Reduction of Surface Residual Lithium Compounds for Single-Crystal LiNi0.6Mn0.2Co0.2O2 via Al2O3 Atomic Layer Deposition and Post-Annealing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterizations
2.3. Electrochemical Measurements
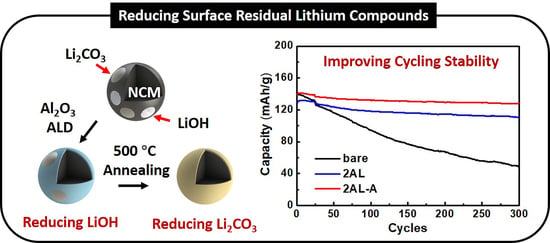
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryu, H.-H.; Park, K.-J.; Yoon, C.S.; Sun, Y.-K. Capacity fading of Ni-rich LiNixCoyMn1-x-yO2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.Q.; Li, L.X.; Sun, C.G.; An, B.G.; Geng, X. Electrochemical properties of LiNi0.6Co0.2Mn0.2O2 cathode materials prepared with different ammonia content. Coatings 2021, 11, 932. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered LiNixCoyMnzO2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Liu, H.; Wolf, M.; Karki, K.; Yu, Y.-S.; Stach, E.A.; Cabana, J.; Chapman, K.W.; Chupas, P.J. Intergranular cracking as a major cause of long-term capacity fading of layered cathodes. Nano Lett. 2017, 17, 3452–3457. [Google Scholar] [CrossRef]
- Jung, R.; Linsenmann, F.; Thomas, R.; Wandt, J.; Solchenbach, S.; Maglia, F.; Stinner, C.; Tromp, M.; Gasteiger, H.A. Nickel, manganese, and cobalt dissolution from Ni-rich NMC and their effects on NMC622-graphite cell. J. Electrochem. Soc. 2019, 166, A378–A389. [Google Scholar] [CrossRef]
- Hatsukade, T.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Origin of carbon dioxide evolved during cycling of nickel-rich layered ncm cathodes. ACS Appl. Mater. Interfaces 2018, 10, 38892–38899. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wang, Z.; Yue, P.; Guo, H.; Wu, F.; Wang, J.; Li, X. Washing effects on electrochemical performance and storage characteristics of LiNi0.8Co0.1Mn0.1O2 as cathode material for lithium-ion batteries. J. Power Sources 2013, 222, 318–325. [Google Scholar] [CrossRef]
- Chen, A.; Wang, K.; Li, J.; Mao, Q.; Xiao, Z.; Zhu, D.; Wang, G.; Liao, P.; He, J.; You, Y.; et al. The formation, detriment and solution of residual lithium compounds on Ni-Rich layered oxides in lithium-ion batteries. Front. Energy Res. 2020, 8, 593009–593024. [Google Scholar] [CrossRef]
- Cho, D.-H.; Jo, C.-H.; Cho, W.; Kim, Y.-J.; Yashiro, H.; Sun, Y.-K.; Myung, S.-T. Effect of residual lithium compounds on layer Ni-rich Li[Ni0.7Mn0.3]O2. J. Electrochem. Soc. 2014, 161, A920–A926. [Google Scholar] [CrossRef]
- Faenza, N.V.; Bruce, L.; Lebens-Higgins, Z.W.; Plitz, I.; Pereira, N.; Piper, L.F.J.; Amatucci, G.G. Editors’ choice—Growth of ambient induced surface impurity species on layered positive electrode materials and impact on electrochemical performance. J. Electrochem. Soc. 2017, 164, A3727–A3741. [Google Scholar] [CrossRef]
- Martinez, A.C.; Grugeon, S.; Cailleu, D.; Courty, M.; Tran-Van, P.; Delobel, B.; Laruelle, S. High reactivity of the nickel-rich LiNi1-x-yMnxCoyO2 layered materials surface towards H2O/CO2 atmosphere and LiPF6-based electrolyte. J. Power Sources 2020, 468, 228204–228213. [Google Scholar] [CrossRef]
- Arai, H.; Okada, S.; Ohtsuka, H.; Ichimura, M.; Yamaki, J. Characterization and cathode performance of Li1−xNi1+xO2 prepared with the excess lithium method. Solid State Ion. 1995, 80, 261–269. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater. 2018, 8, 1702028–1702052. [Google Scholar] [CrossRef]
- Pritzl, D.; Teufl, T.; Freiberg, A.T.S.; Strehle, B.; Sicklinger, J.; Sommer, H.; Hartmann, P.; Gasteiger, H.A. Editors’ choice—Washing of nickel-rich cathode materials for lithium-ion batteries: Towards a mechanistic understanding. J. Electrochem. Soc. 2019, 166, A4056–A4066. [Google Scholar] [CrossRef]
- Jo, J.H.; Jo, C.-H.; Yashiro, H.; Kim, S.-J.; Myung, S.-T. Re-heating effect of Ni-rich cathode material on structure and electrochemical properties. J. Power Sources 2016, 313, 1–8. [Google Scholar] [CrossRef]
- Jo, C.-H.; Cho, D.-H.; Noh, H.-J.; Yashiro, H.; Sun, Y.-K.; Myung, S.T. An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano Res. 2014, 8, 1464–1479. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, Z.; Huang, K.; Chen, Z.; Lv, C.; Dou, H.; Zhang, X. Stabilization of a 4.7 V High-Voltage Nickel-Rich Layered Oxide Cathode for Lithium-Ion Batteries through Boron-Based Surface Residual Lithium-Tuned Interface Modification Engineering. Chemelectrochem 2021, 8, 2014–2021. [Google Scholar] [CrossRef]
- Zhang, S.S.; Ma, L. Oxalic Acid as a cathode additive increasing rate capability of ni-rich layered cathode materials. J. Electrochem. Soc. 2021, 168, 080512–080518. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Warner, J.H.; Manthiram, A. Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries. ACS Energy Lett. 2021, 6, 941–948. [Google Scholar] [CrossRef]
- Li, Q.; Zhuang, W.; Li, Z.; Wu, S.; Li, N.; Gao, M.; Li, W.; Wang, J.; Lu, S. Realizing superior cycle stability of a Ni-Rich layered LiNi0.83Co0.12Mn0.05O2 cathode with a B2O3 surface modification. ChemElectroChem 2020, 7, 998–1006. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Ma, H.; Jeong, H.Y.; Cha, H.; Lee, H.; Yoo, Y.; Park, M.; Cho, J. Controllable solid electrolyte interphase in nickel-rich cathodes by an electrochemical rearrangement for stable lithium-ion batteries. Adv. Mater. 2018, 30, 1704309–1704317. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Bae, C.; Kang, B. Sublimation-induced gas-reacting process for high-energy-density Ni-rich electrode materials. ACS Appl. Mater. Interfaces 2020, 12, 11745–11752. [Google Scholar] [CrossRef]
- Seong, W.M.; Cho, K.H.; Park, J.W.; Park, H.; Eum, D.; Lee, M.H.; Kim, I.S.; Lim, J.; Kang, K. Controlling Residual lithium in high-nickel (>90%) lithium layered oxides for cathodes in lithium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 18662–18669. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Liu, X.; Chen, R. Progress in enhanced fluidization process for particle coating via atomic layer deposition. Chem. Eng. Process. 2021, 159, 108234. [Google Scholar] [CrossRef]
- Cao, K.; Cai, J.; Shan, B.; Chen, R. Surface functionalization on nanoparticles via atomic layer deposition. Sci. Bull. 2020, 65, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, L.; Liu, J.; Adair, K.; Zhao, F.; Sun, Y.; Wu, T.; Bi, X.; Amine, K.; Lu, J.; et al. Atomic/molecular layer deposition for energy storage and conversion. Chem. Soc. Rev. 2021, 50, 3889–3956. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Huang, X.; Liu, T.; Xie, Z.; Wang, Y.; Tian, K.; Bu, L.; Wang, H.; Gao, L.; Zhao, J. Ultrathin Al2O3 coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycleability at extended voltage ranges. Coatings 2019, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.-Z.; Wang, S.-S.; Tai, G.-A.; Zhu, L.; Wang, L.-G.; Zhai, H.-F.; Wu, D.; Li, A.-D.; Li, H. Enhanced electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material by ultrathin ZrO2 coating. J. Alloys Compd. 2016, 657, 593–600. [Google Scholar] [CrossRef]
- Qin, C.; Cao, J.; Chen, J.; Dai, G.; Wu, T.; Chen, Y.; Tang, Y.; Li, A.; Chen, Y. Improvement of electrochemical performance of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode active material by ultrathin TiO2 coating. Dalton Trans. 2016, 45, 9669–9675. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Ren, C.; Tai, G.-A.; Zhang, X.; Li, A.-D.; Wu, D.; Li, H.; Zhou, F. Ultrathin ZnO coating for improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material. J. Power Sources 2014, 266, 433–439. [Google Scholar] [CrossRef]
- Manandhar, K.; Wollmershauser, J.A.; Boercker, J.E.; Feigelson, B.N. Growth per cycle of alumina atomic layer deposition on nano- and micro-powders. J. Vac. Sci. Technol. A 2016, 34, 021519–021528. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Wank, J.R.; George, S.M.; Weimer, A.W. Coating fine nickel particles with Al2O3 Utilizing an atomic layer deposition-fluidized bed reactor (ALD-FBR). J. Am. Ceram. Soc. 2004, 87, 762–765. [Google Scholar] [CrossRef]
- Matero, R.; Rahtu, A.; Ritala, M.; Leskelä, M.; Sajavaara, T. Effect of water dose on the atomic layer deposition rate of oxide thin films. Thin Solid Film. 2000, 368, 1–7. [Google Scholar] [CrossRef]
- Jackson, D.H.K.; Kuech, T.F. Electrochemical effects of annealing on atomic layer deposited Al2O3 coatings on LiNi0.5Mn0.3Co0.2O2. J. Power Sources 2017, 365, 61–67. [Google Scholar] [CrossRef]
- Gao, Y.; Park, J.; Liang, X. Comprehensive Study of Al- and Zr-modified LiNi0.8Mn0.1Co0.1O2 through synergy of coating and doping. ACS Appl. Energy Mater. 2020, 3, 8978–8987. [Google Scholar] [CrossRef]
- Tang, W.J.; Chen, Z.X.; Xiong, F.; Chen, F.; Huang, C.; Gao, Q.; Wang, T.Z.; Yang, Z.H.; Zhang, W.X. An effective etching-induced coating strategy to shield LiNi0.8Co0.1Mn0.1O2 electrode materials by LiAlO2. J. Power Sources 2019, 412, 246–254. [Google Scholar] [CrossRef]
- Liu, S.; Dang, Z.; Liu, D.; Zhang, C.; Huang, T.; Yu, A. Comparative studies of zirconium doping and coating on LiNi0.6Co0.2Mn0.2O2 cathode material at elevated temperatures. J. Power Sources 2018, 396, 288–296. [Google Scholar] [CrossRef]
- Tatara, R.; Karayaylali, P.; Yu, Y.; Zhang, Y.R.; Giordano, L.; Maglia, F.; Jung, R.; Schmidt, J.P.; Lund, I.; Shao-Horn, Y. The effect of electrode-electrolyte interface on the electrochemical impedance spectra for positive electrode in Li-ion battery. J. Electrochem. Soc. 2018, 166, A5090–A5098. [Google Scholar] [CrossRef]
- Wood, K.N.; Teeter, G. XPS on Li-battery-related compounds: Analysis of inorganic sei phases and a methodology for charge correction. ACS Appl. Energy Mater. 2018, 1, 4493–4504. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.P.C.; Kwabi, D.G.; Quinlan, R.A.; Mansour, A.N.; Grimaud, A.; Lee, Y.-L.; Lu, Y.-C.; Shao-Horn, Y. Thermal stability of Li2O2 and Li2O for Li-air batteries: In situ XRD and XPS studies. J. Electrochem. Soc. 2013, 160, A824–A831. [Google Scholar] [CrossRef]
- Chen, L.; Connell, J.G.; Nie, A.; Huang, Z.; Zavadil, K.R.; Klavetter, K.C.; Yuan, Y.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Libera, J.A.; et al. Lithium metal protected by atomic layer deposition metal oxide for high performance anodes. J. Mater. Chem. A 2017, 5, 12297–12309. [Google Scholar] [CrossRef]
- Hoskins, A.L.; McNeary, W.W.; Millican, S.L.; Gossett, T.A.; Lai, A.; Gao, Y.; Liang, X.; Musgrave, C.B.; Weimer, A.W. Nonuniform growth of sub-2 nanometer atomic layer deposited alumina films on lithium nickel manganese cobalt oxide cathode battery materials. ACS Appl. Nano Mater. 2019, 2, 6989–6997. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, P.; Liu, Z.; Ma, B.; Zhou, Y.; Tian, X. Fluorine doping induced crystal space change and performance improvement of single crystalline LiNi0.6Co0.2Mn0.2O2 layered cathode materials. ChemElectroChem 2021, 8, e202100756. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, S.; Rajagopalan, R.; Huang, X.; Ren, Y.; Sun, D.; Wang, H.; Tang, Y. Dual-element-modified single-crystal LiNi0.6Co0.2Mn0.2O2 as a highly stable cathode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 43039–43050. [Google Scholar] [CrossRef]
- Bao, W.; Qian, G.; Zhao, L.; Yu, Y.; Su, L.; Cai, X.; Zhao, H.; Zuo, Y.; Zhang, Y.; Li, H.; et al. Simultaneous enhancement of interfacial stability and kinetics of single-crystal LiNi0.6Mn0.2Co0.2O2 through optimized surface coating and doping. Nano Lett. 2020, 20, 8832–8840. [Google Scholar] [CrossRef]
- Xu, S.; Jing, N.; Hao, H.; Wang, M.; Wang, Z.; Yang, L.; Wang, G.; Chen, J.; Wang, G. Enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2 by a negative-thermal-expansion material at elevated temperature. Energy Technol. 2021, 9, 2100183. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.; Xu, G.; Wang, M.; Gu, Y. Boron-doped single crystal LiNi0.6Mn0.2Co0.2O2 with improved electrochemical performance for lithium-ion batteries. Ionics 2019, 25, 5819–5827. [Google Scholar] [CrossRef]
- Li, G.; You, L.; Wen, Y.; Zhang, C.; Huang, B.; Chu, B.; Wu, J.H.; Huang, T.; Yu, A. Ultrathin Li-Si-O coating layer to stabilize the surface structure and prolong the cycling life of single-crystal LiNi0.6Co0.2Mn0.2O2 cathode materials at 4.5 V. ACS Appl. Mater. Interfaces 2021, 13, 10952–10963. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Xiong, D.; Hao, Y.; Li, J.; Kou, H.; Yan, B.; Li, D.; Lu, S.; Koo, A.; et al. Significantly improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 2018, 44, 111–120. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Banis, M.N.; Lushington, A.; Li, R.; Cai, M.; Sun, X. Atomic layer deposition of solid-state electrolyte coated cathode materials with superior high-voltage cycling behavior for lithium ion battery application. Energy Environ. Sci. 2014, 7, 768–778. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xiang, J.; Yi, G.; Tang, Y.; Shao, H.; Liu, X.; Shan, B.; Chen, R. Reduction of Surface Residual Lithium Compounds for Single-Crystal LiNi0.6Mn0.2Co0.2O2 via Al2O3 Atomic Layer Deposition and Post-Annealing. Coatings 2022, 12, 84. https://doi.org/10.3390/coatings12010084
Li J, Xiang J, Yi G, Tang Y, Shao H, Liu X, Shan B, Chen R. Reduction of Surface Residual Lithium Compounds for Single-Crystal LiNi0.6Mn0.2Co0.2O2 via Al2O3 Atomic Layer Deposition and Post-Annealing. Coatings. 2022; 12(1):84. https://doi.org/10.3390/coatings12010084
Chicago/Turabian StyleLi, Jiawei, Junren Xiang, Ge Yi, Yuanting Tang, Huachen Shao, Xiao Liu, Bin Shan, and Rong Chen. 2022. "Reduction of Surface Residual Lithium Compounds for Single-Crystal LiNi0.6Mn0.2Co0.2O2 via Al2O3 Atomic Layer Deposition and Post-Annealing" Coatings 12, no. 1: 84. https://doi.org/10.3390/coatings12010084
APA StyleLi, J., Xiang, J., Yi, G., Tang, Y., Shao, H., Liu, X., Shan, B., & Chen, R. (2022). Reduction of Surface Residual Lithium Compounds for Single-Crystal LiNi0.6Mn0.2Co0.2O2 via Al2O3 Atomic Layer Deposition and Post-Annealing. Coatings, 12(1), 84. https://doi.org/10.3390/coatings12010084