Production and Characterization of Al-Si Coatings Fabricated by Mechanical Alloying Method on Inconel 625 Superalloy Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
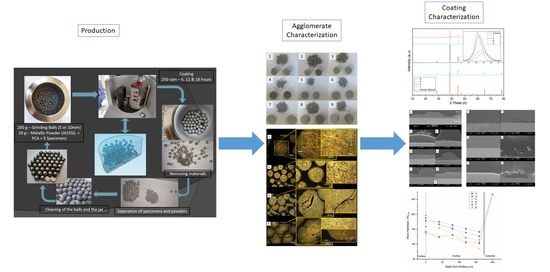
2.2. In Situ Mechanical Alloying and Coating Production
2.3. Characterization
3. Results and Discussion
3.1. Macroscopic Examination
3.2. Characterization of Alloy Particles
3.3. Coating Characterization
4. Conclusions
- Increasing the grinding ball diameter from 5 to 10 mm increases the coating thickness, and provides a homogenized microstructure by introducing a higher energy input on the material’s surface. However, the increased coating thickness in these parameters leads to increased surface roughness values too. Because of the increase, surface roughness values also increase. These values might produce a potential problem on the tribological aspect of potential high temperature, high-speed components.
- Although coating thickness values and mechanical properties improve with the rising milling time, the surface of the coating becomes incompatible with the center or the coating-substrate interface.
- The use of PCA causes cracks and voids in the coatings but enhances the mechanical properties by decreasing grain size. Highest hardness value of 178 ± 41.3 HV1 was attained by using highest amount of PCA (1% stearic acid), and lowest value of 78.8 ± 41.3 HV1 was obtained without using PCA. Therefore, PCA content should be kept at optimal levels to hinder crack formation and for gaining higher hardness values, and milling time needs to be increased when PCA is used.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galetz, M.C. Coatings for superalloys. In Superalloys; InTech: London, UK, 2015; pp. 277–298. [Google Scholar]
- Sivakumar, R.; Mordike, B.L. High temperature coatings for gas turbine blades: A review. Surf. Coat. Technol. 1989, 37, 139–160. [Google Scholar] [CrossRef]
- Jackson, M.R.; Rairden, J. Protective Coatings for Superalloys and the Use of Phase Diagrams; National Bureau of Standards: Gaithersburg, MD, USA, 1978; Volume 1, pp. 423–439. [Google Scholar]
- Sivakumar, R.; Seigle, L.L. On the kinetics of the pack—Aluminization process. Metall. Trans. A 1976, 7, 1073–1079. [Google Scholar] [CrossRef]
- Griffiths, W.T.; Pfeil, L.B. Improvements in Heat Resistant Alloys. UK Patent No. 459,848, 11 January 1937. [Google Scholar]
- Xu, H.; Guo, H.; Gong, S. Thermal barrier coatings. In Developments in High Temperature Corrosion and Protection of Materials; Gao, W., Li, Z., Eds.; Woodhead Publishing: Boca Raton, FL, USA, 2008; pp. 476–491. [Google Scholar]
- Yang, Y.F.; Jiang, C.Y.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Effect of aluminisation characteristics on the microstructure of single phase β-(Ni, Pt) Al coating and the isothermal oxidation behaviour. Corr. Sci. 2016, 106, 43–54. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.R.; Oechner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bul. 2012, 37, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Zhao, Y.G.; Li, W.; Qin, Q.D.; Tian, B.; Hu, S.W. Al–Si coating fused by Al+ Si powders formed on Ti–6Al–4V alloy and its oxidation resistance. Mat. Sci. Eng. A 2006, 430, 142–150. [Google Scholar] [CrossRef]
- Fu, C.; Kong, W.K.; Cao, G.H. Microstructure and oxidation behavior of Al+ Si co-deposited coatings on nickel-based superalloys. Surf. Coat. Technol. 2014, 258, 347–352. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wu, Q.; Li, S.S.; Ma, Y.; Gong, S.K. Preparation and oxidation behaviour of an Al-Si Coating on a Ni3Al based single crystal superalloy IC21. Mater. Sci. Forum. 2013, 747, 575–581. [Google Scholar] [CrossRef]
- Zang, J.; Song, P.; Feng, J.; Xiong, X.; Chen, R.; Liu, G.; Lu, J. Oxidation behaviour of the nickel-based superalloy DZ125 hot-dipped with Al coatings doped by Si. Corr. Sci. 2016, 112, 170–179. [Google Scholar] [CrossRef]
- Kochmańska, A.E. Microstructure of aluminide coatings on Ti6Al4V alloy produced by the slurry method with inorganic binder. Int. J. Mater. Res. 2018, 109, 735–742. [Google Scholar] [CrossRef]
- Grigorieva, R.; Drillet, P.; Mataigne, J.M.; Redjaïmia, A. Phase transformations in the Al-Si coating during the austenitization step. Solid State Phenom. 2011, 172, 784–790. [Google Scholar] [CrossRef]
- Shirvani, K.; Saremi, M.; Nishikata, A.; Tsuru, T. The effect of silicon on cyclic oxidation behavior of aluminide coatings on superalloy IN-738LC. Mater. Sci. Forum. 2004, 461, 335–342. [Google Scholar] [CrossRef]
- Koch, C.C.; Cavin, O.B.; McKamey, C.G.; Scarbrough, J.O. Preparation of ‘‘amorphous’’ Ni60Nb40 by mechanical alloying. Appl. Phys. Lett. 1983, 43, 1017–1019. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S.J.M.R. Novel materials synthesis by mechanical alloying/milling. Int. Mater. Rev. 1998, 43, 101–141. [Google Scholar] [CrossRef]
- Benjamin, J.S. Mechanical alloying. Sci. Am. 1976, 234, 40–49. [Google Scholar] [CrossRef]
- Meng, Y.; Shen, Y.; Chen, C.; Li, Y.; Feng, X. Microstructures and formation mechanism of W–Cu composite coatings on copper substrate prepared by mechanical alloying method. Appl. Surf. Sci. 2013, 282, 757–764. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Deng, R.; Feng, X.; Shen, Y. Microstructure evolution of Cr coatings on Cu substrates prepared by mechanical alloying method. Pow. Technol. 2014, 268, 165–172. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Duan, C.; Feng, X.; Shen, Y. Investigation of Cr–Al composite coatings fabricated on pure Ti substrate via mechanical alloying method: Effects of Cr–Al ratio and milling time on coating, and oxidation behavior of coating. J. Alloys Compd. 2016, 660, 208–219. [Google Scholar] [CrossRef]
- Sudiro, T.; Hia, A.I.J.; Aryanto, D.; Hermanto, B.; Wismogroho, A.S.; Sebayang, P. High temperature cyclic oxidation resistance of 50Cr-50Al coatings mechanically alloyed on low carbon steel. J. Alloys Compd. 2018, 732, 655–665. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.; Shen, Y. Microstructures and properties of TiCp/Al coating synthesized on Ti–6Al–4V alloy substrate using mechanical alloying method. J. Alloys Compd. 2020, 813, 152223. [Google Scholar] [CrossRef]
- Lu, C.; Tian, Y.; Shen, Y.; Feng, X.; Jiang, J. Thermal shock resistance and thermal conductivity of diamond-Cu composite coatings on Cu substrate via mechanical milling method. Surf. Coat. Technol. 2018, 352, 529–540. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, X.; Shen, Y.; Lu, C.; Tian, Y. Oxidation behavior of Cr-AlSi12 composite coatings on Ti-6Al-4V alloy substrate fabricated via high-energy mechanical alloying method. Surf. Coat. Technol. 2019, 367, 212–224. [Google Scholar] [CrossRef]
- Chen, C.L. Influence of V and Heat Treatment on characteristics of WMoNbTaV refractory high-entropy alloy coatings by mechanical alloying. Coatings 2021, 11, 265. [Google Scholar] [CrossRef]
- Othman, A.R.; Sardarinejad, A.; Masrom, A.K. Effect of milling parameters on mechanical alloying of aluminum powders. Int. J. Adv. Manuf. Technol. 2015, 76, 1319–1332. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.; Kaloshkin, S.; Tcherdyntsev, V.; Gorshenkov, M.; Komissarov, A.; Zadorozhnyy, M. Formation of intermetallic Ni–Al coatings by mechanical alloying on the different hardness substrates. J. Alloys Compd. 2014, 586, 373–376. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.Y.; Kaloshkin, S.D.; Churyukanova, M.N.; Borisova, Y.V. Formation of intermetallic Ni-Al coatings by mechanical alloying with different intensities. Metall. Mater. Trans. A 2013, 44, 1779–1784. [Google Scholar] [CrossRef]
- Lü, L.; Lai, M.O. The mechanical alloying process. In Mechanical Alloying; Springer Science & Business Media: Norwell, MA, USA, 1998; pp. 23–67. [Google Scholar]
- Soni, P.R. Mechanical Alloying: Fundamentals and Applications; Cambridge Int Science Publishing: Cambridge, UK, 1999; pp. 15–55. [Google Scholar]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Char. 2007, 58, 883–891. [Google Scholar] [CrossRef]
- Wong, W.; Vo, P.; Irissou, E.; Ryabinin, A.N.; Legoux, J.G.; Yue, S. Effect of particle morphology and size distribution on cold-sprayed pure titanium coatings. J. Therm. Spray Technol. 2013, 22, 1140–1153. [Google Scholar] [CrossRef]
- Brodmann, F.J. Cold spray process parameters: Powders. In The Cold Spray Materials Deposition Process; Champagne, V.K., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2007; pp. 105–116. [Google Scholar]
- Piątkowski, J. The phosphorus interaction on the process forming of primary structure of hypereutectic silumins. Arch. Foundry Eng. 2009, 9, 125–128. [Google Scholar]
- Vijeesh, V.; Prabhu, K.N. Review of microstructure evolution in hypereutectic Al–Si alloys and its effect on wear properties. Trans. Indian Inst. Metal. 2014, 67, 1–18. [Google Scholar]
- Neikov, O.D. Mechanical Alloying. In Handbook of Non-Ferrous Metal Powders; Neikov, O.D., Naboychenko, S.S., Dowson, G., Eds.; Elsevier: Oxford, UK, 2009; pp. 63–79. [Google Scholar]
- Teresiak, A.; Gebert, A.; Savyak, M.; Uhlemann, M.; Mickel, C.; Mattern, N. In situ high temperature XRD studies of the thermal behaviour of the rapidly quenched Mg77Ni18Y5 alloy under hydrogen. J. Alloys Compd. 2005, 398, 156–164. [Google Scholar] [CrossRef]
- Theimer, S.; Graunitz, M.; Schulze, M.; Gaertner, F.; Klassen, T. Optimization adhesion in cold spraying onto hard substrates: A case study for brass coatings. J. Therm. Spray Technol. 2019, 28, 124–134. [Google Scholar] [CrossRef]
- Canakci, A.; Erdemir, F.; Varol, T.; Özkaya, S.; Dalmis, R. Sytentesis of Al-B4C composite coating on low carbon steel by mechanical alloying method. Usak Univ. J. Mater. Sci. 2014, 3, 15–22. [Google Scholar]
- Aryanto, D.; Sudiro, T. Preparation of ferrosilicon-aluminium coating using a mechanical alloying technique: Study of thermal annealing on their structural characteristics. Surf. Coat. Technol. 2018, 337, 35–43. [Google Scholar] [CrossRef]










| Material | Ni | Cr | Mo | Al | Si | Others |
|---|---|---|---|---|---|---|
| Inconel 625 | 60 ± 2 | 21 | 9 | 0.4 max | 0.5 max | bal. |
| Al Powder | - | - | - | 99.9 | - | bal. |
| Si Powder | - | - | - | - | 99 | bal. |
| Sample No | Ball Diameter (mm) | PCA (SA) wt.% | Milling Time (Hour) |
|---|---|---|---|
| 1 | 10 | 0 | 6 |
| 2 | 10 | 0.25 | 6 |
| 3 | 10 | 0.5 | 6 |
| 4 | 5 | 0 | 6 |
| 5 | 5 | 0.25 | 6 |
| 6 | 5 | 0.5 | 6 |
| 7 | 5 | 0.25 | 12 |
| 8 | 5 | 0.5 | 18 |
| 9 | 5 | 1 | 18 |
| Sample | Powder Mixture | 1 (10 mm 6 h 0% PCA) | 4 (5 mm 6 h 0% PCA) | 6 (5 mm 6 h 0.5% PCA) | 8 (5 mm 18 h 0.5% PCA) | |
|---|---|---|---|---|---|---|
| Al (111) Property | ||||||
| Intensity | 23,804 | 14,464 | 20,692 | 9087 | 7282 | |
| 2-Theta (degrees) | 38.64 | 38.70 | 38.68 | 38.76 | 38.82 | |
| d-spacing (Å) | 1.2342 | 1.2326 | 1.2331 | 1.2310 | 1.2294 | |
| FWHM (2θ) | 0.204 | 0.335 | 0.271 | 0.376 | 0.337 | |
| Crystallite size (nm) | 43.98 | 26.26 | 32.46 | 23.40 | 25.38 | |
| Specimen No | Average Thickness (µm) | Roughness (Ra, µm) | Vickers Hardness (HV1) |
|---|---|---|---|
| Substrate | - | - | 273 ± 6.7 |
| 1 | 160.5 ± 22.4 | 19.639 | 91.4 ± 17.4 |
| 2 | 190.4 ± 54.1 | 21.095 | 126 ± 21.9 |
| 3 | 256.7 ± 107.1 | 23.881 | 142 ± 24.3 |
| 4 | 122.3 ± 11.4 | 14.264 | 78.8 ± 12.3 |
| 5 | 126.4 ± 27.6 | 19.571 | 107 ± 18.8 |
| 6 | 128.9 ± 46.3 | 22.314 | 134 ± 24.2 |
| 7 | 176.9 ± 43.5 | 22.792 | 160 ± 21.3 |
| 8 | 182.6 ± 73.7 | 23.311 | 166 ± 32.7 |
| 9 | 164.8 ± 42.1 | 22.405 | 178 ± 41.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köktaş, S.; Önay, A.B.; Kılınç, A.Ç. Production and Characterization of Al-Si Coatings Fabricated by Mechanical Alloying Method on Inconel 625 Superalloy Substrates. Coatings 2021, 11, 1016. https://doi.org/10.3390/coatings11091016
Köktaş S, Önay AB, Kılınç AÇ. Production and Characterization of Al-Si Coatings Fabricated by Mechanical Alloying Method on Inconel 625 Superalloy Substrates. Coatings. 2021; 11(9):1016. https://doi.org/10.3390/coatings11091016
Chicago/Turabian StyleKöktaş, Serhan, Ali Bülent Önay, and Ahmet Çağrı Kılınç. 2021. "Production and Characterization of Al-Si Coatings Fabricated by Mechanical Alloying Method on Inconel 625 Superalloy Substrates" Coatings 11, no. 9: 1016. https://doi.org/10.3390/coatings11091016
APA StyleKöktaş, S., Önay, A. B., & Kılınç, A. Ç. (2021). Production and Characterization of Al-Si Coatings Fabricated by Mechanical Alloying Method on Inconel 625 Superalloy Substrates. Coatings, 11(9), 1016. https://doi.org/10.3390/coatings11091016