Enhanced Historical Limestone Protection by New Organic/Inorganic Additive-Modified Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrophobic Commercial Coatings and Organic/Inorganic Additives
2.2. Samples Characterizations
2.3. UV-Accelerated Aging Tests and Outdoor Exposure
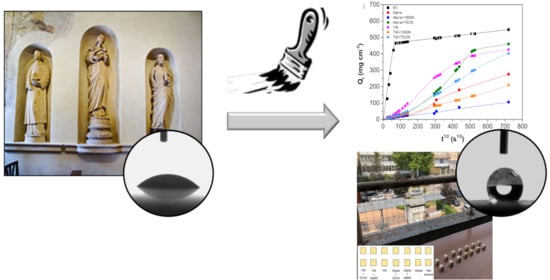
3. Results and Discussion
3.1. Modified Acrylic and Siloxane-Based Protectives Performances
3.2. UV-Accelerated Aging Tests and Exposure in a Real Polluted Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosquera, M.J.; Pinho, L.; Facio, D.S.; Elhaddad, F. New nanomaterials for conservation of cultural heritage: Consolidants, hydrophobic and self-cleaning products. In Science, Technology and Cultural Heritage, Proceedings of the 2nd International Congress on Science and Technology for the Conservation of Cultural Heritage, Sevilla, Spain, 24–27 June 2014; CRC Press: Boca Raton, FL, USA; pp. 121–126.
- Eyssautier-Chuine, S.; Calandra, I.; Vaillant-Gaveau, N.; Fronteau, G.; Thomachot-Schneider, C.; Hubert, J.; Pleck, J.; Gommeaux, M. A new preventive coating for building stones mixing a water repellent and an eco-friendly biocide. Prog. Org. Coat. 2018, 120, 132–142. [Google Scholar] [CrossRef]
- Pino, F.; Fermo, P.; La Russa, M.; Ruffolo, S.; Comite, V.; Baghdachi, J.; Pecchioni, E.; Fratini, F.; Cappelletti, G. Advanced mortar coatings for cultural heritage protection. Durability towards prolonged UV and outdoor exposure. Environ. Sci. Pollut. Res. 2017, 24, 12608–12617. [Google Scholar] [CrossRef]
- Varotsos, C.; Tzanis, C.; Cracknell, A. The enhanced deterioration of the cultural heritage monuments due to air pollution. Environ. Sci. Pollut. Res. 2009, 16, 590–592. [Google Scholar] [CrossRef]
- Charola, A.E. Salts in the deterioration of porous materials: An overview. J. Am. Inst. Conserv. 2018, 39, 327–343. [Google Scholar] [CrossRef]
- Piacenti, F. Chemistry for the conservation of the cultural heritage. Sci. Total. Environ. 1994, 143, 113–120. [Google Scholar] [CrossRef]
- Iñigo, A.C.; García-Talegón, J.; Vicente-Tavera, S.; Martín-González, S.; Casado-Marín, S.; Vargas-Muñoz, M.; Pérez-Rodríguez, J.L. Colour and ultrasound propagation speed changes by different ageing of freezing/thawing and cooling/heating in granitic materials. Cold Reg. Sci. Technol. 2013, 85, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P. Applied microbiology and biotechnology in the conservation of stone cultural heritage materials. Appl. Microbiol. Biotechnol. 2006, 73, 291–296. [Google Scholar] [CrossRef]
- Farkas, O.; Siegesmund, S.; Licha, T.; Török, Á. Geochemical and mineralogical composition of black weathering crusts on limestones from seven different European countries. Environ. Earth Sci. 2018, 77, 1–20. [Google Scholar] [CrossRef]
- La Russa, M.F.; Comite, V.; Aly, N.; Barca, D.; Fermo, P.; Rovella, N.; Antonelli, F.; Tesser, E.; Aquino, M.; Ruffolo, S.A. Black crusts on Venetian built heritage, investigation on the impact of pollution sources on their composition. Eur. Phys. J. Plus 2018, 133, 370. [Google Scholar] [CrossRef]
- Comite, V.; Fermo, P. The effects of air pollution on cultural heritage: The case study of Santa Maria delle Grazie al Naviglio Grande (Milan). Eur. Phys. J. Plus 2018, 133, 556. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; McCarthy, T.J. Self-assembly is not the only reaction possible between alkyltrichlorosilanes and surfaces: Monomolecular and oligomeric covalently attached layers of dichloro- and trichloroalkylsilanes on silicon. Langmuir 2000, 16, 7268–7274. [Google Scholar] [CrossRef]
- Doherty, B.; Pamplona, M.; Selvaggi, R.; Miliani, C.; Matteini, M.; Sgamellotti, A.; Brunetti, B. Efficiency and resistance of the artificial oxalate protection treatment on marble against chemical weathering. Appl. Surf. Sci. 2007, 253, 4477–4484. [Google Scholar] [CrossRef]
- Rizzarelli, P.; La Rosa, C.; Torrisi, A. Testing a fluorinated compound as a protective material for calcarenite. J. Cult. Herit. 2001, 2, 55–62. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Silica/silicone nanofilament hybrid coatings with almost perfect superhydrophobicity. Chem. Phys. Chem. 2013, 14, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Carretti, E.; Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat. 2004, 49, 282–289. [Google Scholar] [CrossRef]
- Mazzola, M.; Frediani, P.; Bracci, S.; Salvini, A. New strategies for the synthesis of partially fluorinated acrylic polymers as possible materials for the protection of stone monuments. Eur. Polym. J. 2003, 39, 1995–2003. [Google Scholar] [CrossRef]
- Sabatini, V.; Farina, H.; Montarsolo, A.; Pargoletti, E.; Ortenzi, M.A.; Cappelletti, G. Fluorinated polyacrylic resins for the protection of cultural heritages: The effect of fluorine on hydrophobic properties and photochemical stability. Chem. Lett. 2018, 47, 280–283. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A Mater. Sci. Process. 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Zielecka, M.; Bujnowska, E.; Bajdor, K. Siloxane-containing polymer matrices as coating materials. J. Coat. Technol. Res. 2007, 4, 275–281. [Google Scholar] [CrossRef]
- Stewart, A.; Schlosser, B.; Douglas, E.P. Surface modification of cured cement pastes by silane coupling agents. ACS Appl. Mater. Interfaces 2013, 5, 1218–1225. [Google Scholar] [CrossRef]
- Rao, Q.; Chen, K.; Wang, C. Facile preparation of self-healing waterborne superhydrophobic coatings based on fluoroalkyl silane-loaded microcapsules. RSC Adv. 2016, 6, 53949–53954. [Google Scholar] [CrossRef]
- Atici, E.G.; Kasapgil, E.; Anac, I.; Erbil, H.Y. Methyltrichlorosilane polysiloxane filament growth on glass using low cost solvents and comparison with gas phase reactions. Thin Solid Films 2016, 616, 101–110. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N.; Savva, A.; Panayiotou, C. Superhydrophobic polymer-particle composite films produced using various particle sizes. Surf. Interface Anal. 2012, 44, 870–875. [Google Scholar] [CrossRef]
- Tsakalof, A.; Manoudis, P.; Karapanagiotis, I.; Chryssoulakis, I.; Panayiotou, C. Assessment of synthetic polymeric coatings for the protection and preservation of stone monuments. J. Cult. Herit. 2007, 8, 69–72. [Google Scholar] [CrossRef]
- Dillon, C.E.; Lagalante, A.F.; Wolbers, R.C. Acrylic emulsion paint films: The effect of solution pH, conductivity, and ionic strength on film swelling and surfactant removal. Stud. Conserv. 2014, 59, 52–62. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Zhang, H.; Liu, X.; Chen, H. Preparation and Properties of Fluorinated Poly(ethyl methacrylate-co-butyl acrylate). Polym. Sci. Ser. B 2019, 61, 163–169. [Google Scholar]
- Zhang, H.; Liu, Q.; Liu, T.; Zhang, B. The preservation damage of hydrophobic polymer coating materials in conservation of stone relics. Prog. Org. Coat. 2013, 76, 1127–1134. [Google Scholar] [CrossRef]
- Pargoletti, E.; Motta, L.; Comite, V.; Fermo, P.; Cappelletti, G. The hydrophobicity modulation of glass and marble materials by different Si-based coatings. Prog. Org. Coat. 2019, 136, 105260. [Google Scholar] [CrossRef] [Green Version]
- Cappelletti, G.; Fermo, P.; Pino, F.; Pargoletti, E.; Pecchioni, E.; Fratini, F.; Ruffolo, S.A.; La Russa, M.F. On the role of hydrophobic Si-based protective coatings in limiting mortar deterioration. Environ. Sci. Pollut. Res. 2015, 22, 17733–17743. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Manoudis, P.N.; Karapanagiotis, I. Fabrication of water repellent coatings using waterborne resins for the protection of the cultural heritage. Macromol. Symp. 2013, 331, 158–165. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Morelli, A.; Pipoli, M.; Frigione, M. Oleo/Hydrophobic Coatings Containing Nano-Particles for the Protection of Stone Materials Having Different Porosity. Coatings 2018, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, A.; De Vecchi, G.P.; Pitturi, L.M.; Vitturi, L. Le pietre tenere dei Colli Berici. In Atti e Memorie dell’Accademia Patavina di Scienze, Lettere ed Arti; Accademia Patavina di Scienze: Padua, Italy, 1976; pp. 69–100. [Google Scholar]
- Marchesini, B.; Biscontin, G.; Frascati, S. Alterazione delle pietre tenere dei colli Berici. In Atti XXVI Congresso ATI; Associazione Termotecnica Italiana: Rome, Italy, 1972; pp. 1–23. [Google Scholar]
- Scrivano, S.; Gaggero, L.; Gisbert Aguilar, J. Micro-porosity and minero-petrographic features influences on decay: Experimental data from four dimension stones. Constr. Build. Mater. 2018, 173, 342–349. [Google Scholar] [CrossRef]
- Scrivano, S.; Gaggero, L. An experimental investigation into the salt-weathering susceptibility of building limestones. Rock Mech. Rock Eng. 2020, 53, 5329–5343. [Google Scholar] [CrossRef]
- Galan, E. Carbonate rocks; alteration and control of stone quality: Some consideration. In Proceedings of the Atti del I Simposio Internernazionale La Conservazione dei Monumenti nel Bacino nel Mediterraneo, Bari, Italy, 7–10 June 1989. [Google Scholar]
- Fermo, P.; Cappelletti, G.; Cozzi, N.; Padeletti, G.; Kaciulis, S.; Brucale, M.; Merlini, M. Hydrophobizing coatings for cultural heritage. A detailed study of resin/stone surface interaction. Appl. Phys. A 2014, 116, 341–348. [Google Scholar] [CrossRef]
- NORMAL 43/93 Misure Colorimetriche di Superfici Opache; CNR-ICR: Roma, Italy, 1993.
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 coatings for Cultural Heritage. Prog. Org. Coat. 2012, 74, 186–191. [Google Scholar] [CrossRef]
- UNI EN 15801. Conservation of Cultural Property. Test Methods. Determination of Water Absorption by Capillarity; UNI: Milan, Italy, 2010. [Google Scholar]
- UNI EN 15803. Conservation of Cultural Property. Test Methods. Determination of Water Vapour Permeability; UNI: Milan, Italy, 2009. [Google Scholar]
- Manoudis, P.N.; Tsakalof, A.; Karapanagiotis, I.; Zuburtikudis, I.; Panayiotou, C. Fabrication of super-hydrophobic surfaces for enhanced stone protection. Surf. Coatings Technol. 2009, 203, 1322–1328. [Google Scholar] [CrossRef]
- Sabatini, V.; Pargoletti, E.; Longoni, M.; Farina, H.; Ortenzi, M.A.; Cappelletti, G. Stearyl methacrylate co-polymers: Towards new polymer coatings for mortars protection. Appl. Surf. Sci. 2019, 488, 213–220. [Google Scholar] [CrossRef]
- Fermo, P.; Goidanich, S.; Comite, V.; Toniolo, L.; Gulotta, D. Study and Characterization of Environmental Deposition on Marble and Surrogate Substrates at a Monumental Heritage Site. Geosciences 2018, 8, 349. [Google Scholar] [CrossRef] [Green Version]
- Zárraga, R.; Cervantes, J.; Salazar-Hernandez, C.; Wheeler, G. Effect of the addition of hydroxyl-terminated polydimethylsiloxane to TEOS-based stone consolidants. J. Cult. Herit. 2010, 11, 138–144. [Google Scholar] [CrossRef]
- Directive 2008/50/EC. Cleaner European Parliament and of the Council of 21 May 2008 on Ambient air Quality and Air for Europe. Available online: https://eur-lex.europa.eu/eli/dir/2008/50/oj (accessed on 9 January 2021).
- Ausset, P.; Crovisier, J.L.; Del Monte, M.; Furlan, V.; Girardet, F.; Hammecker, C.; Jeannette, D.; Lefevre, R.A. Experimental study of limestone and sandstone sulphation in polluted realistic conditions: The Lausanne Atmospheric Simulation Chamber (LASC). Atmos. Environ. 1996, 30, 3197–3207. [Google Scholar] [CrossRef]
- Comite, V.; Pozo-Antonio, J.S.; Cardell, C.; Randazzo, L.; La Russa, M.F.; Fermo, P. A multi-analytical approach for the characterization of black crusts on the facade of an historical cathedral. Microchem. J. 2020, 158, 105121. [Google Scholar] [CrossRef]
- Bernardoni, V.; Vecchi, R.; Valli, G.; Piazzalunga, A.; Fermo, P. PM10 source apportionment in Milan (Italy) using time-resolved data. Sci. Total Environ. 2011, 409, 4788–4795. [Google Scholar] [CrossRef] [PubMed]
- Bove, M.C.; Brotto, P.; Calzolai, G.; Cassola, F.; Cavalli, F.; Fermo, P.; Hjorth, J.; Massabò, D.; Nava, S.; Piazzalunga, A.; et al. PM10 source apportionment applying PMF and chemical tracer analysis to ship-borne measurements in the Western Mediterranean. Atmos. Environ. 2016, 125, 140–151. [Google Scholar] [CrossRef]
- Comite, V.; Álvarez de Buergo, M.; Barca, D.; Belfiore, C.M.; Bonazza, A.; La Russa, M.F.; Pezzino, A.; Randazzo, L.; Ruffolo, S.A. Damage monitoring on carbonate stones: Field exposure tests contributing to pollution impact evaluation in two Italian sites. Constr. Build. Mater. 2017, 152, 907–922. [Google Scholar] [CrossRef]




| Sample | Before Aging | After Aging | ||||||
|---|---|---|---|---|---|---|---|---|
| WCA (°) | <R> (μm) | Qft (mg·cm−2) | CA (mg·cm−2·s−1/2) | % RVP | ΔE* | WCA (°) | ΔE* | |
| NT | 38 ± 8 | 12 ± 2 | 546 | 6.6 | – | – | n.d. | 0.4 |
| Alpha | 134 ± 4 | 9 ± 2 | 275 | 0.4 | 43 | 3.2 | 130 ± 5 | 1.7 |
| Alpha + 1500N | 137 ± 2 | 14 ± 1 | 107 | 0.2 | 46 | 1.4 | 135 ± 5 | 1.4 |
| Alpha + TEOS | 140 ± 4 | 13 ± 2 | 461 | 0.3 | 42 | 4.2 | 125 ± 6 | 1.5 |
| TW | 135 ± 4 | 10 ± 3 | 426 | 0.4 | 26 | 4.6 | 140 ± 5 | 0.5 |
| TW + 1500N | 134 ± 5 | 12 ± 4 | 211 | 0.3 | 30 | 7.4 | 136 ± 2 | 2.9 |
| TW + TEOS | 138 ± 2 | 15 ± 2 | 404 | 0.3 | 17 | 4.2 | 132 ± 1 | 1.3 |
| Sample | Sulphate Concentration (ppm) | ||
|---|---|---|---|
| Surface | 1 mm-in Depth | ∆ | |
| NT | 830 ± 40 | 460 ± 20 | 370 |
| Alpha | 960 ± 50 | 460 ± 20 | 500 |
| Alpha + 1500N | 2500 ± 100 | 430 ± 20 | 2070 |
| Alpha + TEOS | 1580 ± 80 | 710 ± 40 | 870 |
| TW | 1900 ± 100 | 1330 ± 70 | 570 |
| TW + 1500N | 2600 ± 100 | 420 ± 20 | 2180 |
| TW + TEOS | 2300 ± 100 | 670 ± 30 | 1630 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pargoletti, E.; Comite, V.; Fermo, P.; Sabatini, V.; Cappelletti, G. Enhanced Historical Limestone Protection by New Organic/Inorganic Additive-Modified Resins. Coatings 2021, 11, 73. https://doi.org/10.3390/coatings11010073
Pargoletti E, Comite V, Fermo P, Sabatini V, Cappelletti G. Enhanced Historical Limestone Protection by New Organic/Inorganic Additive-Modified Resins. Coatings. 2021; 11(1):73. https://doi.org/10.3390/coatings11010073
Chicago/Turabian StylePargoletti, Eleonora, Valeria Comite, Paola Fermo, Valentina Sabatini, and Giuseppe Cappelletti. 2021. "Enhanced Historical Limestone Protection by New Organic/Inorganic Additive-Modified Resins" Coatings 11, no. 1: 73. https://doi.org/10.3390/coatings11010073
APA StylePargoletti, E., Comite, V., Fermo, P., Sabatini, V., & Cappelletti, G. (2021). Enhanced Historical Limestone Protection by New Organic/Inorganic Additive-Modified Resins. Coatings, 11(1), 73. https://doi.org/10.3390/coatings11010073