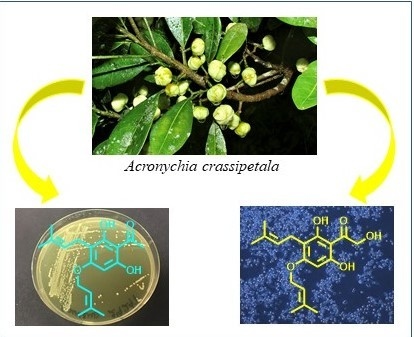
Potent Antibacterial Prenylated Acetophenones from the Australian Endemic Plant Acronychia crassipetala
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antibacterial Assays
3.4.1. ESKAPE Pathogens
3.4.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentrations (MBC)
3.5. Cytotoxic Assays
3.5.1. Cell Culture and Reagents
3.5.2. Cell Growth/Survival Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Locher, C.; Semple, S.J.; Simpson, B.S. Traditional Australian Aboriginal medicinal plants: An untapped resource for novel therapeutic compounds? Future Med. Chem. 2013, 5, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Crisp, M.D.; Laffan, S.; Linder, H.P.; Monro, A. Endemism in the Australian flora. J. Biogeogr. 2001, 28, 183–198. [Google Scholar] [CrossRef]
- Williams, K.J.; Ford, A.; Rosauer, D.F.; De Silva, N.; Mittermeier, R.; Bruce, C.; Larsen, F.W.; Margules, C. Forests of East Australia: The 35th biodiversity hotspot. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 295–310. [Google Scholar] [CrossRef]
- Simpson, B.S.; Bulone, V.; Semple, S.J.; Booker, G.W.; McKinnon, R.A.; Weinstein, P. Arid awakening: New opportunities for Australian plant natural product research. Rangel. J. 2016, 38, 467–478. [Google Scholar] [CrossRef]
- Lassak, E.V.; McCarthy, T. Australian Medicinal Plants; New Holland: Sydney, Australia, 2011. [Google Scholar]
- Carroll, A.R.; Arumugan, G.; Quinn, R.J.; Redburn, J.; Guymer, G.; Grimshaw, P. Grandisine A and B, novel indolizidine alkaloids with human δ-opioid receptor binding affinity from the leaves of the Australian rainforest tree Elaeocarpus grandis. J. Org. Chem. 2005, 70, 1889–1892. [Google Scholar] [CrossRef] [PubMed]
- Boyle, G.M.; D’Souza, M.M.; Pierce, C.J.; Adams, R.A.; Cantor, A.S.; Johns, J.P.; Maslovskaya, L.; Gordon, V.A.; Reddell, P.W.; Parsons, P.G. Intra-lesional injection of the novel PKC activator EBC-46 rapidly ablates tumors in mouse models. PLoS ONE 2014, 9, e108887. [Google Scholar] [CrossRef]
- Stelfonta. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/stelfonta (accessed on 29 April 2020).
- De Ridder, T.R.; Campbell, J.E.; Burke-Schwarz, C.; Clegg, D.; Elliot, E.L.; Geller, S.; Kozak, W.; Pittenger, S.T.; Pruitt, J.B.; Riehl, J.; et al. Randomized controlled clinical study evaluating the efficacy and safety of intratumoral treatment of canine mast cell tumors with tigilanol tiglate (EBC-46). J. Vet. Intern. Med. 2020, 1–15. [Google Scholar] [CrossRef]
- Panizza, B.J.; de Souza, P.; Cooper, A.; Roohullah, A.; Karapetis, C.S.; Lickliter, J.D. Phase I dose-escalation study to determine the safety, tolerability, preliminary efficacy and pharmacokinetics of an intratumoral injection of tigilanol tiglate (EBC-46). EBioMedicine 2019, 50, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.D.; Olsson, M.A.; Choudhury, M.A.; McMillan, D.J.; Cullen, J.K.; Parsons, P.G.; Bernhardt, P.V.; Reddell, P.W.; Ogbourne, S.M. Antibacterial 5α-spirostane saponins from the fruit of Cordyline manners-suttoniae. J. Nat. Prod. 2019, 82, 2809–2817. [Google Scholar] [CrossRef]
- Epifano, F.; Fiorito, S.; Genovese, S. Phytochemistry and pharmacognosy of the genus Acronychia. Phytochemistry 2013, 95, 12–18. [Google Scholar] [CrossRef]
- Hnawia, E.; Hassani, L.; Deharo, E.; Maurel, S.; Waikedre, J.; Cabalion, P.; Bourdy, G.; Valentin, A.; Jullian, V.; Fogliani, B. Antiplasmodial activity of New Caledonia and Vanuatu traditional medicines. Pharm. Biol. 2011, 49, 369–376. [Google Scholar] [CrossRef]
- Wu, T.-S.; Wang, M.-L.; Jong, T.-T.; McPhail, A.T.; McPhail, D.R.; Lee, K.-H. X-Ray crystal structure of acrovestone, a cytotoxic principle from Acronychia pedunculata. J. Nat. Prod. 1989, 52, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, D.; De Rocca Serra, D.; Bighelli, A.; Minh Hoi, T.; Huy Thai, T.; Casanova, J. Composition and antimicrobial activity of the essential oil of Acronychia pedunculata (L.) Miq. from Vietnam. Nat. Prod. Res. 2008, 22, 393–398. [Google Scholar] [CrossRef]
- Dictionary of Natural Products 28.2 Online, Taylor & Francis Group: 2020. Available online: http://dnp.chemnetbase.com/ (accessed on 15 April 2020).
- Li, X.-J.; Zhang, H.-Y. Western-medicine-validated anti-tumor agents and traditional Chinese medicine. Trends Mol. Med. 2008, 1, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.J.; Wu, M.H.; Chen, W.P.; Kuo, S.C.; Su, M.J. Electrophysiological characteristics of antiarrhythmic potential of acrophyllidine, a furoquinoline alkaloid isolated from Acronychia halophylla. Drug Dev. Res. 2000, 50, 170–185. [Google Scholar] [CrossRef]
- Su, C.-R.; Kuo, P.-C.; Wang, M.-L.; Liou, M.-J.; Damu, A.G.; Wu, T.-S. Acetophenone derivatives from Acronychia pedunculata. J. Nat. Prod. 2003, 66, 990–993. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. Leaf essential oils of the Australian species of Acronychia (Rutaceae). J. Essent. Oil Res. 2004, 16, 597–607. [Google Scholar] [CrossRef]
- Tsukayama, M.; Kikuchi, M.; Kawamura, Y. Regioselective synthesis of prenylphenols. Syntheses of naturally occurring 4′-Alkenyloxy-2′, 6′-dihydroxy-3′-(3-methyl-2-butenyl) acetophenones. Chem. Lett. 1994, 23, 1203–1206. [Google Scholar] [CrossRef]
- Kumar, V.; Karunaratne, V.; Sanath, M.; Meegalle, K.; MacLeod, J.K. Two fungicidal phenylethanones from Euodia lunu-ankenda root bark. Phytochemistry 1990, 29, 243–245. [Google Scholar] [CrossRef]
- Alberte, R.S.; Roschek, W.P.; Li, D. Anti-Inflammatory and Anti-Allergy Extracts from Nettle. U.S. Patent Application 12/502,543, 14 January 2010. Available online: https://patents.google.com/patent/US20100009927A1/en (accessed on 29 April 2020).
- Sivakumar, P.M.; Sheshayan, G.; Doble, M. Experimental and QSAR of acetophenones as antibacterial agents. Chem. Biol. Drug Des. 2008, 72, 303–313. [Google Scholar] [CrossRef]
- Gul, H.I.; Denizci, A.A.; Erciyas, E. Antimicrobial evaluation of some Mannich bases of acetophenones and representative quaternary derivatives. Arzneimittelforschung 2002, 52, 773–777. [Google Scholar]
- Tomás-Barberán, F.; Iniesta-Sanmartín, E.; Tomás-Lorente, F.; Rumbero, A. Antimicrobial phenolic compounds from three Spanish Helichrysum species. Phytochemistry 1990, 29, 1093–1095. [Google Scholar] [CrossRef]
- Laks, P.E.; Pruner, M.S. Flavonoid biocides: Structure/activity relations of flavonoid phytoalexin analogues. Phytochemistry 1989, 28, 87–91. [Google Scholar] [CrossRef]
- Instant JChem; Instant JChem 18.8.0; ChemAxon: Budapest, Hungary, 2018.
- Mathekga, A.D.M.; Meyer, J.J.M.; Horn, M.M.; Drewes, S.E. An acylated phloroglucinol with antimicrobial properties from Helichrysum caespititium. Phytochemistry 2000, 53, 93–96. [Google Scholar] [CrossRef]
- Santander, J.; Otto, C.; Lowry, D.; Cuellar, M.; Mellado, M.; Salas, C.; Rothhammer, F.; Echiburu-Chau, C. Specific gram-positive antibacterial activity of 4-hydroxy-3-(3-methyl-2-butenyl) Acetophenone Isolated from Senecio graveolens. Br. Microbiol. Res. J. 2015, 5, 94–106. [Google Scholar] [CrossRef]
- Socolsky, C.; Arena, M.E.; Asakawa, Y.; Bardón, A. Antibacterial prenylated acylphloroglucinols from the fern Elaphoglossum yungense. J. Nat. Prod. 2010, 73, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Hettiarachchi, D.S.; Locher, C.; Longmore, R.B. Antibacterial compounds from the root of the indigenous Australian medicinal plant Carissa lanceolata R.Br. Nat. Prod. Res. 2011, 25, 1388–1395. [Google Scholar] [CrossRef]


| Position | δC | mult. | δH (J in Hz) | NOESY | HMBC |
|---|---|---|---|---|---|
| 1 | 204.4 | C | |||
| 2 | 68.2 | CH2 | 4.63, s | 1 | |
| 1′ | 102.4 | C | |||
| 2′ | 161.5 | C | |||
| 3′ | 107.0 | C | |||
| 4′ | 162.5 | C | |||
| 5′ | 91.3 | CH2 | 6.06, s | 6′-OH, 1‴ | 1′, 3′, 4′, 6′, 1 b |
| 6′ | 160.7 | C | |||
| 1″ | 20.9 | CH2 | 3.11, d (J = 7.2) | 2′-OH, 5″ | 2′, 3′, 4′, 2″, 3″ |
| 2″ | 122.9 | CH | 5.07, t (J = 7.2) | 4″ | 1″, 4″, 5″ |
| 3″ | 129.9 | C | |||
| 4″ | 25.4 | CH3 | 1.59, s | 2″ | 2″, 3″, 5″ |
| 5″ | 17.5 | CH3 | 1.67, s | 1″ | 2″, 3″, 4″ |
| 1‴ | 64.6 | CH2 | 4.52, d (J = 6.4) | 5′, 4‴ | 4′, 2‴, 3‴ |
| 2‴ | 119.2 | CH | 5.40, t (J = 6.4) | 5‴ | 4‴, 5‴ |
| 3‴ | 137.8 | C | |||
| 4‴ | 18.0 | CH3 | 1.70, s | 1‴ | 2‴, 3‴, 5‴ |
| 5‴ | 25.5 | CH3 | 1.75, s | 2‴ | 2‴, 3‴, 4‴ |
| 2-OH | a | ||||
| 2’-OH | 13.45, s | 1″ | 1′, 2′, 3′ | ||
| 6’-OH | 10.97, s | 5′ | 1′, 5′, 6′ |
| Compound | MIC75 (µM) c | MBC (µM) d | ||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus 29247 | S. aureus 25923 | E. faecium 35667 | E. faecium c15 | S. aureus 29247 | S. aureus 25923 | E. faecium 35667 | E. faecium c15 | |
| 1 | a | 78.1 | a | a | a | a | a | a |
| 2 | 5.1 | 2.6 | 20.6 | 20.6 | 20.6 | 20.6 | 20.6 | a |
| Chloramphenicol | 9.7 | 9.7 | 9.7 | 9.7 | b | b | b | b |
| Compound | IC50 (µM) a | ||||
|---|---|---|---|---|---|
| HaCaT | HDF | NFF | HEK293 | HepG2 | |
| 1 | 15.8 | 16.7 | 29.1 | 13.4 | 21.3 |
| 2 | 8.5 | 6.4 | 13.3 | 8.6 | 9.7 |
| Doxorubicin | 0.010 | 0.060 | 0.360 | 0.006 | 0.430 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.D.; Olsson, M.A.; McMillan, D.J.; Cullen, J.K.; Parsons, P.G.; Reddell, P.W.; Ogbourne, S.M. Potent Antibacterial Prenylated Acetophenones from the Australian Endemic Plant Acronychia crassipetala. Antibiotics 2020, 9, 487. https://doi.org/10.3390/antibiotics9080487
Tran TD, Olsson MA, McMillan DJ, Cullen JK, Parsons PG, Reddell PW, Ogbourne SM. Potent Antibacterial Prenylated Acetophenones from the Australian Endemic Plant Acronychia crassipetala. Antibiotics. 2020; 9(8):487. https://doi.org/10.3390/antibiotics9080487
Chicago/Turabian StyleTran, Trong D., Malin A. Olsson, David J. McMillan, Jason K. Cullen, Peter G. Parsons, Paul W. Reddell, and Steven M. Ogbourne. 2020. "Potent Antibacterial Prenylated Acetophenones from the Australian Endemic Plant Acronychia crassipetala" Antibiotics 9, no. 8: 487. https://doi.org/10.3390/antibiotics9080487
APA StyleTran, T. D., Olsson, M. A., McMillan, D. J., Cullen, J. K., Parsons, P. G., Reddell, P. W., & Ogbourne, S. M. (2020). Potent Antibacterial Prenylated Acetophenones from the Australian Endemic Plant Acronychia crassipetala. Antibiotics, 9(8), 487. https://doi.org/10.3390/antibiotics9080487