.R. Chemical constituents of the Combretaceae. Part I. Substituted phenanthrenes and 9,10-dihydrophenanthrenes from the heartwood of Combretum apiculatum. J. Chem. Soc. (C) 1971, 1, 3070–3078. [Google Scholar] [CrossRef]
- Letcher, R.M.; Nhamo, L.R.; Gumiro, I.T. Chemical constituents of the Combretaceae. Part II. Substituted phenanthrenes 9,10-dihydrophenanthrenes and a substituted bibenzyl from the heartwood of Combretum molle. J. Chem. Soc. Perkin Trans. 1972, 1, 206–210. [Google Scholar] [CrossRef]
- Letcher, R.M.; Nhamo, L.R. Chemical constituents of the Combretaceae. Part III. Substituted phenanthrenes 9,10-dihydrophenanthrenes, and bibenzyls from the heartwood of Combretum psidioides. J. Chem. Soc. Perkin Trans. 1972, 1, 2941–2947. [Google Scholar] [CrossRef]
- Letcher, R.M.; Nhamo, L.R. Chemical constituents of the Combretaceae. Part IV. Phenanthrene derivatives from the heartwood of Combretum hereroense. J. Chem. Soc. Perkin Trans. 1973, 1, 1179–1181. [Google Scholar] [CrossRef]
- Pettit, G.R.; Singh, S.B.; Niven, M.L.; Hamel, E.; Lin, C.M.; Alberts, D.S.; Garcia-Kendall, D. Antineoplastic agents, 122. Constituents of Combretum caffrum. J. Nat. Prod. 1987, 50, 386–391. [Google Scholar] [CrossRef]
- Pettit, G.R.; Singh, S.B.; Niven, M.L.; Schmidt, J.M. Cell growth inhibitory dihydrophenanthrene and phenanthrene constituents of the African tree Combretum caffrum. Can. J. Chem. 1988, 66, 406–413. [Google Scholar] [CrossRef]
- Eloff, J.N.; Famakin, J.O.; Katerere, D.R.P. Isolation of an antibacterial stilbene from Combretum woodii (Combretaceae) leaves. Afr. J. Biotechnol. 2005, 4, 1684–5315. [Google Scholar]
- Mushi, N.F.; Innocent, E.; Kidukuli, A.W. Cytotoxic and antimicrobial activities of substituted phenanthrenes from the roots of Combretum adenogonium Steud Ex, A. Rich (Combretaceae). J. Intercult. Ethnopharmacol. 2014, 4, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ogan, A.U. The alkaloids in the leaves of Combretum micranthum. Studies on West African medicinal plants. VII. Planta Med. 1972, 21, 210–217. [Google Scholar] [CrossRef]
- Welch, C.R. Chemistry and Pharmacology of Kinkéliba (Combretum micranthum), a West African Medicinal Plant. Ph.D. Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 2014; 177p. [Google Scholar]
- Brookes, K.B.; Doudoukina, O.V.; Katsoulis, L.C.; Veale, D.J.H. Uteroactive constituents from Combretum kraussii. S. Afr. J. Chem. 1999, 52, 127–132. [Google Scholar]
- Asami, Y.; Ogura, T.; Otake, N.; Mishimura, T.; Xinsheng, Y.; Sakurai, T.; Nagasawa, H.; Sakuda, S.; Tatsuta, K. Isolation and Synthesis of a New Bioactive Ellagic Acid Derivate from Combretum yunnanensis. J. Nat. Prod. 2003, 66, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Samdumu, F.B. Characterization of Antimicrobial Compounds from Combretum paniculatum, a Plant with Proven Anti-HIV Replication Activity. Ph.D. Thesis, Phytomedicine Programme, Department of Paraclinical Sciences, Faculty of Veterinary Sciences, University of Pretoria, Pretoria, South Africa, 2007; 119p. [Google Scholar]
- Jossang, A.; Pousset, J.L.; Bodo, B. Combreglutinin, a hydrolyzable tannin from Combretum glutinosum. J. Nat. Prod. 1994, 57, 732–737. [Google Scholar] [CrossRef]
- Traore, C.M.L. Assessment on Mali’s Medicinal Combretaceae. Pharmacy Thesis, University of Bamako, Bamako, Mali, 1999; 165p. [Google Scholar]
- Elegami, A.A.; El-Nima, E.I.; Tohami, M.E.; Muddathir, A.K. Antimicrobial activity of some species of the family Combretaceae. Phytother. Res. 2002, 16, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, R.T.; Defende, C.P.; da Silva Mirowski, P.; Freire, T.V.; Weber, S.S.; Garcez, W.S.; da Rosa Guterres, Z.; de Fátima Cepa Matos, M.; Garcez, F.R. Myricitrin from Combretum lanceolatum Exhibits Inhibitory Effect on DNA-Topoisomerase Type II and Protective Effect Against In Vivo Doxorubicin-Induced Mutagenicity. J. Med. Food 2020. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, P.; Mwasumbi, L.; Hæggström, C.-A.; Vuorela, H.; Hiltunen, R.; Vuorela, P. Ethnobotanical and antimicrobial investigation of some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J. Ethnopharmacol. 2002, 79, 169–177. [Google Scholar] [CrossRef]
- Fyhrquist, P.; Mwasumbi, L.; Haeggström, C.-A.; Vuorela, H.; Hiltunen, R.; Vuorela, P. Antifungal activity of selected species of Terminalia, Pteleopsis and Combretum (Combretaceae) collected in Tanzania. Pharm. Biol. 2004, 42, 308–317. [Google Scholar] [CrossRef]
- Eloff, J.N. The antibacterial activity of 27 southern African members of the Combretaceae. S. Afr. J. Sci. 1999, 95, 148–152. [Google Scholar]
- Schmelzer, G.H. Combretum zeyheri. In Plant Resources of Tropical Africa; Schmelzer, G.H., Gurib-Fakim, A., Arroo, R., Eds.; Medicinal Plants 2; PROTA Foundation: Wageningen, The Netherlands, 2013. [Google Scholar]
- Mämmelä, P.; Savolainen, H.; Lindroos, L.; Kangas, J.; Vartiainen, T. Analysis of oak tannins by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2000, 891, 75–83. [Google Scholar]
- Piddock, L.J.V.; Williams, K.J.; Ricci, V. Accumulation of rifampicin by Mycobacterium aurum, Mycobacterium smegmatis and Mycobacterium tuberculosis. J. Antimicrob. Chemoth. 2000, 45, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, J.S.; Sharma, D. Mycobacterium smegmatis and tuberculosis. Trends Microbiol. 2002, 10, 68–69. [Google Scholar] [CrossRef]
- Eloff, J.N. A proposal on expressing the antibacterial activity of plant extracts—A small first step in applying scientific knowledge to rural primary health care in South Africa. S. Afr. J. Sci. 2000, 96, 116–118. [Google Scholar]
- Campo, M.; Pinelli, P.; Romani, A. Hydrolyzable Tannins from Sweet Chestnut Fractions obtained by a Sustainable and Eco-friendly Industrial Process. Nat. Prod. Commun. 2014, 11, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Diop, E.H.A.; Queiroz, E.F.; Marcourt, L.; Kicka, S.; Rudaz, S.; Diop, T.; Soldati, T.; Wolfender, J.E. Antimycobacterial activity in a single-cell infection assay of ellagitannins from Combretum aculeatum and their bioavailable metabolites. J. Ethnopharmacol. 2019, 238, 111832. [Google Scholar] [CrossRef]
- Oudane, B.; Boudemagh, D.; Bounekhel, M.; Sobhi, W.; Vidal, M.; Broussy, S. Isolation, characterization, antioxidant activity, and protein precipitating capacity of the hydrolyzable tannin punicalagin from pomegranate yellow peel (Punica granatum). J. Mol. Struct. 2018, 1156, 390–396. [Google Scholar] [CrossRef]
- Pfundstein, B.; Desouky, S.K.L.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compunds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Salih, E.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Kosinska, A.; Diering, S.; Prim, D.; Héritier, J.; Andlauer, W. Phenolic compounds profile of strawberry fruits of Charlotte cultivar. J. Berry Res. 2013, 3, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Puppala, M.; Ponder, J.; Suryanarayana, P.; Reddy, G.B.; Petrash, J.M.; La Barbera, D.V. The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS ONE 2012, 7, e31399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breytenbach, J.C.; Malan, S.F. Pharmacochemical properties of Combretum zeyheri. S. Afr. J. Sci. 1989, 85, 372–374. [Google Scholar]
- Malcolm, S.A.; Sofowora, E.A. Antimicrobial activities of selected Nigerian folk remedies and their constituent plants. Antimicrobial properties of Balanites. Lloydia 1969, 32, 512–517. [Google Scholar]
- Uba, A.; Ibrahim, K.; Agbo, E.B.; Makinde, A.A. In vitro inhibition of Mycobacterium smegmatis and Mycobacterium tuberculosis by some Nigerian Medicinal Plants. East Cent. Afr. J. Pharm. Sci. 2003, 6, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Magwenzi, R.; Nyakunu, C.; Mukanganyama, S. The Effect of selected Combretum species from Zimbabwe on the growth and drug efflux systems of Mycobacterium aurum and Mycobacterium smegmatis. J. Microb. Biochem. Technol. 2014, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Masoko, P.; Nxumalo, K.M. Validation of Antimycobacterial Plants used by Traditional Healers in Three Districts of Limpopo Province (South Africa). Evid.-Based Compl. Alt. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Eldeen, I.M.S.; van Staaden, J. Cyclooxygenase inhibition and antimycobacterial effects of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2008, 74, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Eldeen, I.M.S.; Van Staaden, J. Antimycobacterial activity of some trees used in South African traditional medicine. S. Afr. J. Bot. 2007, 73, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Pires, D.; Aínsa, J.A.; Gracia, B.; Mulhovo, S.; Duarte, A.; Anes, E.; Ferreira, M.-J.U. Antimycobacterial evaluation and preliminary phytochemical investigation of selected medicinal plants traditionally used in Mosambique. J. Ethnopharmacol. 2011, 137, 114–120. [Google Scholar] [CrossRef]
- Nyambuya, T.; Mautsa, R.; Mukanganyama, S. Alkaloid extracts from Combretum zeyheri inhibit the growth of Mycobacterium smegmatis. BMC Complem. Altern. Med. 2017, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Coulidiati, T.H.; HMillogo-Kone ALamien-Meda MYougbare-Ziebrou, J. Millogo-Rasolodimby and O. G. Nacoulma. Antioxidant and Antibacterial Activities of Two Combretum Species from Burkina Faso. Res. J. Med. Plant 2011, 5, 42–53. [Google Scholar]
- Silva, O.; Gomes, E.T.; Wolfender, J.L.; Marston, A.; Hostettman, K. Application of high performance liquid chromatography coupled with ultraviolet spectroscopy and electrospray mass spectrometry to the characterization of ellagitannins from Terminalia macroptera roots. Pharm. Res. 2000, 17, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.; Viegas, S.; de Mello-Sampayo, C.; Joao, P.; Costa, M.; Serrano, R.; Cabrita, J.; Gomes, E.T. Anti-Helicobacter pylori activity of Terminalia macroptera root. Fitoterapia 2012, 83, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Hsu, F.-L.; Cheng, J.-T. Antihypertensive activity of corilagin and chebulinic acid, tannins from Lumnitzera racemosa. J. Nat. Prod. 1993, 56, 629–632. [Google Scholar] [CrossRef]
- Guo, J.S.; Wang, S.X.; Li, X.; Zhu, T.R. Studies on the antibacterial constituents of Geranium sibiricum L. Acta Pharmacol. Sin. 1987, 22, 28–32. [Google Scholar]
- Hatano, T.; Kusuda, M.; Inada, K.; Ogawa T-o Shiota, S.; Tsuchiya, T.; Yoshida, T. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 2005, 66, 2047–2055. [Google Scholar] [CrossRef]
- Shiota, S.; Shimizu, M.; Sugiyama, J.; Morita, Y.; Mizushima, T.; Tsushiya, T. Mechanisms of action of Corilagin and Tellimagrandin I that remarkably potentiate the activity of β-lactams against methicillin-resistant Staphyloccus aureus. Microbiol. Immunol. 2004, 48, 67–73. [Google Scholar] [CrossRef]
- Li, N.; Luo, M.; Fu, Y.; Zu, Y.; Wang, W.; Zhang, L.; Yao, L.; Zhao, C.; Sun, Y. Effect of Corilagin on Membrane Permeability of Esherichia coli, Staphylococcus aureus and Candida albicans. Phytother. Res. 2013, 10, 1517–1523. [Google Scholar]
- Anokwuru, C.P.; Sinisi, A.; Samie, A.; Taglialatela-Scafati, O. Antibacterial and antioxidant constituents of Acalypha wilkensiana. Nat. Prod. Res. 2015, 29, 1180–1183. [Google Scholar] [CrossRef]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.-L.; Mocek, U.M.; Kittel, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.M.; Hardy, W.D.; et al. Isolation and Characterization of Antimicrobial Compounds in Plant Extracts against Multidrug-Resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e61594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Shiota, S.; Mizushima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Marked potentiation of activity of beta-lactams against methicillin-resistant Staphylococcus aureus by corilagin. Antimicrob. Agents Chemother. 2001, 45, 3198–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, Y.; Toida, T.; Kusano, G.; Imai, J. Specific inhibition of formation of acid fastness in mycobacteria by 3,3′-di-O-methylellagic acid. Experientia 1979, 35, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Shilpi, J.A.; Ali, M.T.; Saha, S.; Hasan, S.; Gray, A.I.; Seidel, V. Molecular docking studies on InhA, MabA and PanK enzymes from Mycobacterium tuberculosis of ellagic acid derivatives from Ludwigia adscendens and Trewia nudiflora. Silico Pharmacol. 2015, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Machumi, F.; Midiwo, J.O.; Jacob, M.R.; Khan, S.I.; Tekwani, B.L.; Zhang, J.; Walker, L.A.; Muhammad, I. Phytochemical, Antimicrobial and Antiplasmodial Investigations of Terminalia brownii. Nat. Prod. Commun. 2013, 8, 761–764. [Google Scholar] [CrossRef] [Green Version]
- Pettit, G.R.; Singh, S.B. Isolation, structure and synthesis of combretastatin A2, A3 and B2. Can. J. Chem. 1987, 65, 2390–2396. [Google Scholar] [CrossRef]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Phytochemical and antimicrobial investigations of stilbenoids and flavonoids isolated from three species of Combretaceae. Fitoterapia 2012, 83, 932–940. [Google Scholar] [CrossRef]
- Sun, T.; Qin, B.; Gao, M.; Yin, Y.; Wang, C.; Zang, S.; Li, X.; Zhang, C.; Xin, Y.; Jiang, T. Effects of epigallocatechin gallate on the cell-wall structure of Mycobacterium smegmatis mc2 155. Nat. Prod. Res. 2015, 29, 2122–2124. [Google Scholar] [CrossRef]
- Anand, P.K.; Kaul, D.; Sharma, M. Green tea polyphenol inhibits Mycobacterium tuberculosis survival within human macrophages. Int. J. Biochem. Cell Biol. 2006, 38, 600–609. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of Catechin as One of the Flavonoids from Combretum albiflorum Bark Extract That Reduces the Production of Quorum-Sensing Controlled Virulence Factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Masoko, P.; Picard, J.; Eloff, J.N. The antifungal activity of twenty-four Southern African Combretum species (Combretaceae). S. Afr. J. Bot. 2007, 73, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Fyhrquist, P.; Laakso, I.; Garcia Marco, S.; Julkunen-Tiitto, R.; Hiltunen, R. Antimycobacterial activity of ellagitannin and ellagic acid derivative rich crude extracts and fractions of five selected species of Terminalia used for treatment of infectious diseases in African traditional medicine. S. Afr. J. Bot. 2014, 90, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Julkunen-Tiitto, R.; Rousi, M.; Bryant, J.; Sorsa, S.; Keinänen, M.; Sikanen, H. Chemical diversity of several Betulaceae species: Comparison of phenolics and terpenoids in northern birch stems. Trees 1996, 11, 16–22. [Google Scholar] [CrossRef]
- Taulavuori, K.; Julkunen-Tiitto, R.; Hyöky, V.; Taulavuori, E. Blue mood for superfood. J. Nat. Prod. Commun. 2013, 8, 791–794. [Google Scholar] [CrossRef] [Green Version]
- Brenton, A.G.; Godfrey, A.R. Accurate Mass Measurement: Terminology and Treatment of Data. J. Am. Soc. Mass Spectrom. 2010, 21, 1821–1835. [Google Scholar] [CrossRef] [Green Version]
- Collins, L.A.; Franzblau, S.G. Microplate Alamar Blue Assay versus BACTEC 460 System for High-Troughput Screening of Compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimyc. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Muñoz, C.; Vaillant, F. Metabolic Fate of Ellagitannins: Implications for Health, and Research Perspectives for Innovative Functional Foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef]








| Combretum Species | Uses of Plant Species in TM | Way of Preparation in Traditional Medicine | Antibacterial and Antimycobacterial Effects | Identified Compounds |
|---|---|---|---|---|
| C. padoides Engl. & Diels, Thicket bush-willow (P. Fyhrquist & L. Mwasumbi Voucher 1697070) | Leaves and roots: For treatment of snakebites, wounds, hookworms, bloody diarrhea, malaria and conjunctivitis [30,37,41]. | Water extracts of leaves for snakebites and root decoctions for hookworms. Crushed leaves for wounds [30,37,41]. | Extracts of the leaves gave excellent inhibitory effects against E. coli and E. aerogenes at 0.8 mg/mL [80]; Stem bark extracts gave good antibacterial and antifungal effects [78,79]. 1α,23β-dihydroxy-12-oleanen-29-oic-acid-23β-O-α-4-acetylrhamnopyranoside and 1,22-dihydroxy-12-oleanen-30-oic acid from leaves gave antibacterial effects against S. aureus and E. coli [50]. Crude MeOH extract of stem bark and its BuOH fraction inhibit Mycobacterium smegmatis (AI is 50.5% of the inhibitory effect of rifampicin, MIC 1250–2500 µg/mL, IZD 26.5 mm). * | Leaves contain rhamnose based mono- and bi-desmosidic triterpenoids [46,47], leaves contain oleanane-type triterpenoid glycoside, 1α,23β-dihydroxy-12-oleanen-29-oic-acid-23β-O-α-4-acetylrhamnopyranoside and two known triterpenoids; 1,22-dihydroxy-12-oleanen-30-oic acid and 24-ethylcholesta-7,22,25-trien-O-β-d-glucopyranoside [50]. Punicalagin and a corilagin derivative and twenty-four other unknown ellagitannins, five gallotannins and five ellagic acid derivatives; ellagic acid arabinoside and methyl ellagic acid xyloside in an antimycobacterial BuOH extract of stem bark. * |
| C. psidioides Welw. Velvet bush-willow C. psidioides, continued (P. Fyhrquist & L. Mwasumbi Voucher 1697037) | Leaves and roots: Aphrodisiac, diarrhea, malaria, back pain, galactogogue [34]. Leaves and roots used for diarrhea, muscle pain, oedema [78]. | Decoctions of roots, leaf extracts or leaves mixed with maize porridge (Ugali) for treatment of diarrhea and oedema [78]. | Broad-spectrum antibacterial profile in our earlier investigation [78] as well as some antifungal effects [79]. Crude methanol extract and its butanol and CHCl3 fractions were growth inhibitory against Mycobacterium smegmatis (MIC 625-2500 µg/mL). * | Substituted phenanthrenes and bibenzyls from heartwood and stem bark [63]; Oleanane and ursane pentacyclic triterpene glucosides from root bark [48]. Combretastatin B-2 was tentatively identified in a methanol extract of the stem bark. Sixteen ellagitannins including corilagin and its isomer and punicalagin, six gallotannins including 1,6-di-O-galloyl-β-d-glucose, epigallocatechin gallate, four ellagic acid derivatives and protocatechuic acid were identified in a butanol extract of the stem bark. * |
| C. zeyheri Sond., Large-fruited bush-willow (P. Fyhrquist & L. Mwasumbi Voucher 1697020) | Leaves, roots and stem bark: Wounds, cough (TB?), malaria, diarrhea, inflammation, scorpion sting, back pain, dysentery, hook worms, tooth ache, eye lotion [35,37,38]. Diarrhea, cancer (stomach tumors) [78]. | Smoke of burnt leaves inhaled for cough (TB?), water extracts of dried leaves for colic, crushed leaves for rheumatism and joint pain [35]; hot water decoctions of roots for diarrhea, dysentery and ankylostomiasis [37]; Pounded roots cooked in porridge for hookworms and dysentery, ground roots cooked and applied to wounds, root decoctions for stomach-ache, cough, pneumonia, vomiting, stomach ulcers and diarrhea, leaf infusions for cough, stem bark infusion for leprosy [80]. Roots, leaves and stem bark made into decoctions or mixed in maize porridge for diarrhea and stomach tumors [78]. | Stem bark and leaf extracts inhibitory against several bacteria [81,82]; Fruit, stem bark and root extracts show good antibacterial potential. Triterpenoids from leaves evaluated for anti-Candida effects; terminolic acid was found to be the most active compound. SAR: oleanane and ursane type triterpenoids were the most active ones [54]. We found that extracts of stem bark and roots inhibit the growth of Mycobacterium smegmatis, the BuOH fraction of the roots being especially active (IZ 23 mm).* | Triterpenoids and saponins from the leaves [47]; Three unidentified antimicrobial compounds were isolated from the leaves and stem bark [82]; Ursolic acid, maslinic acid, 2α,3β-dihydroxyurs- 12-en-28-oic acid, 6β-hydroxymaslinic acid and terminolic acid from leaves [54]. A root butanol extract contained six ellagic acid derivatives including methyl-ellagic acid xyloside, di-methyl-ellagic acid xyloside and 3,3′-Di-O-methyl-4-O-(n′′-O-galloyl-β-d-xylopyranosyl) ellagic acid, fifteen ellagitannins including punicalagin and nine gallotannins including hexagalloylglucose.* |
| Combretum Species, Extracts and Fractions | M. Smegmatis ATCC 14468 | AI |
|---|---|---|
| C. padoides SCr | 26.5 ± 0.5 | 0.50 |
| C. padoides WI | 18.0 ± 2.0 | 0.34 |
| C. padoides BuOH | 26.5 ± 0.5 | 0.50 |
| C. padoides CHCl3 | 17.5 ± 0.5 | 0.33 |
| C. zeyheri SCr 12 | 20.5 ± 0.5 | 0.39 |
| C. zeyheri WI | 0.0 | 0.00 |
| C. zeyheri Ws | 17.0 ± 3.0 | 0.32 |
| C. zeyheri BuOH | 19.0 ± 2.0 | 0.36 |
| C. zeyheri CHCl3 | 14.0 ± 0.0 | 0.27 |
| C. zeyheri SCr 43 | 21.0 ± 0.0 | 0.40 |
| C. zeyheri WS | 18.5 ± 0.5 | 0.35 |
| C. zeyheri WI | 17.5 ± 0.5 | 0.33 |
| C. zeyheri BuOH | 20.5 ± 0.5 | 0.39 |
| C. zeyheri CHCl3 | 0.0 | 0.00 |
| C. zeyheri RCr 33 | 16.0 ± 0.8 | 0.30 |
| C. zeyheri WS | 0.0 | 0.00 |
| C. zeyheri WI | 16.0 ± 1.6 | 0.30 |
| C. zeyheri BuOH | 23.0 ± 1.6 | 0.44 |
| C. zeyheri CHCl3 | 14.0 ± 0.0 | 0.27 |
| C. psidioides SCr | 29.00 ± 0.5 | 0.53 |
| C. psidioides BuOH | 21.5 ± 2.0 | 0.41 |
| C. psidioides WI | 0.0 | 0.00 |
| C. psidioides CHCl3 | 25.5 ± 0.7 | 0.48 |
| C. psidioides L Cr | 0.0 | 0.00 |
| Rifampicin | 52.5 ± 0.5 | 1.00 |
| Extracts | M. Smegmatis ATCC 14468 | Total Activity (mL/g) |
|---|---|---|
| C. psidioides stem bark: | ||
| crude methanol extract | 625 (IC94) | 313.44 |
| butanol soluble fraction | 2500 (IC95) | 98.76 |
| chloroform soluble fraction | 2500 (IC90) | 22.89 |
| C. padoides stem bark: | ||
| crude methanol extract | 1250 (IC93) | 85.12 |
| butanol soluble fraction | 2500 (IC97) | 190.12 |
| Pure compounds: | ||
| Corilagin * | 1000 (IC94) | |
| Ellagic acid ** | 500 (IC98) | |
| Rifampicin | 3.90 (IC91) |
| Compounds | Molecular Formula | Rt HPLC-DAD or GC* (min) | Rt UHPLC (min) | Measured [M-H]− (m/z) or * GC-MS | Exact Mass (calc.) | ppm Value | UVλmax (in MeOH) | Peak Area % (280 nm) |
|---|---|---|---|---|---|---|---|---|
| Gallic acid | C7H6O5 | 1.636 | 1.334 | 169.0149 | 170.0213 | 8.2834 | 216, 272 | 9.2848 |
| Flavonoid? | 1.933 | 210, 224, 278 | 0.1800 | |||||
| Protocatechuic acid | C7H6O4 | 3.718 | 2.733 | 153.0196 | 154.0264 | 6.5352 | 210, 218, 260, 294 | 4.6595 |
| O-hydroxycinnamic acid like | 4.628 | 210, 230, 280, 310 | 0.7723 | |||||
| Unknown ellagitannin | 7.187 | 214, 258, 376 | ||||||
| Unknown ellagitannin | 7.731 | 216, 260, 378 | 4.0268 | |||||
| Sanguiin H-4 | C27H22O18 | 8.208 | 4.448 | 633.0746 | 634.0798 | 4.1070 | 216, 258, 376 | 5.7862 |
| Gallotannin | 8.979 | 258, 218 | 0.1717 | |||||
| Epigallocatechin gallate | C22H18O11 | 9.427 | 458.0843 | 210, 276 | 0.5409 | |||
| Unknown ellagitannin | 9.673 | 216, 258, 378 | 2.5987 | |||||
| 1,6-Di-O-galloyl-β-d-Glucose | C20H20O14 | 10.022 | 4.700 | 483.0801 | 484.0846 | 6.8312 | 216, 276 | 2.7681 |
| Unknown ellagitannin | 10.406 | 214, 252, 384 | 0.1968 | |||||
| Unknown ellagitannin | 10.778 | 216, 260, 374 | 4.4198 | |||||
| Galloylglucose | 10.975 | 216, 276 | 2.0395 | |||||
| Unknown ellagitannin | 11.178 | 214, 260, 381 | 0.0556 | |||||
| Unknown ellagitannin | 11.454 | 218, 256, 378 | 0.8819 | |||||
| Corilagin | C27H22O18 | 12.244 | 633.0750 | 634.0798 | 4.7388 | 215, 258, 380 | 5.7058 | |
| Epigallocatechin like (flavan-3-ol) | 13.391 | 210, 282, 320 | 0.5197 | |||||
| Combretastatin B-2 | C17H20O5 | *13.489 | *304 | 304.1311 | 6.9049 | |||
| Galloylglucose | 14.281 | 216, 276 | 4.2740 | |||||
| β-Punicalagin | C48H28O30 | 14.956 | 5.427 | 1083.1541 | 1084.0654 | 25.6045 | 218, 256, 380 | 6.9297 |
| Epigallocatechin like | 17.368 | 210, 284 | ||||||
| Ellagic acid derivative | 17.565 | 254, 380 | 3.3214 | |||||
| Gallotannin | 17.591 | 216, 282 | ||||||
| Ellagic acid derivative | 18.935 | 254, 362 | 0.3382 | |||||
| 3′-O-methyl-4-O-(β-d-xylopyranosyl) ellagic acid (main compound) | C20H16O12 | 19.846 | 8.201 | 447.0574 | 448.0636 | 3.5790 | 254, 364 | 8.4825 |
| Gallotannin (not PGG) | 21.184 | 939.1000 | 214, 280 | 0.1463 | ||||
| Unknown ellagitannin | 21.815 | 218, 248, 368 | 0.5505 | |||||
| Ellagic acid derivative | 24.922 | 254,362 | 3.1564 | |||||
| Unknown ellagitannin | 26.444 | 210, 264, 398 | 0.0953 | |||||
| Unknown ellagitannin | 27.163 | 218, 254, 360 | 0.3147 | |||||
| Unknown ellagitannin | 28.605 | 218, 256, 362 | 2.0192 | |||||
| Unknown ellagitannin | 31.560 | 216, 256, 360 | 1.8907 | |||||
| Unknown ellagitannin | 3.688 | 218, 256, 360 | 0.9801 | |||||
| Hexagalloylglucose | C48H36O30 | 38.299 | 1092.1278 | 218, 280 | 0.1879 |
| Compounds | Molecular Formula | Rt HPLC-DAD (min) | Rt UHPLC (min) | Measured [M-H]− (m/z) | Exact Mass (calc.) | ppm Value | UVλmax (in MeOH) | Peak Area % (280 nm) |
|---|---|---|---|---|---|---|---|---|
| 1-O-galloyl-β-d-glucose (syn. β-glucogallin) | C13H16O10 | 1.577 | 331.0685 | 332.0738 | 7.5514 | 216, 276 | 0.1894 | |
| Gallic acid | C7H6O5 | 1.763 | 1.306 | 169.0157 | 170.0213 | 13.0167 | 216, 272 | 1.2354 |
| Ellagitannin | 3.245 | 215, 260, 380 | 1.8848 | |||||
| Ellagitannin | 7.815 | 3.170 | 466.0264 | 214, 258, 380 | 3.1453 | |||
| Ellagitannin | 8.408 | 216, 258, 376 | 1.4324 | |||||
| Ellagitannin | 8.829 | 3.387 | 1083.0591 | 216, 256, 374 | 8.3917 | |||
| Gallotannin | 9.370 | 216, 252 | 2.6083 | |||||
| Ellagitannin | 9.740 | 210, 258, 380 | 0.7464 | |||||
| Corilagin derivative | C27H22O18 | 10.114 | 3.768 | 633.0750 | 634.0798 | 4.7388 | 216, 258, 379 | 10.9106 |
| Ellagitannin | 10.413 | 220, 256, 374 | 0.4238 | |||||
| α-Punicalagin anomer or Punicacortein D | C48H28O30 | 10.903 | 3.853 | 1083.0587 | 1084.0654 | 1.0156 | 216, 260, 376 | 14.9380 |
| Ellagitannin | 11.382 | 3.903 | 1083.0581 | 216, 258, 376 | 3.9407 | |||
| Ellagitannin | 11.764 | 216, 258, 380 | 0.1157 | |||||
| Ellagitannin | 12.073 | 218, 258, 378 | 4.6576 | |||||
| Ellagitannin | 12.385 | 224, 256, 374 | 0.7334 | |||||
| Ellagitannin | 12.619 | 210, 260, 382 | 0.2170 | |||||
| Ellagitannin | 12.858 | 214, 256, 378 | 2.7210 | |||||
| Unknown | 13.373 | 214, 248, 292 | 0.1398 | |||||
| Flavonoid; Ampelopsine like | 13.643 | 210, 280 | 0.5307 | |||||
| Ellagitannin | 14.054 | 218, 258, 368 | 0.1152 | |||||
| Ellagitannin | 14.500 | 220, 260, 376 | 0.8036 | |||||
| Flavonoid | 15.137 | 210, 276, 370 | 0,9277 | |||||
| Ellagitannin | 15.693 | 216, 256, 380 | 5.3916 | |||||
| Ellagitannin | 16.015 | 210, 256, 364 | 0.2770 | |||||
| Ellagitannin | 16.219 | 216, 260, 378 | 0.7760 | |||||
| Ellagitannin | 16.849 | 216, 284, 382 | 0.3067 | |||||
| Ellagitannin | 17.668 | 218, 260, 378 | 0.8513 | |||||
| Gallotannin | 17.975 | 218, 280 | 0.2328 | |||||
| Ellagic acid derivative | 18.556 | 254, 380 | 0.4644 | |||||
| Stilbene like compound? | 18.809 | 210, 235, 382 | 0.9999 | |||||
| Gallotannin | 18.991 | 210, 280 | 0.4259 | |||||
| Ellagic acid derivative | 19.960 | 254, 362 | 0.2292 | |||||
| Ellagitannin | 20.253 | 4.502 | 1085.0719 | 210, 254, 362 | 0.5726 | |||
| Ellagic acid arabinoside | C19H14O12 | 20.792 | 8.316 | 433.0391 | 434.0480 | -2.5402 | 254, 362 | 1.6928 |
| 3′-O-methyl-4-O-(β-d-xylopyranosyl) ellagic acid | C20H16O12 | 21.055 | 8.616 | 447.0564 | 448.0636 | 1.3421 | 254, 368 | 4.4746 |
| Gallotannin (not PGG) | 22.163 | 214, 284 | 0.1343 | |||||
| Ellagic acid derivative | 26.258 | 254, 360 | 1.5195 | |||||
| Ellagitannin | 28.596 | 216, 254, 362 | 0.1066 | |||||
| Ellagitannin | 30.121 | 216, 254, 362 | 1.5374 | |||||
| Ellagitannin | 33.134 | 216, 256, 360 | 2.2061 | |||||
| Ellagitannin | 36.269 | 218, 256, 362 | 0.8344 |
| Compounds | Molecular Formula | Rt HPLC-DAD (min) | Rt UHPLC (min) | Measured [M-H]− (m/z) | Exact Mass (calc.) | ppm Value | UVλmax (in MeOH) | Peak Area % (280 nm) |
|---|---|---|---|---|---|---|---|---|
| Gallic acid | C7H6O5 | 1.760 | 1.173 | 169.0140 | 170.0213 | 2.9583 | 216, 272 | 1.1995 |
| Gallic acid derivative | 2.191 | 216, 274 | 0.6905 | |||||
| Protocatechuic acid | C7H6O4 | 3.865 | 2.593 | 153.0198 | 154.0264 | 218, 220, 260, 294 | 0.1245 | |
| Cinnamic acid like | 7.025 | 0.1205 | ||||||
| Gallotannin or gallic acid derivative | 8.516 | 216, 274 | 3.3768 | |||||
| Corilagin isomer | 8.835 | 216, 254, 374 | 2.8165 | |||||
| Gallotannin | 9.325 | 216, 258 | 0.1997 | |||||
| Ellagitannin | 10.080 | 218, 258, 378 | 0.0607 | |||||
| Gallotannin | 10.481 | 216, 275 | 3.2150 | |||||
| Ellagitannin | 11.190 | 216, 256, 378 | 0.2296 | |||||
| Gallotannin | 11.486 | 216, 277 | 2.2688 | |||||
| Ellagitannin | 11.984 | 220, 256, 378 | 0.3862 | |||||
| Ellagitannin | 12.804 | 216, 256, 380 | 2.2389 | |||||
| Eriodictyol like | 13.899 | 210, 279 | 2.2785 | |||||
| Ellagitannin | 14.531 | 222, 258, 380 | 0.1015 | |||||
| Gallotannin | 15.008 | 5.352 | 647.0894 | 216, 276 | 20.0817 | |||
| Punicalagin | C48H28O30 | 15.645 | 5.485 | 1083.0574 | 1084.0654 | −0.1847 | 216, 256, 381 | 4.3927 |
| Batatasin like | 16.068 | 210, 272 | 0.0939 | |||||
| Ellagitannin | 16.562 | 256, 372 | 0.0601 | |||||
| Salidrosid and arbutin like | 17.497 | 217, 274, 276, 298 | 0.7553 | |||||
| Ellagic acid derivative | 18.559 | 5.718 | 435.1310 | 254, 380 | 0.4557 | |||
| Lignan? | 19.014 | 210, 272 | 2.3806 | |||||
| Lignan? | 19.462 | 210, 274 | 0.0977 | |||||
| Lignan? | 20.178 | 210, 276 | 3.1006 | |||||
| Ellagic acid derivative | 20.773 | 5.818 | 581.1903 | 254, 362 | 0.4712 | |||
| Procyanidin B-3 like | 23.022 | 212, 274 | 2.8707 | |||||
| Ellagic acid derivative | 24.693 | 8.033 | 435.1310 | 250, 366 | 1.4412 | |||
| Ellagitannin | 26.230 | 210, 254, 360 | 1.2651 | |||||
| Lignan? | 26.778 | 210, 274 | 1.3692 | |||||
| Procyanidin B-3 like | 27.670 | 212, 276 | 1.0954 | |||||
| Stilbene or lignan? | 27.956 | 212, 264 | 0.8470 | |||||
| Procyanidin B-3 like | 28.803 | 212, 272 | 1.2008 | |||||
| Methyl-ellagic acid xyloside | C20H16O12 | 29.922 | 8.582 | 447.0566 | 448.0636 | 1.7895 | 210, 274 | 1.5613 |
| Ellagitannin | 31.578 | 0.0677 | ||||||
| Ellagitannin | 32.116 | 216, 256, 366 | 0.1269 | |||||
| Ellagitannin | 33.103 | 216, 256, 360 | 0.7444 | |||||
| Di-methyl-ellagic acid xyloside | C21H18O12 | 34.243 | 9.931 | 461.0741 | 462.0792 | 5.8559 | 251, 366 | 0.9611 |
| Ellagitannin | 34.998 | 10.192 | 763.0788 | 226, 268, 370 | 0.2394 | |||
| 3,3′-Di-O-methyl-4-O-(n′′-O-galloyl-β-d-xylopyranosyl) ellagic acid | C28H22O16 | 36.257 | 12.312 | 613.0849 | 614.0900 | 4.4039 | 218, 256, 358 | 0.2287 |
| Gallotannin or ellagitannin according to the UV abs max | 36.931 | 12.829 | 727.0566; 765.0566 | 216, 252, 366 | 1.1001 | |||
| Hexagalloylglucose | C48H36O30 | 39.583 | 1092.1278 | 216, 278 | 5.0497 | |||
| Ellagitannin | 39.956 | 13.994 | 817.4212 | 210, 252, 363 | 0.4127 | |||
| Gallotannin Ellagitannin? | 44.414 | 17.000 | 725.4118 | 216, 274 | 0.3647 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fyhrquist, P.; Salih, E.Y.A.; Helenius, S.; Laakso, I.; Julkunen-Tiitto, R. HPLC-DAD and UHPLC/QTOF-MS Analysis of Polyphenols in Extracts of the African Species Combretum padoides, C. zeyheri and C. psidioides Related to Their Antimycobacterial Activity. Antibiotics 2020, 9, 459. https://doi.org/10.3390/antibiotics9080459
Fyhrquist P, Salih EYA, Helenius S, Laakso I, Julkunen-Tiitto R. HPLC-DAD and UHPLC/QTOF-MS Analysis of Polyphenols in Extracts of the African Species Combretum padoides, C. zeyheri and C. psidioides Related to Their Antimycobacterial Activity. Antibiotics. 2020; 9(8):459. https://doi.org/10.3390/antibiotics9080459
Chicago/Turabian StyleFyhrquist, Pia, Enass Y. A. Salih, Satu Helenius, Into Laakso, and Riitta Julkunen-Tiitto. 2020. "HPLC-DAD and UHPLC/QTOF-MS Analysis of Polyphenols in Extracts of the African Species Combretum padoides, C. zeyheri and C. psidioides Related to Their Antimycobacterial Activity" Antibiotics 9, no. 8: 459. https://doi.org/10.3390/antibiotics9080459
APA StyleFyhrquist, P., Salih, E. Y. A., Helenius, S., Laakso, I., & Julkunen-Tiitto, R. (2020). HPLC-DAD and UHPLC/QTOF-MS Analysis of Polyphenols in Extracts of the African Species Combretum padoides, C. zeyheri and C. psidioides Related to Their Antimycobacterial Activity. Antibiotics, 9(8), 459. https://doi.org/10.3390/antibiotics9080459